Adenovirus Infection in Pediatric Hematopoietic Cell Transplantation: A Challenge Still Open for Survival
Abstract
1. Introduction
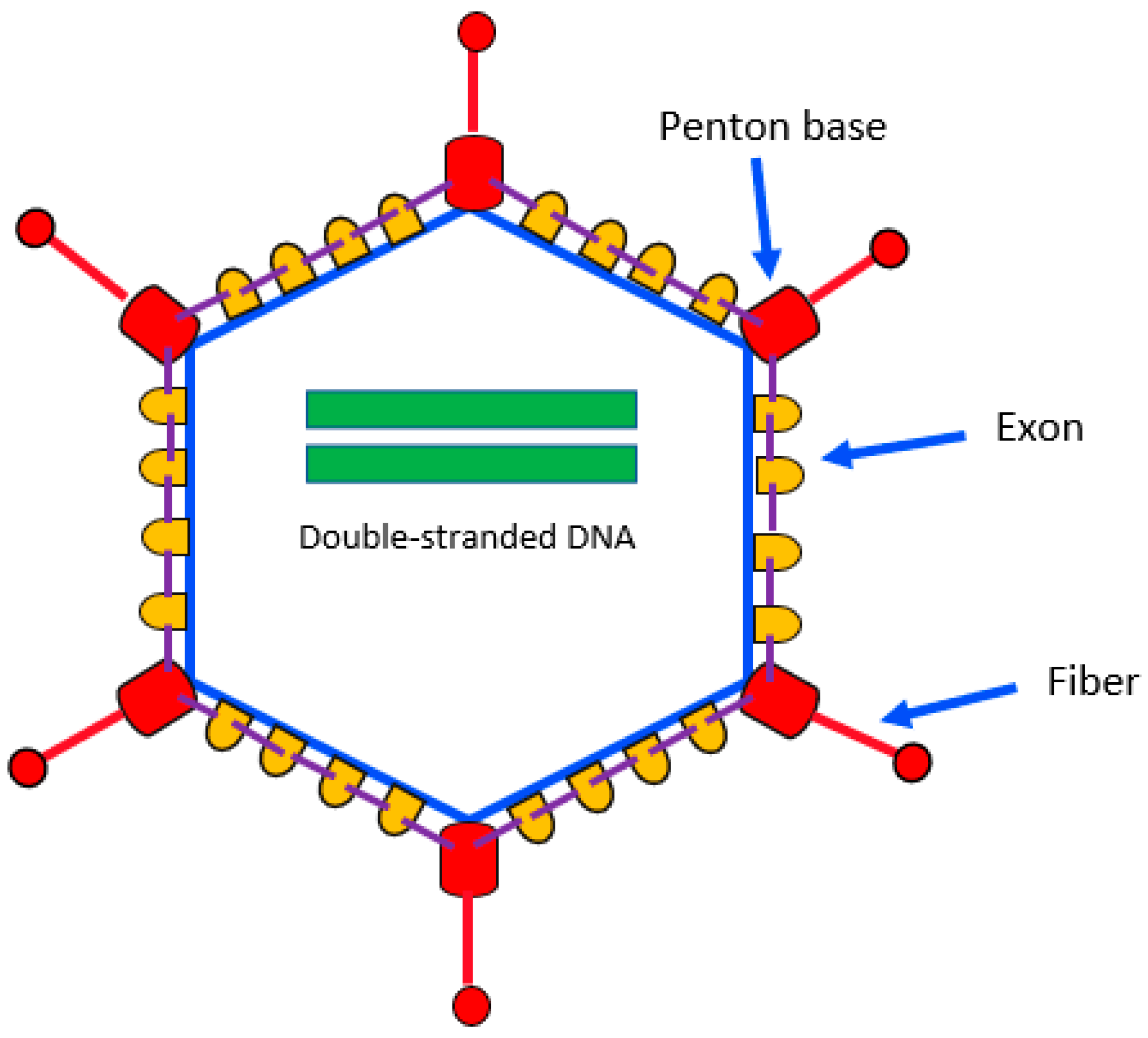
2. Biology
3. Diagnosis
4. Clinical Symptoms and Definitions
5. Risk Factors
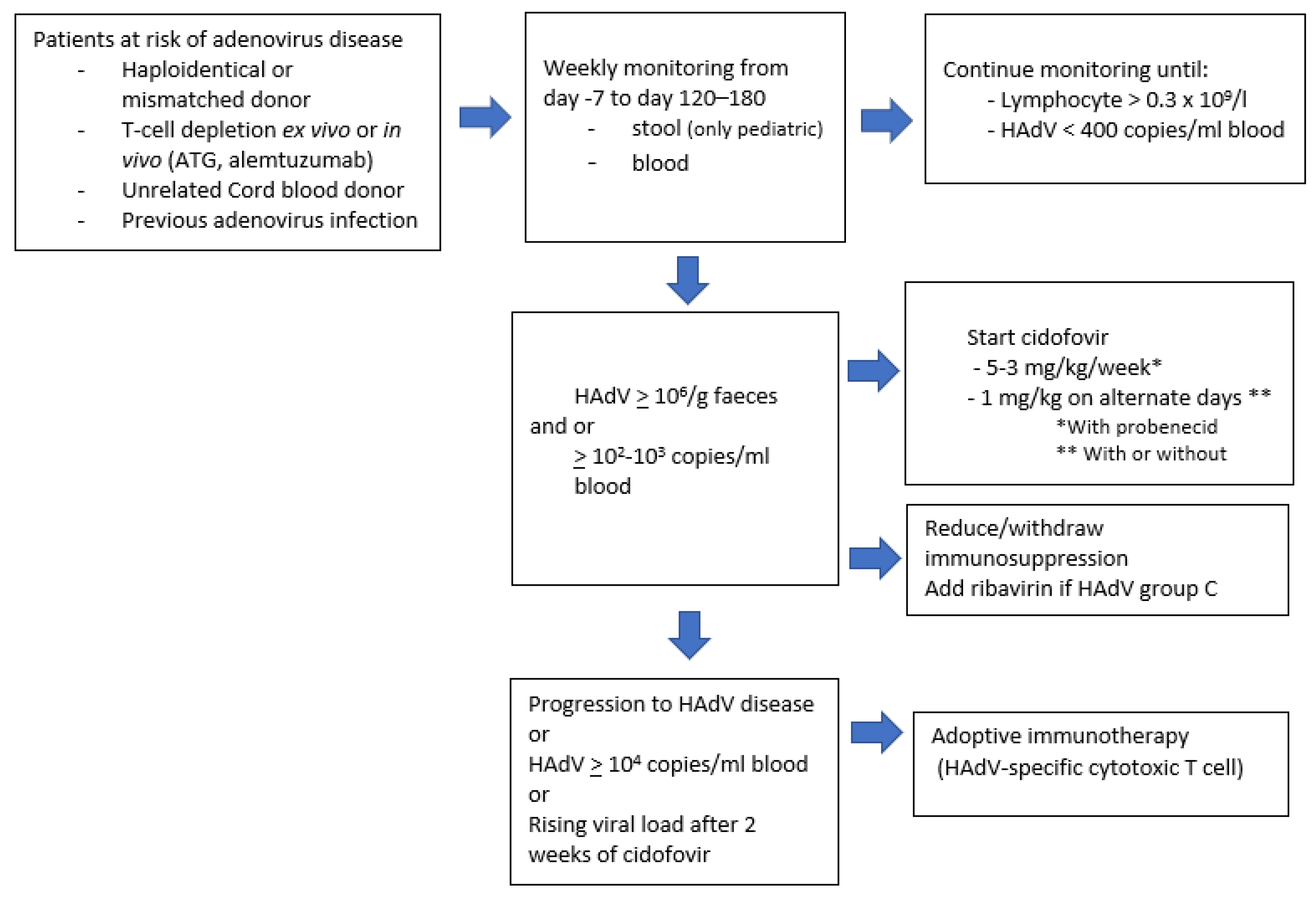
6. Prevention
7. Treatment
8. Immunotherapy
Author Contributions
Funding
Conflicts of Interest
References
- Hill, J.A.; Mayer, B.T.; Xie, H.; Leisenring, W.M.; Huang, M.-L.; Stevens-Ayers, T.; Milano, F.; Delaney, C.; Sorror, M.L.; Sandmaier, B.M.; et al. The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood 2017, 129, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Su, Q.-Q.; Zuo, T.-T.; Chen, Y.-B. Adenovirus diseases: A systematic review and meta-analysis of 228 case reports. Infection 2021, 49, 1–13. [Google Scholar] [CrossRef]
- Keramari, S.; Poutoglidou, F.; Poutoglidis, A.; Sotiropoulos, D.; Savopoulos, C.; Chlichlia, K.; Chatzis, S.; Xagorari, A.; Kaiafa, G. Adenoviral Infections in Bone Marrow Transplanted Adult Patients: A Review of the 44 Cases Reported in the Last 25 Years. Cureus 2021, 13, e19865. [Google Scholar] [CrossRef] [PubMed]
- Kosulin, K.; Pichler, H.; Lawitschka, A.; Geyeregger, R.; Lion, T. Diagnostic Parameters of Adenoviremia in Pediatric Stem Cell Transplant Recipients. Front. Microbiol. 2019, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, G.A.; Dvorak, C.C.; Dadwal, S.; Maron, G.; Prasad, V.K.; Giller, R.; Abdel-Azim, H.; Sadanand, A.; Casciano, R.; Chandak, A.; et al. Practice patterns and incidence of adenovirus infection in allogeneic hematopoietic cell transplant recipients: Multicenter survey of transplant centers in the United States. Transpl. Infect. Dis. 2020, 22, e13283. [Google Scholar] [CrossRef]
- Cesaro, S.; Berger, M.; Tridello, G.; Mikulska, M.; Ward, K.N.; Ljungman, P.; Van Der Werf, S.; Averbuch, D.; Styczynski, J. Infectious Disease Working Party of EBMT A survey on incidence and management of adenovirus infection after allogeneic HSCT. Bone Marrow Transplant. 2019, 54, 1275–1280. [Google Scholar] [CrossRef]
- Sedláček, P.; Petterson, T.; Robin, M.; Sivaprakasam, P.; Vainorius, E.; Brundage, T.; Chandak, A.; Mozaffari, E.; Nichols, G.; Voigt, S. Incidence of Adenovirus Infection in Hematopoietic Stem Cell Transplantation Recipients: Findings from the AdVance Study. Biol. Blood Marrow Transplant. 2019, 25, 810–818. [Google Scholar] [CrossRef]
