The Relationship between the M1/M2 Macrophage Polarization and the Degree of Ossicular Erosion in Human Acquired Cholesteatoma: An Immunohistochemical Study
Abstract
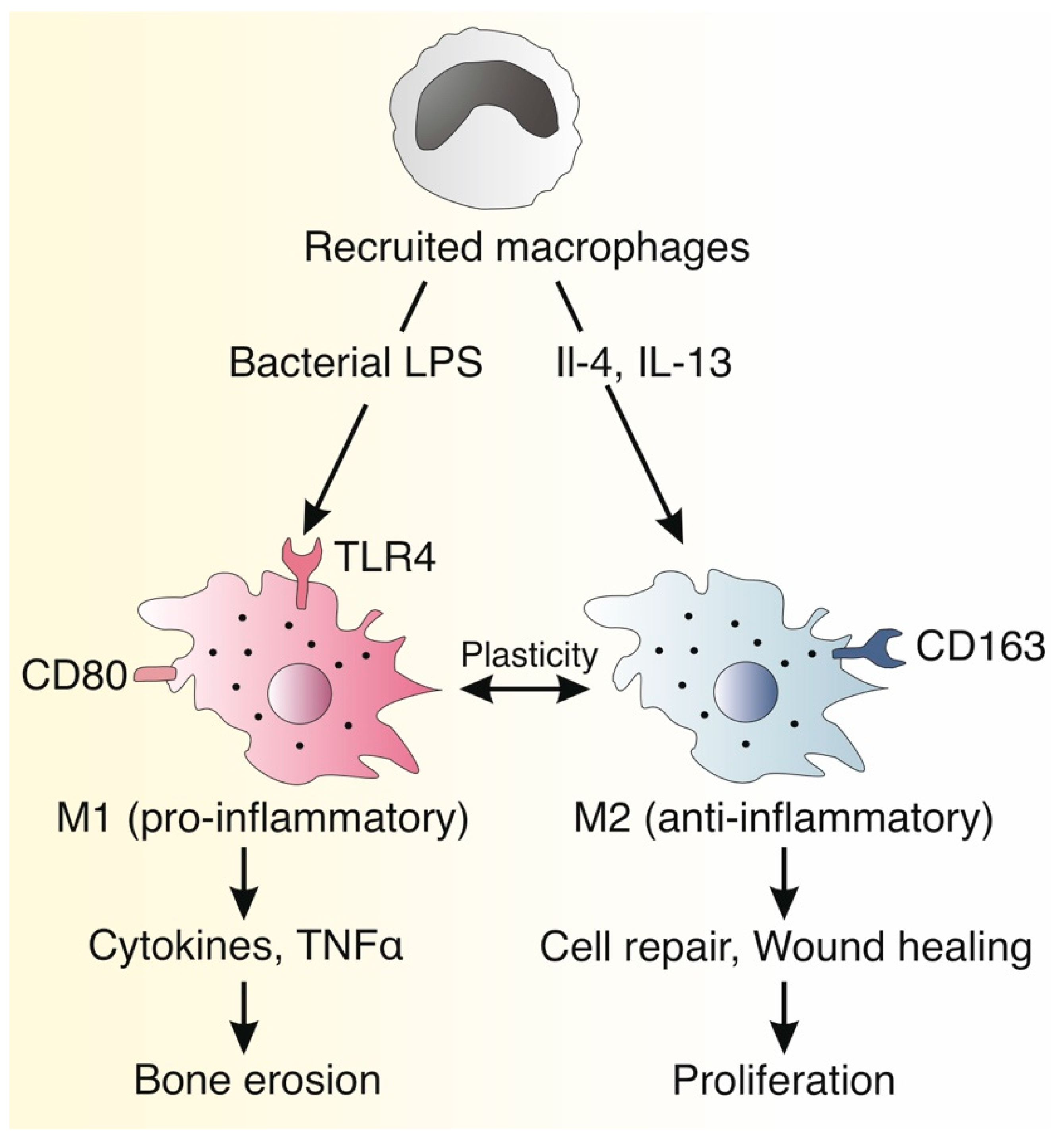
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics and Clinical Profile
3.2. The Expression of Macrophage Markers in the Cholesteatoma Specimens
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maksimovic, Z.; Rukovanjski, M. Intracranial complications of cholesteatoma. Acta Otorhinolaryngol. Belg. 1993, 47, 33–36. [Google Scholar]
- Kuo, C.L.; Shiao, A.S.; Yung, M.; Sakagami, M.; Sudhoff, H.; Wang, C.H.; Hsu, C.H.; Lien, C.F. Updates and knowledge gaps in cholesteatoma research. BioMed Res. Int. 2015, 2015, 854024. [Google Scholar] [CrossRef]
- Lee, J.A.; Fuller, S.R.; Nguyen, S.A.; Meyer, T.A. Factors affecting complications and comorbidities in children with cholesteatoma. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110080. [Google Scholar] [CrossRef]
- Sudhoff, H.; Tos, M. Pathogenesis of attic cholesteatoma: Clinical and immunohistochemical support for combination of retraction theory and proliferation theory. Am. J. Otol. 2000, 21, 786–792. [Google Scholar]
- Olszewska, E.; Wagner, M.; Bernal-Sprekelsen, M.; Ebmeyer, J.; Dazert, S.; Hildmann, H.; Sudhoff, H. Etiopathogenesis of cholesteatoma. Eur. Arch. Otorhinolaryngol. 2004, 261, 6–24. [Google Scholar] [CrossRef]
- Semaan, M.T.; Megerian, C.A. The pathophysiology of cholesteatoma. Otolaryngol. Clin. N. Am. 2006, 39, 1143–1159. [Google Scholar] [CrossRef] [PubMed]
- Vikram, B.K.; Udayashankar, S.G.; Naseeruddin, K.; Venkatesha, B.K.; Manjunath, D.; Savantrewwa, I.R. Complications in primary and secondary acquired cholesteatoma: A prospective comparative study of 62 ears. Am. J. Otolaryngol. 2008, 29, 1–6. [Google Scholar] [CrossRef]
- Stankovic, M.D. Audiologic results of surgery for cholesteatoma: Short- and long-term follow-up of influential factors. Otol. Neurotol. 2008, 29, 933–940. [Google Scholar] [CrossRef]
