Circulating Osteogenic Progenitor Cells Enhanced with Teriparatide or Denosumab Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Biochemical Measurements
2.3. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.4. Cytometry Analysis of COPs
2.5. Bone Mineral Density
2.6. Statistical Analysis
3. Results
3.1. Basal Anthropometric Parameters, BTMs, and BMD in the Healthy Population
3.2. Percentage of COP Cells in Healthy Population
3.3. Basal Anthropometric Parameters and BTMs and BMD in OP Patients
3.4. Percentage of COP Cells in Osteoporosis Patients
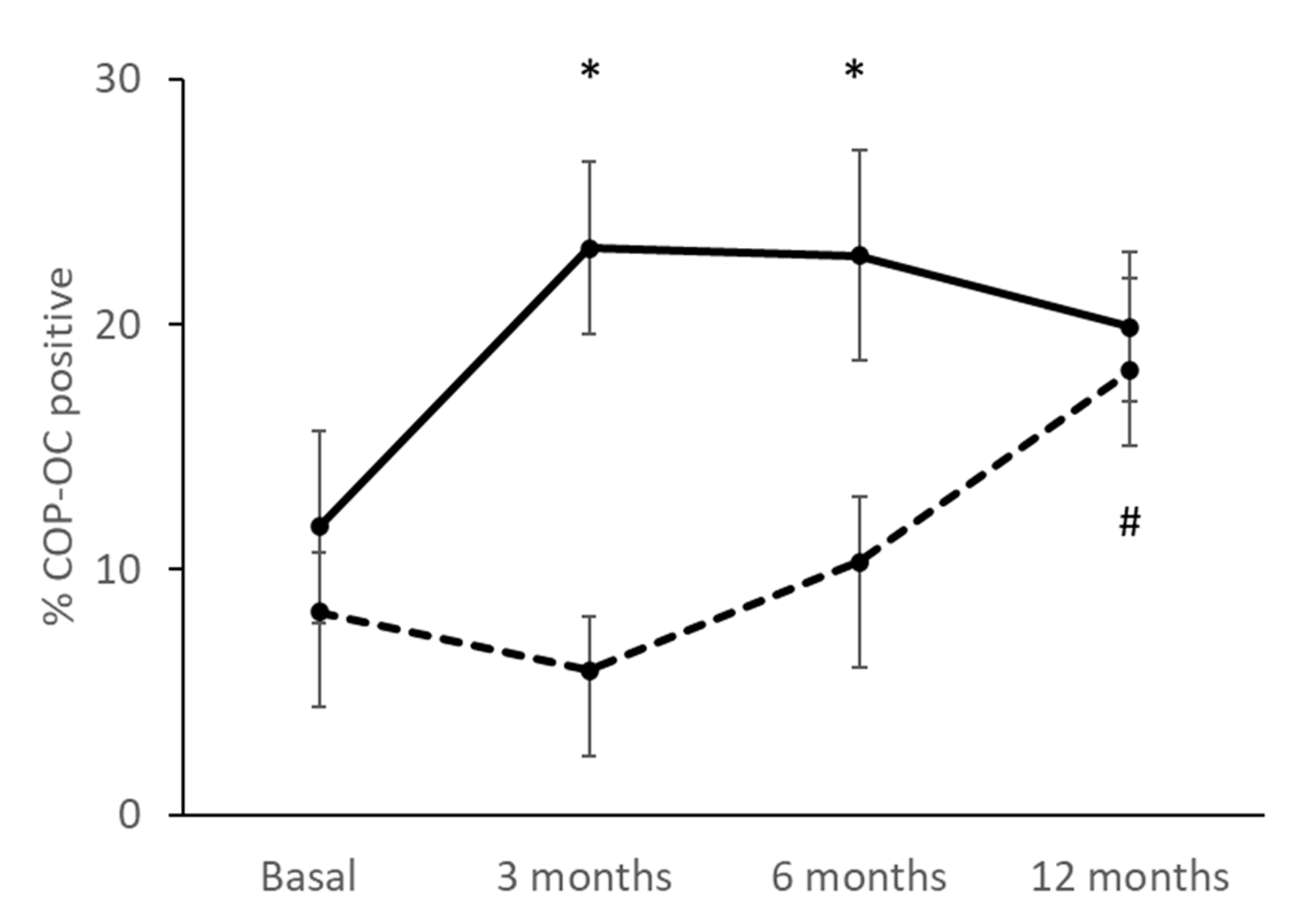
3.5. Percentage of COPs Correlating with the Treatment
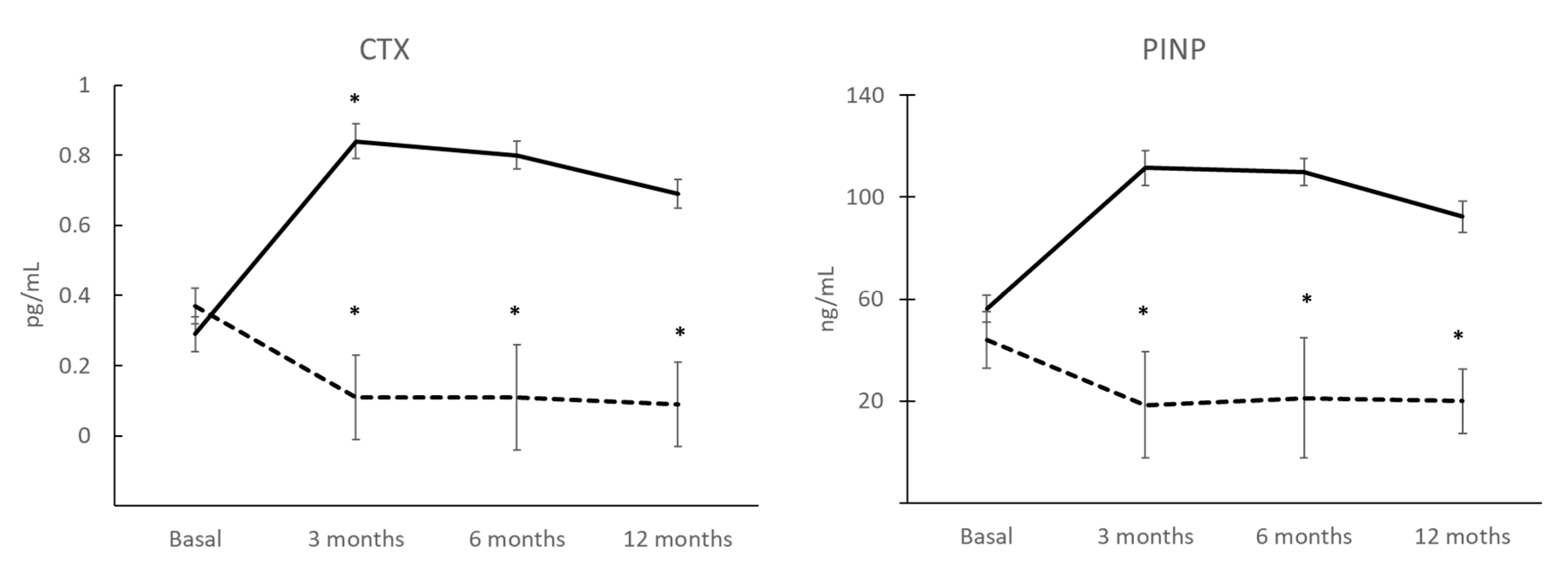
3.6. BTMs after 12 Months of Treatment and Correlation with COP
3.7. BMD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feehan, J.; Nurgali, K.; Apostolopoulos, V.; Al Saedi, A.; Duque, G. Circulating osteogenic precursor cells: Building bone from blood. eBioMedicine 2019, 39, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Otsuru, S.; Tamai, K.; Yamazaki, T.; Yoshikawa, H.; Kaneda, Y. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem. Biophys. Res. Commun. 2007, 354, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Vasanji, A.; Drazba, J.A.; Butler, R.S.; Muschler, G.F. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J. Orthop. Res. 2008, 26, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.R.; McDonald, L.T.; Pellegrini, V.D.; Cray, J.J.; Larue, A.C. Identification of circulating murine CD34+OCN+ cells. Cytotherapy 2018, 20, 1371–1380. [Google Scholar] [CrossRef]
- Eghbali-Fatourechi, G.Z.; Lamsam, J.; Fraser, D.; Nagel, D.; Riggs, B.L.; Khosla, S. Circulating osteoblast-lineage cells in humans. N. Engl. J. Med. 2005, 352, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Eghbali-Fatourechi, G.Z.; Modder, U.I.; Charatcharoenwitthaya, N.; Sanyal, A.; Undale, A.H.; Clowes, J.A.; Tarara, J.E.; Khosla, S. Characterization of circulating osteoblast lineage cells in humans. Bone 2007, 40, 1370–1377. [Google Scholar] [CrossRef]
- Hong, H.S.; Lee, J.; Lee, E.; Kwon, Y.S.; Lee, E.; Ahn, W.; Jiang, M.H.; Kim, J.C.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, G.Y.; Delorme, B.; Lopez, A.; Hérault, O.; Bonnet, P.; Charbord, P.; Eder, V.; Domenech, J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells 2006, 24, 2202–2208. [Google Scholar] [CrossRef]
