HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Ethics
2.2. Analysis of Cellular mRNA Expression
2.3. Analysis of Immune Landscape Features
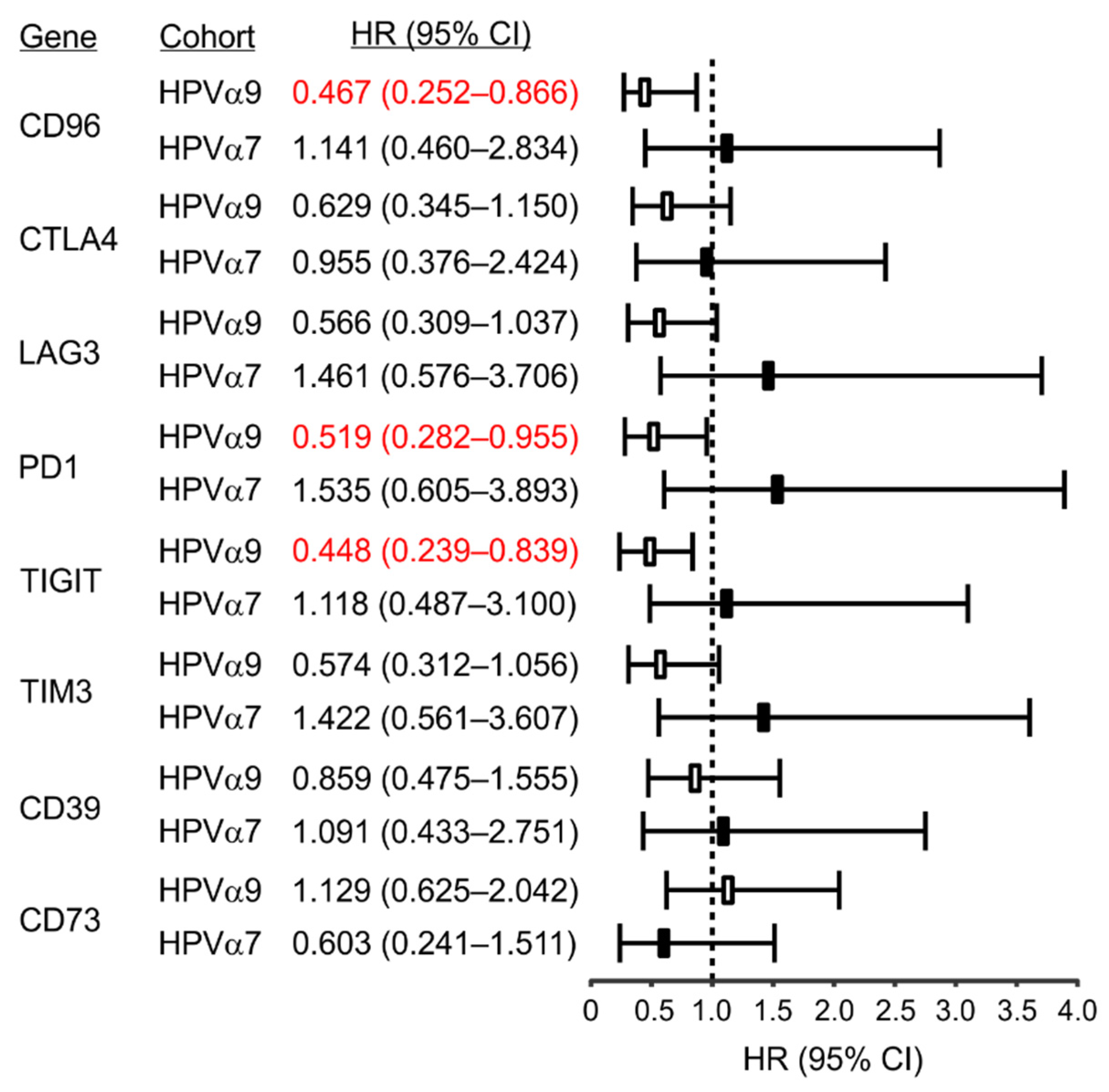
2.4. Survival Analysis
3. Results
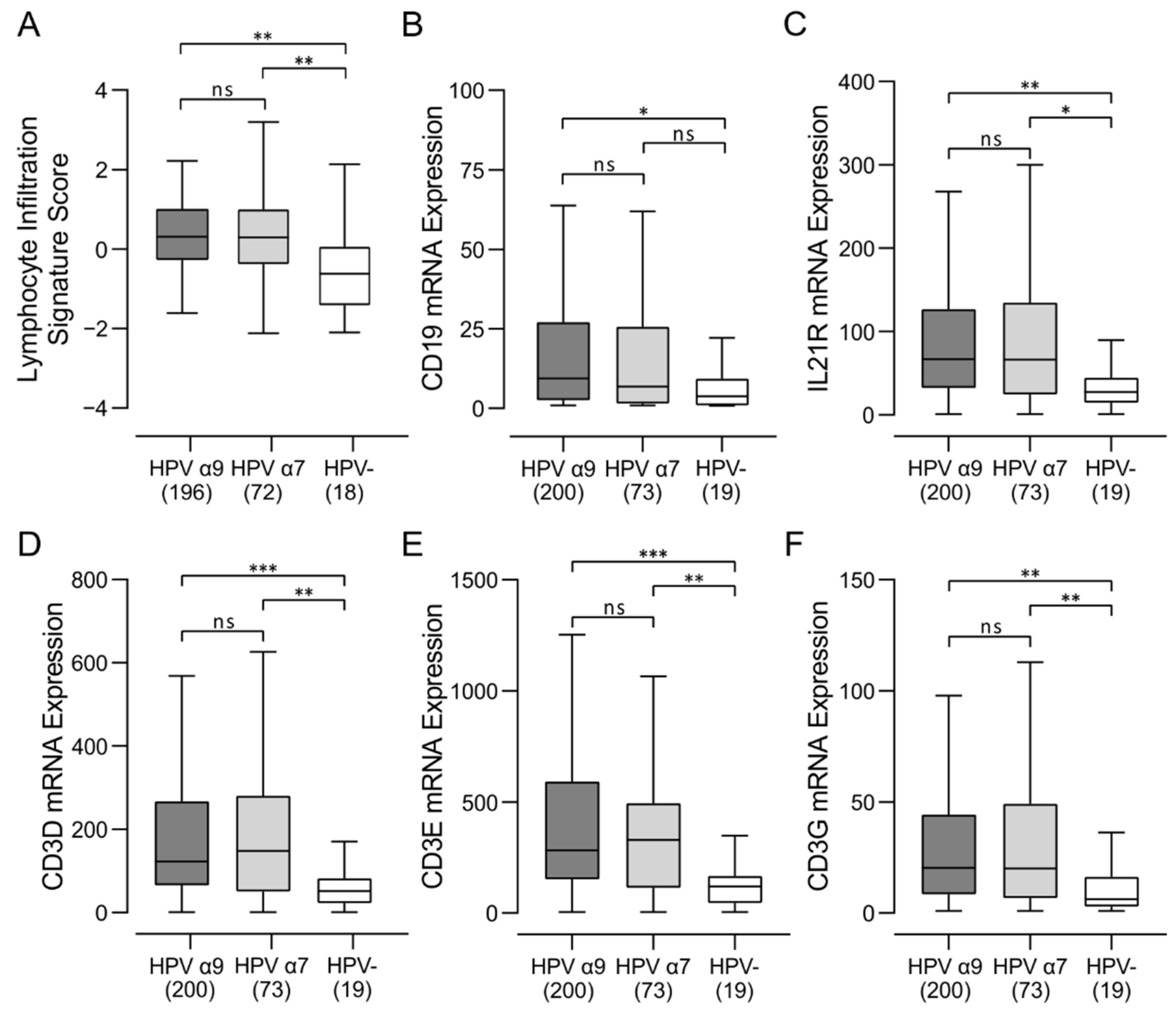
3.1. HPV-Positive and HPV-Negative CC Exhibit Strong Differences in Lymphocyte Infiltration
3.2. Higher Levels of B, T, and NK Lymphocytes Are Present in HPV-Positive CC
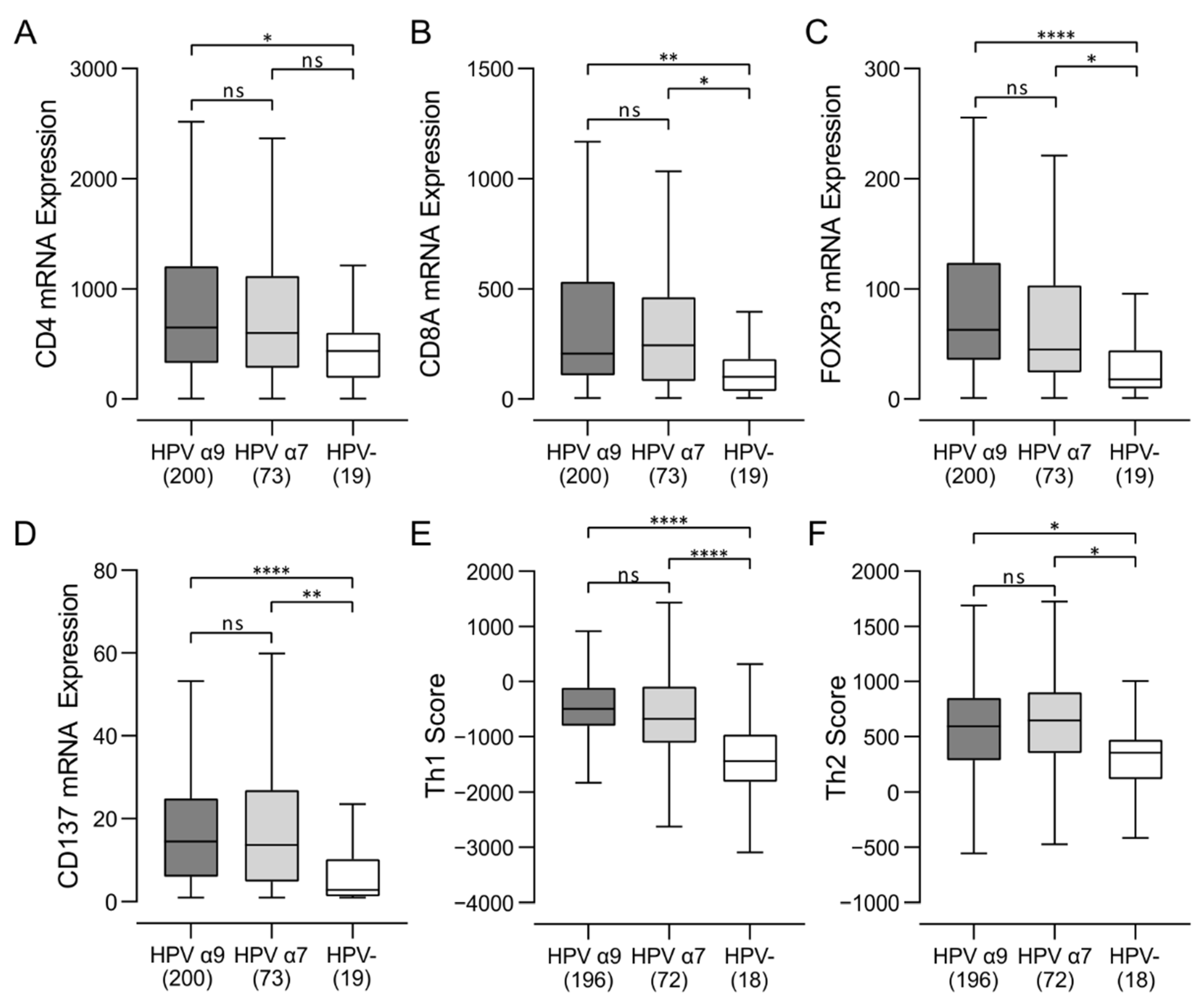
3.3. Higher Levels of CD4+, CD8+ T Cells, and T Regulatory Cells Were Present in HPV-Positive CC
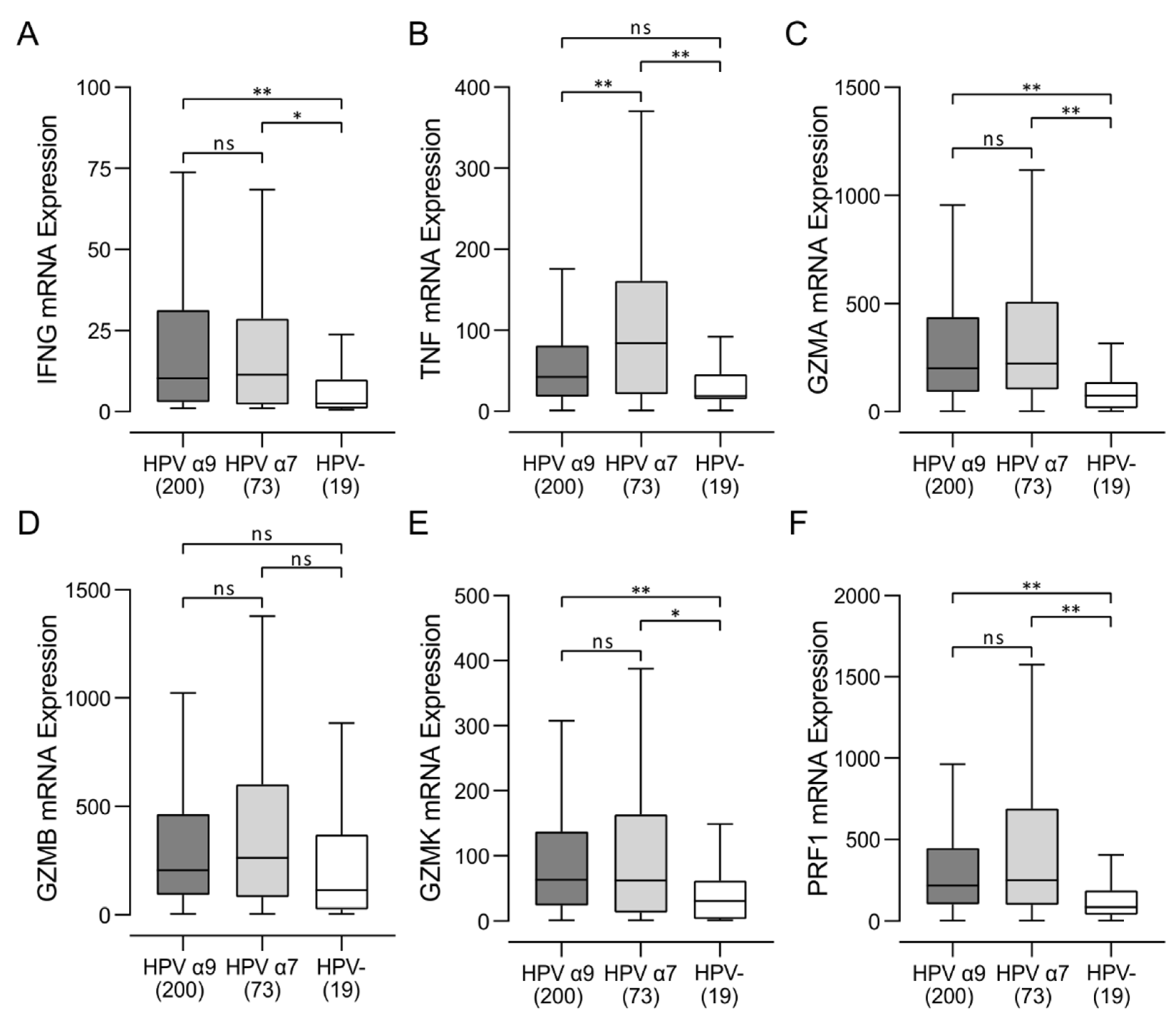
3.4. HPV-Positive CC Expressed Higher Levels of Multiple T-Cell Effector Molecules Than HPV-Negative CC with Characteristics of a T-Cell-Inflamed Phenotype
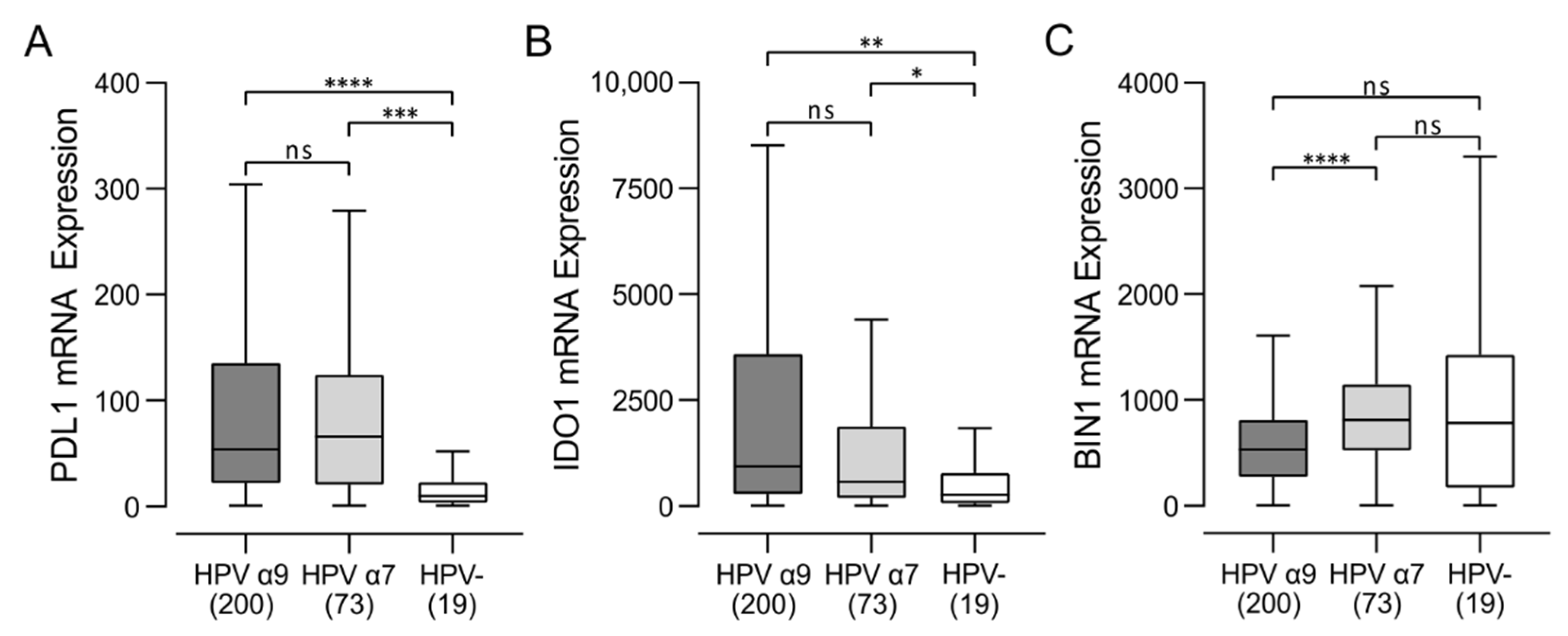
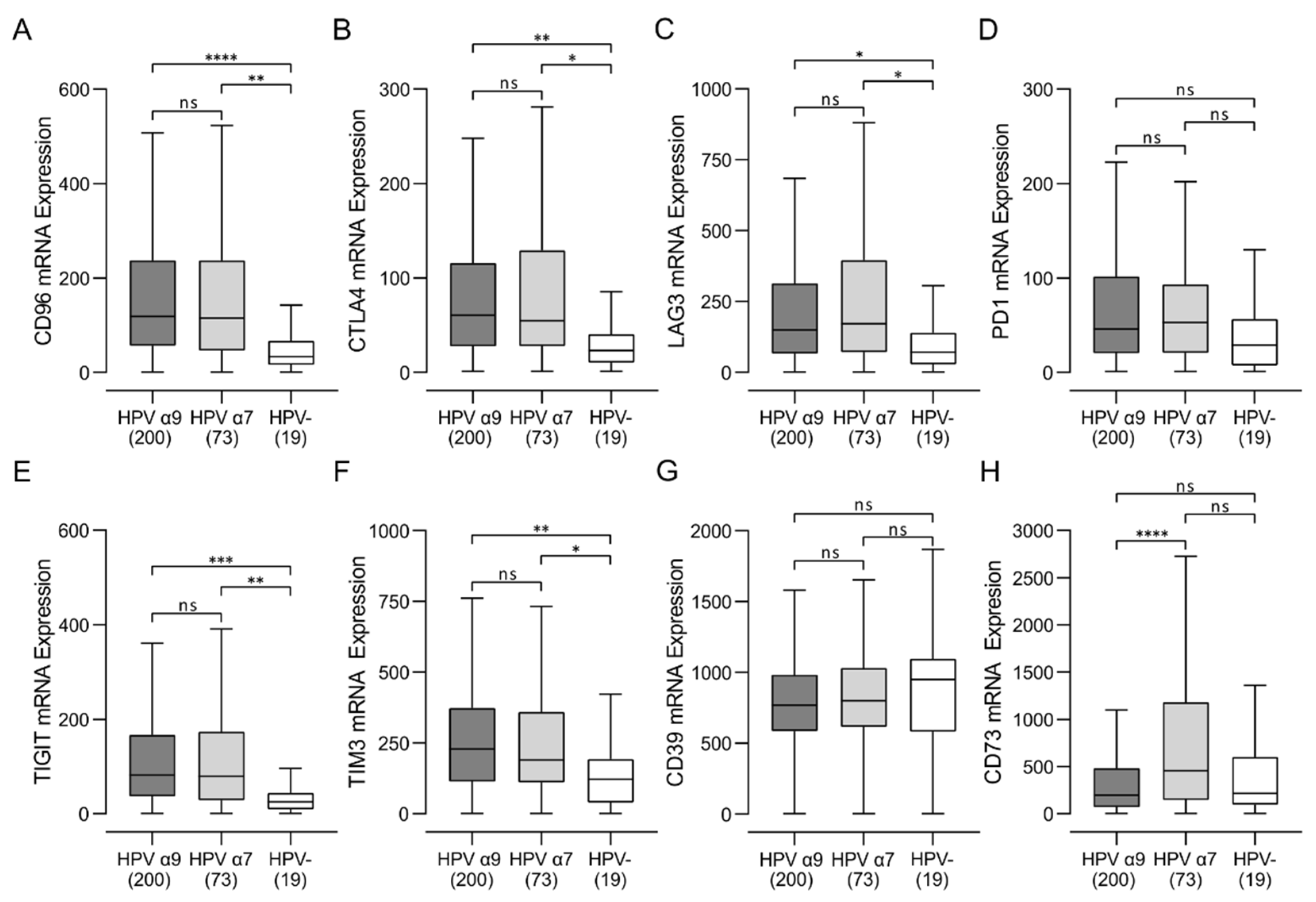
3.5. HPV-Positive CC Express High Levels of Multiple Immune Checkpoint Markers
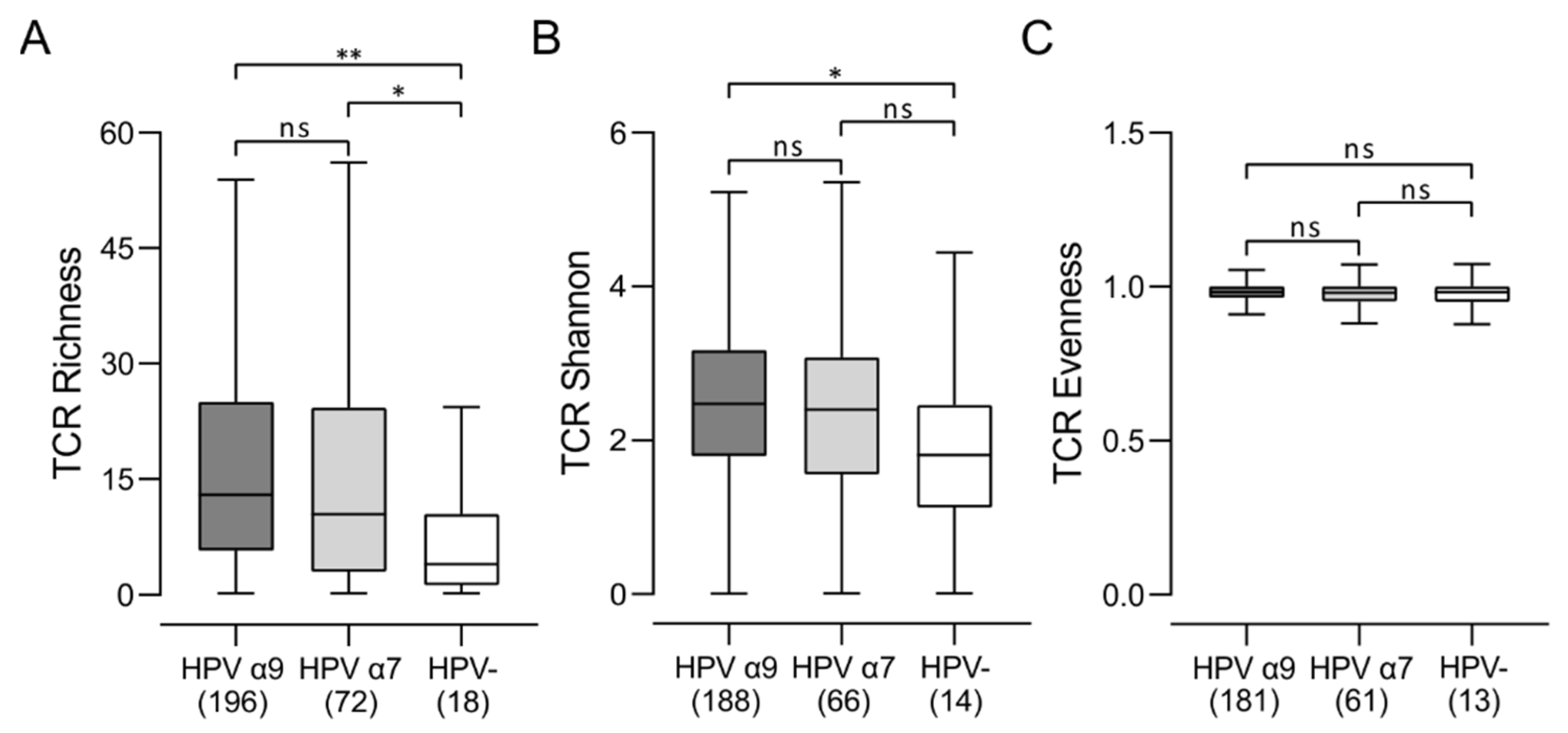
3.6. Comparison of the T-Cell Receptor Repertoire between HPV-Positive and HPV-Negative CC
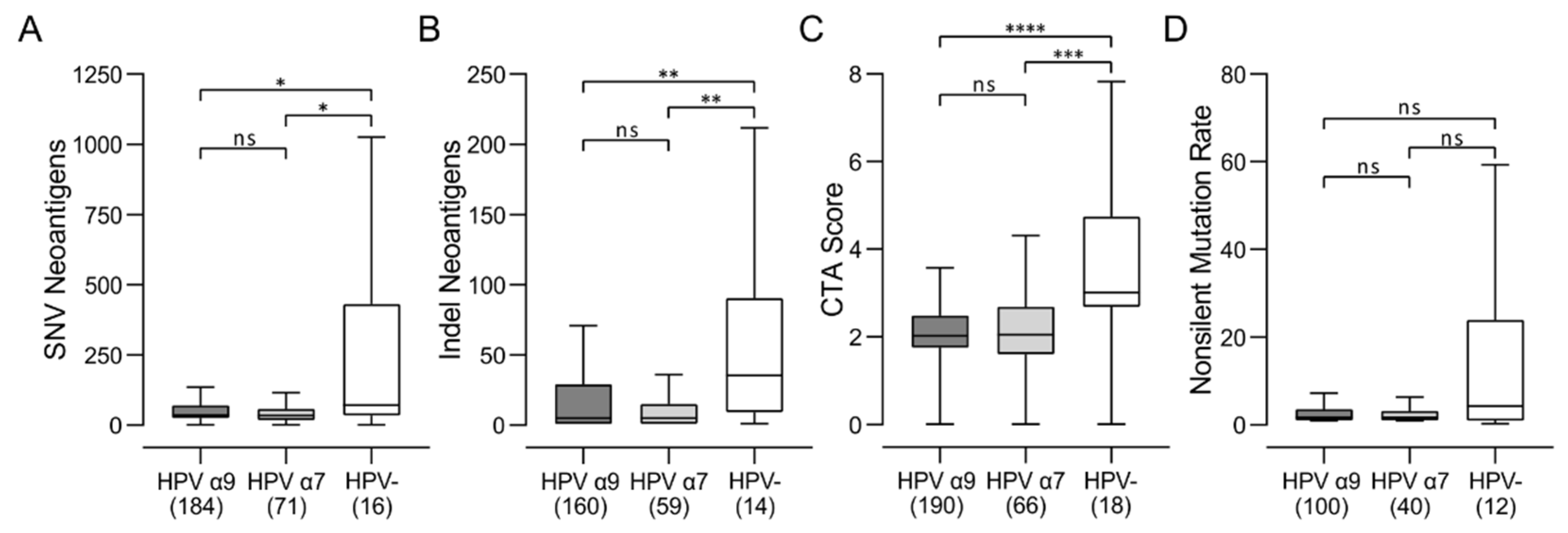
3.7. HPV-Negative CC Express High Levels of Potential Neoantigens
3.8. Comparison of the Immune Landscape between HPV α9-Positive CC with Integrated or Non-Integrated Viral Genomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjose, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Fernandes, A.; Viveros-Carreno, D.; Hoegl, J.; Avila, M.; Pareja, R. Human papillomavirus-independent cervical cancer. Int. J. Gynecol. Cancer 2022, 32, 1–7. [Google Scholar] [CrossRef]