- Martins, O.; Victor, J.; Selesnick, S. The relationship between individual ossicular status and conductive hearing loss in cholesteatoma. Otol. Neurotol. 2012, 33, 387–392. [Google Scholar] [CrossRef]
- Blom, E.F.; Gunning, M.N.; Kleinrensink, N.J.; Lokin, A.S.; Bruijnzeel, H.; Smit, A.L.; Grolman, W. Influence of ossicular chain damage on hearing after chronic otitis media and cholesteatoma surgery: A systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 974–982. [Google Scholar] [CrossRef]
- Toner, J.G.; Smyth, G.D. Surgical treatment of cholesteatoma: A comparison of three techniques. Am. J. Otol. 1990, 11, 247–249. [Google Scholar] [PubMed]
- Ajalloueyan, M. Experience with surgical management of cholesteatomas. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 931–933. [Google Scholar] [CrossRef]
- Kuo, C.L.; Liao, W.H.; Shiao, A.S. A review of current progress in acquired cholesteatoma management. Eur. Arch. Otorhinolaryngol. 2015, 272, 3601–3609. [Google Scholar] [CrossRef]
- Moller, P.R.; Pedersen, C.N.; Grosfjeld, L.R.; Faber, C.E.; Djurhuus, B.D. Recurrence of cholesteatoma—a retrospective study including 1,006 patients for more than 33 years. Int. Arch. Otorhinolaryngol. 2020, 24, e18–e23. [Google Scholar] [CrossRef]
- Hermann, R.; Blanc, J.; Fieux, M.; Desternes, G.; Coudert, A.; Truy, E. Multi-operated cholesteatoma: When two surgeries are not enough. Eur. Arch. Otorhinolaryngol. 2021, 278, 665–673. [Google Scholar] [CrossRef]
- Lim, D.J.; Saunders, W.H. Acquired cholesteatoma: Light and electron microscopic observations. Ann. Otol. Rhinol. Laryngol. 1972, 81, 2–12. [Google Scholar] [CrossRef]
- Dornelles, C.; Meurer, L.; da Costa, S.S.; Schweiger, C. Histologic description of acquired cholesteatomas: Comparison between children and adults. Braz. J. Otorhinolaryngol. 2006, 72, 641–648. [Google Scholar] [CrossRef]
- Alves, A.L.; Pereira, C.S.B.; Ribeiro, F.A.Q.; Fregnani, J. Analysis of histopathological aspects in acquired middle ear cholesteatoma. Braz J. Otorhinolaryngol. 2008, 74, 835–841. [Google Scholar] [CrossRef]