- Shieh, W.-J. Human adenovirus infections in pediatric population-An update on clinico-pathologic correlation. Biomed. J. 2022, 45, 38–49. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019, 593, 3571–3582. [Google Scholar] [CrossRef]
- Biserni, G.B.; Scarpini, S.; Dondi, A.; Biagi, C.; Pierantoni, L.; Masetti, R.; Sureshkumar, S.; Rocca, A.; Lanari, M. Potential Diagnostic and Prognostic Biomarkers for Adenovirus Respiratory Infection in Children and Young Adults. Viruses 2021, 13, 1885. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, Z.; Ma, Q.; Liu, Q.; Lu, X.; Liu, W.; Liao, X.; Zhou, R.; Su, X.; Luo, Q. Prevalence of neutralizing antibodies to common respiratory viruses in intravenous immunoglobulin and in healthy donors in southern China. J. Thorac. Dis. 2016, 8, 803–812. [Google Scholar] [CrossRef]
- Lion, T.; Baumgartinger, R.; Watzinger, F.; Matthes-Martin, S.; Suda, M.; Preuner, S.; Futterknecht, B.; Lawitschka, A.; Peters, C.; Potschger, U.; et al. Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood 2003, 102, 1114–1120. [Google Scholar] [CrossRef]
- Matthes-Martin, S.; Feuchtinger, T.; Shaw, P.J.; Engelhard, D.; Hirsch, H.H.; Cordonnier, C.; Ljungman, P.; Fourth European Conference on Infections in Leukemia. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: Summary of ECIL-4 (2011). Transpl. Infect. Dis. 2012, 14, 555–563. [Google Scholar] [CrossRef]
- Ganzenmueller, T.; Heim, A. Adenoviral load diagnostics by quantitative polymerase chain reaction: Techniques and application. Rev. Med. Virol. 2012, 22, 194–208. [Google Scholar] [CrossRef]
- Matthes-Martin, S.; Boztug, H.; Lion, T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev. Anti-Infect. Ther. 2013, 11, 1017–1028. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Leen, A.M.; Boelens, J.J. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood 2010, 116, 5476–5485. [Google Scholar] [CrossRef]
- Echavarria, M.; Maldonado, D.; Elbert, G.; Videla, C.; Rappaport, R.; Carballal, G. Use of PCR to demonstrate presence of adenovirus species B, C, or F as well as coinfection with two adenovirus species in children with flu-like symptoms. J. Clin. Microbiol. 2006, 44, 625–627. [Google Scholar] [CrossRef]