- Weinstock, H.; Berman, S.; Cates, W., Jr. Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspect. Sex. Reprod. Health 2004, 36, 6–10. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Li, Z.; Xirasagar, S.; Maes, P.; Kaminsky, D.; Liou, D.; Sun, Q.; Kaur, R.; Huyen, Y.; McBride, A.A. The Papillomavirus Episteme: A major update to the papillomavirus sequence database. Nucleic Acids Res. 2017, 45, D499–D506. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomavirus infections—A major cause of human cancers. Biochim. Biophys. Acta 1996, 1288, F55–F78. [Google Scholar] [CrossRef]
- Riou, G.; Favre, M.; Jeannel, D.; Bourhis, J.; Le Doussal, V.; Orth, G. Association between poor prognosis in early-stage invasive cervical carcinomas and non-detection of HPV DNA. Lancet 1990, 335, 1171–1174. [Google Scholar] [CrossRef]
- Rodriguez-Carunchio, L.; Soveral, I.; Steenbergen, R.D.; Torne, A.; Martinez, S.; Fuste, P.; Pahisa, J.; Marimon, L.; Ordi, J.; del Pino, M. HPV-negative carcinoma of the uterine cervix: A distinct type of cervical cancer with poor prognosis. BJOG 2015, 122, 119–127. [Google Scholar] [CrossRef]
- Onuki, M.; Matsumoto, K.; Tenjimbayashi, Y.; Tasaka, N.; Akiyama, A.; Sakurai, M.; Minaguchi, T.; Oki, A.; Satoh, T.; Yoshikawa, H. Human papillomavirus genotype and prognosis of cervical cancer: Favorable survival of patients with HPV16-positive tumors. Papillomavirus Res. 2018, 6, 41–45. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Lagheden, C.; Eklund, C.; Nordqvist Kleppe, S.; Andrae, B.; Elfstrom, K.M.; Dillner, J.; Sparen, P.; Sundstrom, K. High-risk human papillomavirus status and prognosis in invasive cervical cancer: A nationwide cohort study. PLoS Med. 2018, 15, e1002666. [Google Scholar] [CrossRef]
- Xing, B.; Guo, J.; Sheng, Y.; Wu, G.; Zhao, Y. Human Papillomavirus-Negative Cervical Cancer: A Comprehensive Review. Front. Oncol. 2020, 10, 606335. [Google Scholar] [CrossRef]
- Yoshida, H.; Shiraishi, K.; Kato, T. Molecular Pathology of Human Papilloma Virus-Negative Cervical Cancers. Cancers 2021, 13, 6351. [Google Scholar] [CrossRef] [PubMed]