- Klenke, C.; Janowski, S.; Borck, D.; Widera, D.; Ebmeyer, J.; Kalinowski, J.; Leichtle, A.; Hofestädt, R.; Upile, T.; Kaltschmidt, C.; et al. Identification of novel cholesteatoma-related gene expression signatures using full-genome microarrays. PLoS ONE 2012, 7, e52718. [Google Scholar] [CrossRef] [PubMed]
- Schilling, V.; Bujia, J.; Negri, B.; Schulz, P.; Kastenbauer, E. Immunologically activated cells in aural cholesteatoma. Am. J. Otolaryngol. 1991, 12, 249–253. [Google Scholar] [CrossRef]
- Negri, B.; Schilling, V.; Bujia, J.; Schulz, P.; Kastenbauer, E. Immunotype findings in macrophages in aural cholesteatomas. Eur. Arch. Otorhinolaryngol. 1992, 249, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R.; Sayed, R.H.; Abu-Dief, E.E. Immune cell profile in invasive cholesteatomas: Preliminary findings. Exp. Mol. Pathol. 2010, 88, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Hume, D.A. The many alternative faces of macrophage activation. Front. Immunol. 2015, 6, 370. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 2016, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage polarization in chronic inflammatory diseases: Killers or builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef]
- Nazareth, N.; Magro, F.; Silva, J.; Duro, M.; Gracio, D.; Coelho, R.; Appelberg, R.; Macedo, G.; Sarmento, A. Infliximab therapy increases the frequency of circulating CD16(+) monocytes and modifies macrophage cytokine response to bacterial infection. Clin. Exp. Immunol. 2014, 177, 703–711. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Wang, S.; Zhang, P.; Wang, Q.; Xiao, J.; Zhang, C.; Zheng, X.; Xu, X.; Xue, S.; et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019, 27, 1176–1189.e5. [Google Scholar] [CrossRef]
- Han, X.; Ding, S.; Jiang, H.; Liu, G. Roles of macrophages in the development and treatment of gut inflammation. Front. Cell Dev. Biol. 2021, 9, 625423. [Google Scholar] [CrossRef]