- Rayne, F.; Wittkop, L.; Bader, C.; Kassab, S.; Tumiotto, C.; Berciaud, S.; Wodrich, H.; Lafon, M.-E. Typadeno Study Members Rapid Adenovirus typing method for species identification. J. Virol. Methods 2017, 249, 156–160. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Mautner, V.; Osman, H.; Collingham, K.E.; Fegan, C.D.; Klapper, P.E.; Moss, P.A.H.; Milligan, D.W. Adenovirus infections following allogeneic stem cell transplantation: Incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood 2002, 100, 1619–1627. [Google Scholar] [CrossRef]
- Thomas, S.J.; Young, R.T.; Steinbach, W.J.; Lugo, D.J. Risks and outcomes of adenovirus disease in pediatric hematopoietic stem cell transplant recipients-Comparison of current antiviral treatment options. Transpl. Infect. Dis. 2021, 23, e13505. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chung, D.; Xiao, K.; Papadopoulos, E.B.; Barker, J.N.; Small, T.N.; Giralt, S.A.; Zheng, J.; Jakubowski, A.A.; Papanicolaou, G.A. Adenovirus viremia and disease: Comparison of T cell-depleted and conventional hematopoietic stem cell transplantation recipients from a single institution. Biol. Blood Marrow Transplant. 2013, 19, 387–392. [Google Scholar] [CrossRef]
- Lion, T.; Kosulin, K.; Landlinger, C.; Rauch, M.; Preuner, S.; Jugovic, D.; Pötschger, U.; Lawitschka, A.; Peters, C.; Fritsch, G.; et al. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia 2010, 24, 706–714. [Google Scholar] [CrossRef]
- Feghoul, L.; Chevret, S.; Cuinet, A.; Dalle, J.-H.; Ouachée, M.; Yacouben, K.; Fahd, M.; Guérin-El Khourouj, V.; Roupret-Serzec, J.; Sterkers, G.; et al. Adenovirus infection and disease in paediatric haematopoietic stem cell transplant patients: Clues for antiviral pre-emptive treatment. Clin. Microbiol. Infect. 2015, 21, 701–709. [Google Scholar] [CrossRef]
- Jeulin, H.; Salmon, A.; Bordigoni, P.; Venard, V. Diagnostic value of quantitative PCR for adenovirus detection in stool samples as compared with antigen detection and cell culture in haematopoietic stem cell transplant recipients. Clin. Microbiol. Infect. 2011, 17, 1674–1680. [Google Scholar] [CrossRef][Green Version]