- Hang, D.; Jia, M.; Ma, H.; Zhou, J.; Feng, X.; Lyu, Z.; Yin, J.; Cui, H.; Yin, Y.; Jin, G.; et al. Independent prognostic role of human papillomavirus genotype in cervical cancer. BMC Infect. Dis. 2017, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.M.; Daling, J.R.; Shera, K.A.; Madeleine, M.M.; McKnight, B.; Galloway, D.A.; Porter, P.L.; McDougall, J.K. Human papillomavirus and prognosis of invasive cervical cancer: A population-based study. J. Clin. Oncol. 2001, 19, 1906–1915. [Google Scholar] [CrossRef]
- Ruiz, F.J.; Inkman, M.; Rashmi, R.; Muhammad, N.; Gabriel, N.; Miller, C.A.; McLellan, M.D.; Goldstein, M.; Markovina, S.; Grigsby, P.W.; et al. HPV transcript expression affects cervical cancer response to chemoradiation. JCI Insight 2021, 6, e138734. [Google Scholar] [CrossRef] [PubMed]
- Basukala, O.; Banks, L. The Not-So-Good, the Bad and the Ugly: HPV E5, E6 and E7 Oncoproteins in the Orchestration of Carcinogenesis. Viruses 2021, 13, 1892. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhou, Q.; Luo, A.; Li, X.; Li, K.; Li, W.; Yu, M.; Amanullah, M.; Lu, B.; Lu, W.; et al. Integrated analysis of virus and host transcriptomes in cervical cancer in Asian and Western populations. Genomics 2021, 113, 1554–1564. [Google Scholar] [CrossRef]
- Chen, L.; Luan, S.; Xia, B.; Liu, Y.; Gao, Y.; Yu, H.; Mu, Q.; Zhang, P.; Zhang, W.; Zhang, S.; et al. Integrated analysis of HPV-mediated immune alterations in cervical cancer. Gynecol. Oncol. 2018, 149, 248–255. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Borcoman, E.; Le Tourneau, C. Keynote-158 study, FDA granted accelerated approval of pembrolizumab for the treatment of patients with advanced PD-L1-positive cervical cancer. Ann. Transl. Med. 2020, 8, 1611. [Google Scholar] [CrossRef] [PubMed]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.D.; Chen, Z.; Saller, C.; Tarvin, K.; Carvalho, A.L.; Scapulatempo-Neto, C.; Silveira, H.C.; Fregnani, J.H.; Creighton, C.J.; Anderson, M.L.; et al. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Banister, C.E.; Liu, C.; Pirisi, L.; Creek, K.E.; Buckhaults, P.J. Identification and characterization of HPV-independent cervical cancers. Oncotarget 2017, 8, 13375–13386. [Google Scholar] [CrossRef]
- Brant, A.C.; Menezes, A.N.; Felix, S.P.; Almeida, L.M.; Moreira, M.A.M. Preferential expression of a HPV genotype in invasive cervical carcinomas infected by multiple genotypes. Genomics 2020, 112, 2942–2948. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e814. [Google Scholar] [CrossRef]
- Gameiro, S.F.; Kolendowski, B.; Zhang, A.; Barrett, J.W.; Nichols, A.C.; Torchia, J.; Mymryk, J.S. Human papillomavirus dysregulates the cellular apparatus controlling the methylation status of H3K27 in different human cancers to consistently alter gene expression regardless of tissue of origin. Oncotarget 2017, 8, 72564–72576. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e411. [Google Scholar] [CrossRef]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Corrales, L.; Williams, J.; Horton, B.; Sivan, A.; Spranger, S. Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment. Adv. Exp. Med. Biol. 2017, 1036, 19–31. [Google Scholar] [CrossRef]
- Muller, A.J.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005, 11, 312–319. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Hasko, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Augustin, R.C.; Leone, R.D.; Naing, A.; Fong, L.; Bao, R.; Luke, J.J. Next steps for clinical translation of adenosine pathway inhibition in cancer immunotherapy. J. Immunother. Cancer 2022, 10, e004089. [Google Scholar] [CrossRef] [PubMed]