- Fontes Lima, A.; Carvalho Moreira, F.; Sousa Menezes, A.; Esteves Costa, I.; Azevedo, C.; Miguel, S.B.; Dias, L. Is pediatric cholesteatoma more aggressive in children than in adults? A comparative study using the EAONO/JOS classification. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110170. [Google Scholar] [CrossRef]
- Preciado, D.A. Biology of cholesteatoma: Special considerations in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Haraguchi, S.; Hiori, M.; Shimada, J.; Ohmori, Y. Tumor-associated macrophages in oral premalignant lesions coexpress CD163 and STAT1 in a Th1-dominated microenvironment. BMC Cancer 2015, 15, 573. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.L.; Rios, E.; Duraes, C.; Ribeiro, R.; Machado, J.C.; Mantovani, A.; Barbosa, M.A.; Carneiro, F.; Oliveira, M.J. The two faces of tumor-associated macrophages and their clinical significance in colorectal cancer. Front. Immunol. 2019, 10, 1875. [Google Scholar] [CrossRef]
- Nisenbaum, E.; Misztal, C.; Szczupak, M.; Thielhelm, T.; Peña, S.; Mei, C.; Goncalves, S.; Bracho, O.; Ma, R.; Ivan, M.E.; et al. Tumor-Associated Macrophages in Vestibular Schwannoma and Relationship to Hearing. OTO Open 2021, 5, 2473974x211059111. [Google Scholar] [CrossRef] [PubMed]
- Merkus, P.; Tije, F.A.T.; Stam, M.; Tan, F.M.L.; Pauw, R.J. Implementation of the “EAONO/JOS definitions and classification of middle ear cholesteatoma”—from STAM to STAMCO. J. Int. Adv. Otol. 2017, 13, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Rosito, L.S.; Netto, L.S.; Teixeira, A.R.; da Costa, S.S. Sensorineural Hearing Loss in Cholesteatoma. Otol Neurotol. 2016, 37, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Gulustan, F.; Yazici, Z.M.; Sayin, I.; Abakay, M.A.; Gunes, S.; Akidil, A.O. Evaluation of the Presence of Sensorineural Hearing Loss and the Relationship With Intraoperative Findings in Cholesteatoma. Ear. Nose Throat. J. 2021, 100, 249s–252s. [Google Scholar] [CrossRef]