- Kosulin, K.; Berkowitsch, B.; Matthes, S.; Pichler, H.; Lawitschka, A.; Pötschger, U.; Fritsch, G.; Lion, T. Intestinal Adenovirus Shedding Before Allogeneic Stem Cell Transplantation Is a Risk Factor for Invasive Infection Post-transplant. EBioMedicine 2018, 28, 114–119. [Google Scholar] [CrossRef]
- Kosulin, K.; Geiger, E.; Vécsei, A.; Huber, W.-D.; Rauch, M.; Brenner, E.; Wrba, F.; Hammer, K.; Innerhofer, A.; Pötschger, U.; et al. Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin. Microbiol. Infect. 2016, 22, 381.e1–381.e8. [Google Scholar] [CrossRef]
- Mynarek, M.; Ganzenmueller, T.; Mueller-Heine, A.; Mielke, C.; Gonnermann, A.; Beier, R.; Sauer, M.; Eiz-Vesper, B.; Kohstall, U.; Sykora, K.-W.; et al. Patient, virus, and treatment-related risk factors in pediatric adenovirus infection after stem cell transplantation: Results of a routine monitoring program. Biol. Blood Marrow Transplant. 2014, 20, 250–256. [Google Scholar] [CrossRef]
- Zecca, M.; Wynn, R.; Dalle, J.-H.; Feuchtinger, T.; Vainorius, E.; Brundage, T.M.; Chandak, A.; Mozaffari, E.; Nichols, G.; Locatelli, F. Association between adenovirus viral load and mortality in pediatric allo-HCT recipients: The multinational AdVance study. Bone Marrow Transplant. 2019, 54, 1632–1642. [Google Scholar] [CrossRef]
- González-Vicent, M.; Verna, M.; Pochon, C.; Chandak, A.; Vainorius, E.; Brundage, T.; Mozaffari, E.; Nichols, G.; Rao, K. Current practices in the management of adenovirus infection in allogeneic hematopoietic stem cell transplant recipients in Europe: The AdVance study. Eur. J. Haematol. 2019, 102, 210–217. [Google Scholar] [CrossRef]
- Hiwarkar, P.; Kosulin, K.; Cesaro, S.; Mikulska, M.; Styczynski, J.; Wynn, R.; Lion, T. Management of adenovirus infection in patients after haematopoietic stem cell transplantation: State-of-the-art and real-life current approach: A position statement on behalf of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation. Rev. Med. Virol. 2018, 28, e1980. [Google Scholar] [CrossRef]
- Lenaerts, L.; Naesens, L. Antiviral therapy for adenovirus infections. Antivir. Res. 2006, 71, 172–180. [Google Scholar] [CrossRef]