- Tang, Y.; Xia, H.; Kang, L.; Sun, Q.; Su, Z.; Hao, C.; Xue, Y. Effects of intermittent parathyroid hormone 1–34 administration on circulating mesenchymal stem cells in postmenopausal osteoporotic women. Med. Sci. Monit. 2019, 25, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Alm, J.J.; Koivu, H.M.; Heino, T.J.; Hentunen, T.A.; Laitinen, S.; Aro, H.T. Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J. Orthop. Res. 2010, 28, 1634–1642. [Google Scholar] [CrossRef]
- D’Amelio, P.; Cristofaro, M.A.; Grimaldi, A.; Ravazzoli, M.; Pluviano, F.; Grosso, E.; Pescarmona, G.P.; Isaia, G.C. The role of circulating bone cell precursors in fracture healing. Calcif. Tissue Int. 2010, 86, 463–469. [Google Scholar] [CrossRef]
- Albiero, M.; Bonora, B.M.; Fadini, G.P. Diabetes pharmacotherapy and circulating stem/progenitor cells. State of the art and evidence gaps. Curr. Opin. Pharmacol. 2020, 55, 151–156. [Google Scholar] [CrossRef]
- Al, P.; Al Saedi, A.; Singh, L.; Bermeo, S.; Vogrin, S.; Phu, S.; Suriyaarachchi, P.; Pignolo, R.J.; Duque, G. Age, gender, and percentage of circulating osteoprogenitor (COP) cells: The COP Study. Exp. Gerontol. 2017, 96, 68–72. [Google Scholar]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Trial Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Valenti, M.T.; Zanatta, M.; Donatelli, L.; Lo, C.V. Circulating mesenchymal stem cells with abnormal osteogenic differentiation in patients with osteoporosis. Arthritis Rheum. 2009, 60, 3356–3365. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Makras, P.; Yavropoulou, M.P.; Tabacco, G.; Naciu, A.M.; Palermo, A. Denosumab Discontinuation and the Rebound Phenomenon: A Narrative Review. J. Clin. Med. 2021, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P. The Utility of Biomarkers in Osteoporosis Management. Mol. Diagn. Ther. 2017, 21, 401–418. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Tabacco, G.; Bilezikian, J.P. Osteoanabolic and dual action drugs. Br. J. Clin. Pharmacol. 2019, 85, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Diez-Perez, A.; Naylor, K.E.; Abrahamsen, B.; Agnusdei, D.; Brandi, M.L.; Cooper, C.; Dennison, E.; Eriksen, E.F.; Gold, D.T.; Guañabens, N.; et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos. Int. 2017, 28, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Giner, M.; Montoya, M.J.; Vázquez, M.A.; Miranda, M.; Pérez-Cano, R. Preliminary study of osteoblasts in peripheral blood in the population of infants and adolescents. Rev. Osteoporos. Metab. Miner. 2011, 3, 31–34. [Google Scholar]