- Chole, R.A.; Faddis, B.T. Evidence for microbial biofilms in cholesteatomas. Arch. Otolaryngol. Head Neck. Surg. 2002, 128, 1129–1133. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, C.; Xu, J.; Wang, Q.; Shen, L.; Ou, X.; Liu, H.; Han, X.; Wang, J.; Ding, W.; et al. Bacterial and fungal infections promote the bone erosion progression in acquired cholesteatoma revealed by metagenomic next-generation sequencing. Front. Microbiol. 2021, 12, 761111. [Google Scholar] [CrossRef]
- Ricciardiello, F.; Cavaliere, M.; Mesolella, M.; Iengo, M. Notes on the microbiology of cholesteatoma: Clinical findings and treatment. Acta Otorhinolaryngol. Ital. 2009, 29, 197–202. [Google Scholar] [PubMed]
- Likus, W.; Siemianowicz, K.; Markowski, J.; Wiaderkiewicz, J.; Kostrzab-Zdebel, A.; Jura-Szoltys, E.; Dziubdziela, W.; Wiaderkiewicz, R.; Los, M.J. Bacterial infections and osteoclastogenesis regulators in men and women with cholesteatoma. Arch. Immunol. Ther. Exp. 2016, 64, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Albino, A.P.; Kimmelman, C.P.; Parisier, S.C. Cholesteatoma: A molecular and cellular puzzle. Am. J. Otol. 1998, 19, 7–19. [Google Scholar] [PubMed]
- Milewski, C. Role of perimatrix fibroblasts in development of acquired middle ear cholesteatoma. A hypothesis. HNO 1998, 46, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.A.; de Heer, E.; Ten Dijke, P.; Grote, J.J. Transforming growth factor beta and wound healing in human cholesteatoma. Laryngoscope 2008, 118, 94–98. [Google Scholar] [CrossRef]
- Fang, L.; Chen, L.; Lin, B.; Han, L.; Zhu, K.; Song, Q. Analysis of inflammatory and homeostatic roles of tissue-resident macrophages in the progression of cholesteatoma by RNA-Seq. Immunol. Investig. 2021, 50, 609–621. [Google Scholar] [CrossRef]
- Imai, R.; Sato, T.; Iwamoto, Y.; Hanada, Y.; Terao, M.; Ohta, Y.; Osaki, Y.; Imai, T.; Morihana, T.; Okazaki, S.; et al. Osteoclasts modulate bone erosion in cholesteatoma via RANKL signaling. J. Assoc. Res. Otolaryngol. 2019, 20, 449–459. [Google Scholar] [CrossRef]