- Morfin, F.; Dupuis-Girod, S.; Mundweiler, S.; Falcon, D.; Carrington, D.; Sedlacek, P.; Bierings, M.; Cetkovsky, P.; Kroes, A.C.M.; van Tol, M.J.D.; et al. In vitro susceptibility of adenovirus to antiviral drugs is species-dependent. Antivir. Ther. 2005, 10, 225–229. [Google Scholar] [CrossRef]
- Symeonidis, N.; Jakubowski, A.; Pierre-Louis, S.; Jaffe, D.; Pamer, E.; Sepkowitz, K.; O’Reilly, R.J.; Papanicolaou, G.A. Invasive adenoviral infections in T-cell-depleted allogeneic hematopoietic stem cell transplantation: High mortality in the era of cidofovir. Transpl. Infect. 2007, 9, 108–113. [Google Scholar] [CrossRef]
- Chandorkar, A.; Anderson, A.D.; Morris, M.I.; Natori, Y.; Jimenez, A.; Komanduri, K.V.; Camargo, J.F. Viral kinetics and outcomes of adenovirus viremia following allogeneic hematopoietic cell transplantation. Clin. Transplant. 2021, 35, e14481. [Google Scholar] [CrossRef]
- Lacy, S.A.; Hitchcock, M.J.; Lee, W.A.; Tellier, P.; Cundy, K.C. Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys. Toxicol. Sci. 1998, 44, 97–106. [Google Scholar] [CrossRef]
- Lash, L.H.; Lee, C.A.; Wilker, C.; Shah, V. Transporter-dependent cytotoxicity of antiviral drugs in primary cultures of human proximal tubular cells. Toxicology 2018, 404–405, 10–24. [Google Scholar] [CrossRef]
- Neofytos, D.; Ojha, A.; Mookerjee, B.; Wagner, J.; Filicko, J.; Ferber, A.; Dessain, S.; Grosso, D.; Brunner, J.; Flomenberg, N.; et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol. Blood Marrow Transplant. 2007, 13, 74–81. [Google Scholar] [CrossRef]
- Florescu, D.F.; Keck, M.A. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev. Anti Infect. Ther. 2014, 12, 1171–1178. [Google Scholar] [CrossRef]
- Grimley, M.S.; Chemaly, R.F.; Englund, J.A.; Kurtzberg, J.; Chittick, G.; Brundage, T.M.; Bae, A.; Morrison, M.E.; Prasad, V.K. Brincidofovir for Asymptomatic Adenovirus Viremia in Pediatric and Adult Allogeneic Hematopoietic Cell Transplant Recipients: A Randomized Placebo-Controlled Phase II Trial. Biol. Blood Marrow Transplant. 2017, 23, 512–521. [Google Scholar] [CrossRef]