- Iwakura, T.; Lee, S.Y.; Miwa, M.; Niikura, T.; Sakai, Y.; Oe, K.; Koh, A.; Koga, T.; Kuroda, R.; Kurosaka, M. Analysis of circulating mesenchymal progenitor cells in arterial and venous blood after fracture. J. Tissue Eng. Regen. Med. 2013, 7, 501–504. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Villa, F.; Pfeffer, U.; Quarto, R.; Cancedda, R.; Tasso, R. Circulating healing (CH) cells expressing BST2 are functionally activated by the injury-regulated systemic factor HGFA. Stem Cell Res. Ther. 2018, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Al Saedi, A.; Phu, S.; Vogrin, S.; Gunawardene, P.; Duque, G. Association between Circulating Osteoprogenitor Cells and Sarcopenia. Gerontology 2021, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Spies, V.; Mehling, I.; Gruszka, D.; Rommens, P.M.; Hofmann, A. Mobilization of CD34+-progenitor cells in patients with severe trauma. PLoS ONE 2014, 9, e97369. [Google Scholar] [CrossRef]
- Feehan, J.; Kassem, M.; Pignolo, R.J.; Duque, G. Bone From Blood: Characteristics and Clinical Implications of Circulating Osteogenic Progenitor (COP) Cells. J. Bone Miner. Res. 2021, 36, 12–23. [Google Scholar] [CrossRef]
- Feehan, J.; Smith, C.; Tripodi, N.; Degabrille, E.; Al Saedi, A.; Vogrin, S.; Duque, G.; Levinger, I. Higher Levels of Circulating Osteoprogenitor Cells Are Associated with Higher Bone Mineral Density and Lean Mass in Older Adults: A Cross-Sectional Study. JBMR Plus 2021, 5, e10561. [Google Scholar] [CrossRef]
- Pasqualini, L.; Ministrini, S.; Lombardini, R.; Bagaglia, F.; Paltriccia, R.; Pippi, R.; Collebrusco, L.; Reginato, E.; Sbroma Tomaro, E.; Marini, E.; et al. Effects of a 3-month weight-bearing and resistance exercise training on circulating osteogenic cells and bone formation markers in postmenopausal women with low bone mass. Osteoporos. Int. 2019, 30, 797–806. [Google Scholar] [CrossRef]