- Si, Y.; Chen, Y.B.; Chen, S.J.; Zheng, Y.Q.; Liu, X.; Liu, Y.; Jiang, H.L.; Xu, G.; Li, Z.H.; Huang, Q.H.; et al. TLR4 drives the pathogenesis of acquired cholesteatoma by promoting local inflammation and bone destruction. Sci. Rep. 2015, 5, 16683. [Google Scholar] [CrossRef]
- Hamed, M.A.; Nakata, S.; Sayed, R.H.; Ueda, H.; Badawy, B.S.; Nishimura, Y.; Kojima, T.; Iwata, N.; Ahmed, A.R.; Dahy, K.; et al. Pathogenesis and bone resorption in acquired cholesteatoma: Current knowledge and future prospectives. Clin. Exp. Otorhinolaryngol. 2016, 9, 298–308. [Google Scholar] [CrossRef]
- Xie, S.; Wang, X.; Ren, J.; Liu, W. The role of bone resorption in the etiopathogenesis of acquired middle ear cholesteatoma. Eur. Arch. Otorhinolaryngol. 2017, 274, 2071–2078. [Google Scholar] [CrossRef]
- Sastry, K.V.; Sharma, S.C.; Mann, S.B.; Ganguly, N.K.; Panda, N.K. Aural cholesteatoma: Role of tumor necrosis factor-alpha in bone destruction. Am. J. Otol. 1999, 20, 158–161. [Google Scholar] [PubMed]
- Vitale, R.F.; Fde, A.R. The role of tumor necrosis factor-alpha (TNF-alpha) in bone resorption present in middle ear cholesteatoma. Braz. J. Otorhinolaryngol. 2007, 73, 117–121. [Google Scholar] [CrossRef]
- van der Bruggen, T.; Nijenhuis, S.; van Raaij, E.; Verhoef, J.; van Asbeck, B.S. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect. Immun. 1999, 67, 3824–3829. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.D.; Ceci, J.D.; Tsatsanis, C.; Kontoyiannis, D.; Stamatakis, K.; Lin, J.H.; Patriotis, C.; Jenkins, N.A.; Copeland, N.G.; Kollias, G.; et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 2000, 103, 1071–1083. [Google Scholar] [CrossRef]
- Reis, J.; Guan, X.Q.; Kisselev, A.F.; Papasian, C.J.; Qureshi, A.A.; Morrison, D.C.; Van Way, C.W.; Vogel, S.N.; Qureshi, N. LPS-induced formation of immunoproteasomes: TNF-alpha and nitric oxide production are regulated by altered composition of proteasome-active sites. Cell Biochem. Biophys. 2011, 60, 77–88. [Google Scholar] [CrossRef]
- Peek, F.A.; Huisman, M.A.; Berckmans, R.J.; Sturk, A.; Van Loon, J.; Grote, J.J. Lipopolysaccharide concentration and bone resorption in cholesteatoma. Otol. Neurotol. 2003, 24, 709–713. [Google Scholar] [CrossRef]