- Hiwarkar, P.; Amrolia, P.; Sivaprakasam, P.; Lum, S.H.; Doss, H.; O’Rafferty, C.; Petterson, T.; Patrick, K.; Silva, J.; Slatter, M.; et al. Brincidofovir is highly efficacious in controlling adenoviremia in pediatric recipients of hematopoietic cell transplant. Blood 2017, 129, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Perruccio, K.; Menconi, M.; Galaverna, F.; Pagliara, D.; Carraro, F.; Fagioli, F.; Calore, E.; Biffi, A.; Baretta, V.; Massei, M.S.; et al. Safety and efficacy of brincidofovir for Adenovirus infection in children receiving allogeneic stem cell transplantation: An AIEOP retrospective analyses. Bone Marrow Transplant. 2021, 56, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Winston, D.J.; Chemaly, R.F.; Mullane, K.M.; Shore, T.B.; Papanicolaou, G.A.; Chittick, G.; Brundage, T.M.; Wilson, C.; Morrison, M.E.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial of Oral Brincidofovir for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 369–381. [Google Scholar] [CrossRef]
- Zandvliet, M.L.; Falkenburg, J.H.F.; van Liempt, E.; Veltrop-Duits, L.A.; Lankester, A.C.; Kalpoe, J.S.; Kester, M.G.D.; van der Steen, D.M.; van Tol, M.J.; Willemze, R.; et al. Combined CD8+ and CD4+ adenovirus hexon-specific T cells associated with viral clearance after stem cell transplantation as treatment for adenovirus infection. Haematologica 2010, 95, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Hromas, R.; Cornetta, K.; Srour, E.; Blanke, C.; Broun, E.R. Donor leukocyte infusion as therapy of life-threatening adenoviral infections after T-cell-depleted bone marrow transplantation. Blood 1994, 84, 1689–1690. [Google Scholar] [CrossRef]
- Bollard, C.M.; Heslop, H.E. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood 2016, 127, 3331–3340. [Google Scholar] [CrossRef]
- Feucht, J.; Opherk, K.; Lang, P.; Kayser, S.; Hartl, L.; Bethge, W.; Matthes-Martin, S.; Bader, P.; Albert, M.H.; Maecker-Kolhoff, B.; et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood 2015, 125, 1986–1994. [Google Scholar] [CrossRef]
- Tzannou, I.; Papadopoulou, A.; Naik, S.; Leung, K.; Martinez, C.A.; Ramos, C.A.; Carrum, G.; Sasa, G.; Lulla, P.; Watanabe, A.; et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2017, 35, 3547–3557. [Google Scholar] [CrossRef]
- Withers, B.; Clancy, L.; Burgess, J.; Simms, R.; Brown, R.; Micklethwaite, K.; Blyth, E.; Gottlieb, D. Establishment and Operation of a Third-Party Virus-Specific T Cell Bank within an Allogeneic Stem Cell Transplant Program. Biol. Blood Marrow Transplant. 2018, 24, 2433–2442. [Google Scholar] [CrossRef]