- Kidman, J.; Principe, N.; Watson, M.; Lassmann, T.; Holt, R.A.; Nowak, A.K.; Lesterhuis, W.J.; Lake, R.A.; Chee, J. Characteristics of TCR Repertoire Associated With Successful Immune Checkpoint Therapy Responses. Front. Immunol. 2020, 11, 587014. [Google Scholar] [CrossRef] [PubMed]
- Rosati, E.; Dowds, C.M.; Liaskou, E.; Henriksen, E.K.K.; Karlsen, T.H.; Franke, A. Overview of methodologies for T-cell receptor repertoire analysis. BMC Biotechnol. 2017, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Zehbe, I.; Kaufmann, A.M.; Schmidt, M.; Hohn, H.; Maeurer, M.J. Human papillomavirus 16 E6-specific CD45RA+ CCR7+ high avidity CD8+ T cells fail to control tumor growth despite interferon-gamma production in patients with cervical cancer. J. Immunother. 2007, 30, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Greenfield, W.; Moerman-Herzog, A.; Coleman, H.N. Cross-Reactivity, Epitope Spreading, and De Novo Immune Stimulation Are Possible Mechanisms of Cross-Protection of Nonvaccine Human Papillomavirus (HPV) Types in Recipients of HPV Therapeutic Vaccines. Clin. Vaccine Immunol. 2015, 22, 679–687. [Google Scholar] [CrossRef]
- Fratta, E.; Coral, S.; Covre, A.; Parisi, G.; Colizzi, F.; Danielli, R.; Nicolay, H.J.; Sigalotti, L.; Maio, M. The biology of cancer testis antigens: Putative function, regulation and therapeutic potential. Mol. Oncol. 2011, 5, 164–182. [Google Scholar] [CrossRef]
- Gjerstorff, M.F.; Andersen, M.H.; Ditzel, H.J. Oncogenic cancer/testis antigens: Prime candidates for immunotherapy. Oncotarget 2015, 6, 15772–15787. [Google Scholar] [CrossRef]
- Fleischmann, M.; Chatzikonstantinou, G.; Fokas, E.; Wichmann, J.; Christiansen, H.; Strebhardt, K.; Rodel, C.; Tselis, N.; Rodel, F. Molecular Markers to Predict Prognosis and Treatment Response in Uterine Cervical Cancer. Cancers 2021, 13, 5748. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Lameiras, S.; Deloger, M.; Morel, A.; Vacher, S.; Lecerf, C.; Dupain, C.; Jeannot, E.; Girard, E.; Baulande, S.; et al. Human papilloma virus (HPV) integration signature in Cervical Cancer: Identification of MACROD2 gene as HPV hot spot integration site. Br. J. Cancer 2021, 124, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef]
- Shamseddine, A.A.; Burman, B.; Lee, N.Y.; Zamarin, D.; Riaz, N. Tumor Immunity and Immunotherapy for HPV-Related Cancers. Cancer Discov. 2021, 11, 1896–1912. [Google Scholar] [CrossRef]
- van der Burg, S.H.; Piersma, S.J.; de Jong, A.; van der Hulst, J.M.; Kwappenberg, K.M.; van den Hende, M.; Welters, M.J.; Van Rood, J.J.; Fleuren, G.J.; Melief, C.J.; et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc. Natl. Acad. Sci. USA 2007, 104, 12087–12092. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.C.; Lin, R.H.; Lien, H.C.; Ho, H.N.; Hsu, S.M.; Huang, S.C. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J. Immunol. 2001, 167, 2972–2978. [Google Scholar] [CrossRef] [PubMed]
- Bais, A.G.; Beckmann, I.; Lindemans, J.; Ewing, P.C.; Meijer, C.J.; Snijders, P.J.; Helmerhorst, T.J. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J. Clin. Pathol. 2005, 58, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.F.; Ghasemi, F.; Barrett, J.W.; Koropatnick, J.; Nichols, A.C.; Mymryk, J.S.; Maleki Vareki, S. Treatment-naive HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology 2018, 7, e1498439. [Google Scholar] [CrossRef]
- Heeren, A.M.; Rotman, J.; Stam, A.G.M.; Pocorni, N.; Gassama, A.A.; Samuels, S.; Bleeker, M.C.G.; Mom, C.H.; Zijlmans, H.; Kenter, G.G.; et al. Efficacy of PD-1 blockade in cervical cancer is related to a CD8(+)FoxP3(+)CD25(+) T-cell subset with operational effector functions despite high immune checkpoint levels. J. Immunother. Cancer 2019, 7, 43. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Qiu, J.; Qu, X.; Peng, J.; Lu, C.; Zhang, M.; Zhang, M.; Qi, X.; Li, G.; et al. Targeting CD96 overcomes PD-1 blockade resistance by enhancing CD8+ TIL function in cervical cancer. J. Immunother. Cancer 2022, 10, e003667. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, D.; Zhu, Y.; Pang, N.; Ding, J. The role of Tim-3/Galectin-9 pathway in T-cell function and prognosis of patients with human papilloma virus-associated cervical carcinoma. FASEB J. 2021, 35, e21401. [Google Scholar] [CrossRef]
- Kassardjian, A.; Moatamed, N.A. Expression of immune checkpoint regulators, cytotoxic T lymphocyte antigen 4 (CTLA-4), and CD137 in cervical carcinoma. Int. J. Clin. Exp. Pathol. 2021, 14, 1038–1047. [Google Scholar] [PubMed]
- Zhang, A.W.; McPherson, A.; Milne, K.; Kroeger, D.R.; Hamilton, P.T.; Miranda, A.; Funnell, T.; Little, N.; de Souza, C.P.E.; Laan, S.; et al. Interfaces of Malignant and Immunologic Clonal Dynamics in Ovarian Cancer. Cell 2018, 173, 1755–1769.e1722. [Google Scholar] [CrossRef] [PubMed]
- Schietinger, A.; Philip, M.; Krisnawan, V.E.; Chiu, E.Y.; Delrow, J.J.; Basom, R.S.; Lauer, P.; Brockstedt, D.G.; Knoblaugh, S.E.; Hammerling, G.J.; et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity 2016, 45, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Lanitis, E.; Dangaj, D.; Irving, M.; Coukos, G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann. Oncol. 2017, 28, xii18–xii32. [Google Scholar] [CrossRef]
- Ferrall, L.; Lin, K.Y.; Roden, R.B.S.; Hung, C.F.; Wu, T.C. Cervical Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2021, 27, 4953–4973. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell-cancer cycle: Advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Imai, C.; Iwamoto, S.; Campana, D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005, 106, 376–383. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Doran, S.L.; Stevanovic, S.; Adhikary, S.; Gartner, J.J.; Jia, L.; Kwong, M.L.M.; Faquin, W.C.; Hewitt, S.M.; Sherry, R.M.; Yang, J.C.; et al. T-Cell Receptor Gene Therapy for Human Papillomavirus-Associated Epithelial Cancers: A First-in-Human, Phase I/II Study. J. Clin. Oncol. 2019, 37, 2759–2768. [Google Scholar] [CrossRef]
- van Hall, T.; Andre, P.; Horowitz, A.; Ruan, D.F.; Borst, L.; Zerbib, R.; Narni-Mancinelli, E.; van der Burg, S.H.; Vivier, E. Monalizumab: Inhibiting the novel immune checkpoint NKG2A. J. Immunother. Cancer 2019, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yanez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Enomoto, T.; Kast, W.M.; McCormack, M.; Tan, D.S.P.; Wu, X.; Gonzalez-Martin, A. Integration of immunotherapy into treatment of cervical cancer: Recent data and ongoing trials. Cancer Treat. Rev. 2022, 106, 102385. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, A.M.; Salnikov, M.; Gameiro, S.F.; Maleki Vareki, S.; Mymryk, J.S. HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct. J. Clin. Med. 2022, 11, 4825. https://doi.org/10.3390/jcm11164825
Evans AM, Salnikov M, Gameiro SF, Maleki Vareki S, Mymryk JS. HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct. Journal of Clinical Medicine. 2022; 11(16):4825. https://doi.org/10.3390/jcm11164825
Chicago/Turabian StyleEvans, Andris M., Mikhail Salnikov, Steven F. Gameiro, Saman Maleki Vareki, and Joe S. Mymryk. 2022. "HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct" Journal of Clinical Medicine 11, no. 16: 4825. https://doi.org/10.3390/jcm11164825
APA StyleEvans, A. M., Salnikov, M., Gameiro, S. F., Maleki Vareki, S., & Mymryk, J. S. (2022). HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct. Journal of Clinical Medicine, 11(16), 4825. https://doi.org/10.3390/jcm11164825