- Schurmann, M.; Oppel, F.; Shao, S.; Volland-Thurn, V.; Kaltschmidt, C.; Kaltschmidt, B.; Scholtz, L.U.; Sudhoff, H. Chronic inflammation of middle ear cholesteatoma promotes its recurrence via a paracrine mechanism. Cell Commun. Signal. 2021, 19, 25. [Google Scholar] [CrossRef]
- Leichtle, A.; Leffers, D.; Daerr, M.G.; Draf, C.; Kurabi, A.; Ryan, A.F.; Rupp, J.; Bruchhage, K.L. Immunomodulation in cholesteatoma. Laryngorhinootologie 2022, 101, 310–319. [Google Scholar] [CrossRef]
- Schurmann, M.; Greiner, J.F.W.; Volland-Thurn, V.; Oppel, F.; Kaltschmidt, C.; Sudhoff, H.; Kaltschmidt, B. Stem cell-induced inflammation in cholesteatoma is inhibited by the TLR4 antagonist LPS-RS. Cells 2020, 9, 199. [Google Scholar] [CrossRef]
- Degboe, Y.; Rauwel, B.; Baron, M.; Boyer, J.F.; Ruyssen-Witrand, A.; Constantin, A.; Davignon, J.L. Polarization of rheumatoid macrophages by TNF targeting through an IL-10/STAT3 mechanism. Front. Immunol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Linder, T.E.; Shah, S.; Martha, A.S.; Roosli, C.; Emmett, S.D. Introducing the “ChOLE” classification and its comparison to the EAONO/JOS consensus classification for cholesteatoma staging. Otol. Neurotol. 2019, 40, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.; Tono, T.; Olszewska, E.; Yamamoto, Y.; Sudhoff, H.; Sakagami, M.; Mulder, J.; Kojima, H.; Incesulu, A.; Trabalzini, F.; et al. EAONO/JOS joint consensus statements on the definitions, classification and staging of middle ear cholesteatoma. J. Int. Adv. Otol. 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aquino, J.E.; Cruz Filho, N.A.; de Aquino, J.N. Epidemiology of middle ear and mastoid cholesteatomas: Study of 1146 cases. Braz J. Otorhinolaryngol. 2011, 77, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Gengenbacher, M.; Sebald, H.J.; Villiger, P.M.; Hofstetter, W.; Seitz, M. Infliximab inhibits bone resorption by circulating osteoclast precursor cells in patients with rheumatoid arthritis and ankylosing spondylitis. Ann. Rheum. Dis. 2008, 67, 620–624. [Google Scholar] [CrossRef]