- Rubinstein, J.D.; Lutzko, C.; Leemhuis, T.; Zhu, X.; Pham, G.; Ray, L.M.; Thomas, S.; Dourson, C.; Wilhelm, J.; Lane, A.; et al. Scheduled Administration of Virus-Specific T cells for Viral Prophylaxis After Pediatric Allogeneic Stem Cell Transplant. Blood Adv. 2022, 6, 2897–2907. [Google Scholar] [CrossRef]
| Species | Serotypes/Genotypes |
|---|---|
| A | 12, 18, 31, 61 |
| B | 3, 7, 11, 14, 16, 21, 34, 35, 50, 55, 66, 68, 76–79 |
| C | 1,2,5,6,57,89 |
| D | 8–10, 13, 15, 17, 19, 20, 22–30, 32, 33, 36–39,42–49, 51, 53, 54, 56, 58, 59, 60, 63, 64, 65, 67,69–75, 80–88, 90–103 |
| E | 4 |
| F | 40, 41 |
| G | 52 |
| HAdV Infection | |
|---|---|
| Children | Allo-HCT with ex vivo or in vivo T-cell depletion Allo-HCT with an unrelated donor Allo-HCT with unrelated cord blood donor Moderate-severe, grade III-IV acute graft versus host disease Lymphopenia ≤ 0.2 × 109/L HAdV ≥ 103 copies/mL stool HAdV shedding in the stool before HCT |
| Adults | Allo-HCT with unrelated cord blood donor or haploidentical donor Moderate -severe, grade III-IV, acute graft versus host disease Treatment with alemtuzumab |
| HAdV disease | |
| Children and adults | HAdV viremia ≥ 103 copies/mL blood ≥2 sites/organs HAdV positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesaro, S.; Porta, F. Adenovirus Infection in Pediatric Hematopoietic Cell Transplantation: A Challenge Still Open for Survival. J. Clin. Med. 2022, 11, 4827. https://doi.org/10.3390/jcm11164827
Cesaro S, Porta F. Adenovirus Infection in Pediatric Hematopoietic Cell Transplantation: A Challenge Still Open for Survival. Journal of Clinical Medicine. 2022; 11(16):4827. https://doi.org/10.3390/jcm11164827
Chicago/Turabian StyleCesaro, Simone, and Fulvio Porta. 2022. "Adenovirus Infection in Pediatric Hematopoietic Cell Transplantation: A Challenge Still Open for Survival" Journal of Clinical Medicine 11, no. 16: 4827. https://doi.org/10.3390/jcm11164827
APA StyleCesaro, S., & Porta, F. (2022). Adenovirus Infection in Pediatric Hematopoietic Cell Transplantation: A Challenge Still Open for Survival. Journal of Clinical Medicine, 11(16), 4827. https://doi.org/10.3390/jcm11164827