- Chao, J.; Dewyer, N.; McKenna, M.J. Spontaneous resolution of cholesteatoma in a patient on long-term infliximab. Ann. Otol. Rhinol. Laryngol. 2019, 128, 365–368. [Google Scholar] [CrossRef]

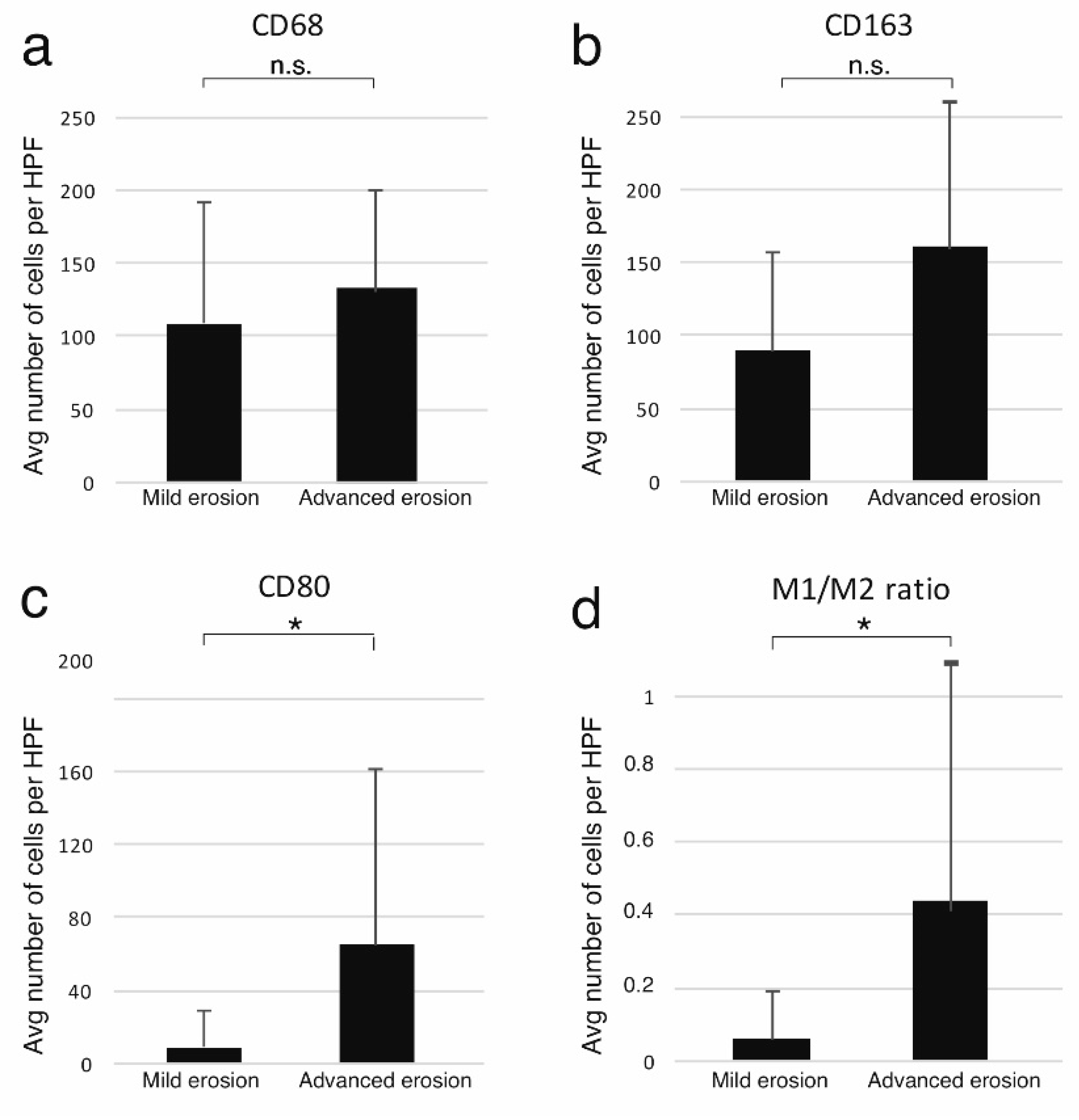
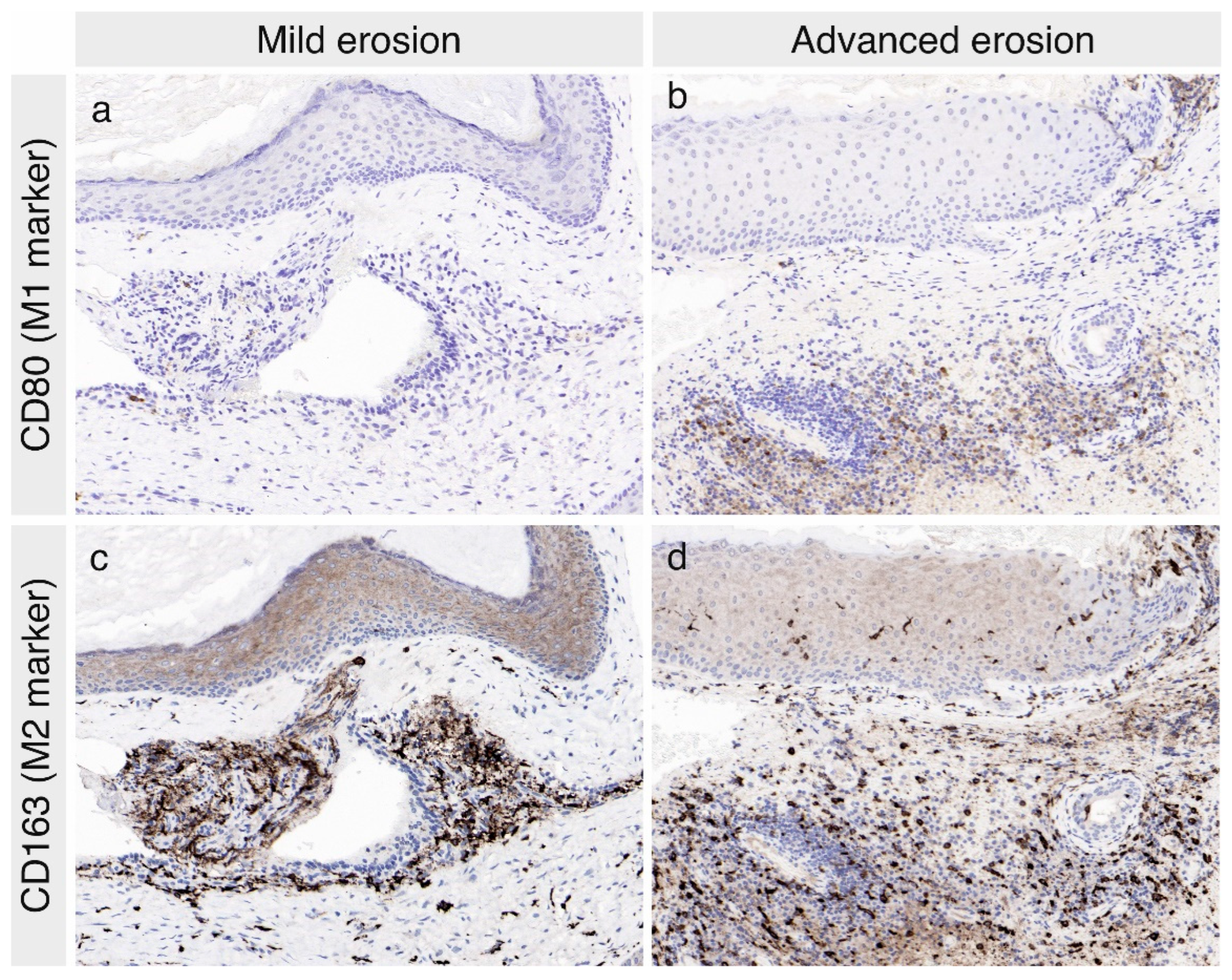
| Mild Erosion | Advanced Erosion | p-Value | |
|---|---|---|---|
| Air conduction threshold | 43.4 dB (±14.7) | 49.6 dB (±19.4) | 0.44 |
| Bone conduction threshold | 20.7 (±18.5) | 21.7 (±16.8) | 0.95 |
| Air-bone gap | 22.5 (±15.0) | 27.8 (±9.5) | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassiouni, M.; Arens, P.; Zabaneh, S.I.; Olze, H.; Horst, D.; Roßner, F. The Relationship between the M1/M2 Macrophage Polarization and the Degree of Ossicular Erosion in Human Acquired Cholesteatoma: An Immunohistochemical Study. J. Clin. Med. 2022, 11, 4826. https://doi.org/10.3390/jcm11164826
Bassiouni M, Arens P, Zabaneh SI, Olze H, Horst D, Roßner F. The Relationship between the M1/M2 Macrophage Polarization and the Degree of Ossicular Erosion in Human Acquired Cholesteatoma: An Immunohistochemical Study. Journal of Clinical Medicine. 2022; 11(16):4826. https://doi.org/10.3390/jcm11164826
Chicago/Turabian StyleBassiouni, Mohamed, Philipp Arens, Samira Ira Zabaneh, Heidi Olze, David Horst, and Florian Roßner. 2022. "The Relationship between the M1/M2 Macrophage Polarization and the Degree of Ossicular Erosion in Human Acquired Cholesteatoma: An Immunohistochemical Study" Journal of Clinical Medicine 11, no. 16: 4826. https://doi.org/10.3390/jcm11164826
APA StyleBassiouni, M., Arens, P., Zabaneh, S. I., Olze, H., Horst, D., & Roßner, F. (2022). The Relationship between the M1/M2 Macrophage Polarization and the Degree of Ossicular Erosion in Human Acquired Cholesteatoma: An Immunohistochemical Study. Journal of Clinical Medicine, 11(16), 4826. https://doi.org/10.3390/jcm11164826