- Pirro, M.; Leli, C.; Fabbriciani, G.; Manfredelli, M.R.; Callarelli, L.; Bagaglia, F.; Scarponi, A.M.; Mannarino, E. Association between circulating osteoprogenitor cell numbers and bone mineral density in postmenopausal osteoporosis. Osteoporos. Int. 2010, 21, 297–306. [Google Scholar] [CrossRef]
- Yu, E.W.; Kumbhani, R.; Siwila-Sackman, E.; DeLelys, M.; Preffer, F.I.; Leder, B.Z.; Wu, J.Y. Teriparatide (PTH 1–34) treatment increases peripheral hematopoietic stem cells in postmenopausal women. J. Bone Miner. Res. 2014, 29, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwiński, E.; Fahrleitner-Pammer, A.; Kendler, M.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Lyu, H.; Zhao, S.S.; Yoshida, K.; Tedeschi, S.K.; Xu, C.; Nigwekar, S.U.; Leder, B.Z.; Solomon, D.H. Comparison of Teriparatide and Denosumab in Patients Switching From Long-Term Bisphosphonate Use. J. Clin. Endocrinol. Metab. 2019, 104, 5611–5620. [Google Scholar] [CrossRef]
- Langdahl, B.L. Overview of treatment approaches to osteoporosis. Br. J. Pharmacol. 2021, 178, 1891–1906. [Google Scholar] [CrossRef]


| Children | Young Adults | Senior Adults | |
|---|---|---|---|
| n = 13 | n = 13 | n = 9 | |
| Age (years) | 10 ± 1 | 29 ± 3 | 68 ± 8 |
| Gender (female/male) | 6/7 | 7/6 | 6/3 |
| Body mass index (kg/m2) | 20.41 ± 4.5 * | 22.80 ± 2.3 * | 25.9 ± 3.3 |
| P1NP (ng/mL) | 485.9 ± 154.1 * | 42.9 ± 3.7 | 41.5 ± 13.9 |
| CTX (pg/mL) | 1.22 ± 0.103 | 0.36 ± 0.04 | 0.31 ± 0.03 |
| 25OHVD3 (ng/mL) | 24.06 ± 7.4 | 23.7 ± 1.6 | 32.44 ± 5.9 |
| Ca (mg/dL) | 9.8 ±0.1 | 9.4 ± 0.1 | 9.6 ± 0.1 |
| P (mg/dL) | 4.5 ± 0.1 | 3.4 ± 0.1 | 3.5 ± 0.1 |
| Femoral neck (gHA/cm2) | 0.723 ± 0.92 | 0.828 ± 0.14 | 0.622 ± 0.06 |
| Hip (gHA/cm2) | 0.813 ± 0.09 | 0.8612 ± 0.31 | 0.897 ± 0.12 |
| Lumbar spine (gHA/cm2) | 0.673 ± 0.09 | 0.969 ± 0.72 | 0.805 ± 0.15 |
| TPTD (n = 7) | DmAb (n = 10) | p | |
|---|---|---|---|
| Age (years) | 58 ± 2 | 66 ± 10 | 0.142 |
| Body mass index (kg/m2) | 24.5 ± 3.7 | 26.3 ± 5.4 | 0.574 |
| CTX (pg/mL) | 0.28 ± 0.05 | 0.37 ± 0.05 | 0.302 |
| P1NP (ng/mL) | 56.4 ± 11.1 | 44.01 ± 5.3 | 0.274 |
| IGF-1 (ng/mL) | 158.2 ± 30.1 | 127.7 ± 18.2 | 0.393 |
| 25OHVD3 (ng/mL) | 34.1 ± 7.9 | 35.5 ± 10.7 | 0.924 |
| TPTD (n = 7) | p | |||
|---|---|---|---|---|
| Basal | 12 Months | % Change | ||
| Femoral neck (gHA/cm2) | 0.64 ± 0.12 | 0.66 ± 0.17 | 3.1 | 0.696 |
| Hip (gHA/cm2) | 0.82 ± 0.09 | 0.82 ± 0.11 | 0 | 0.321 |
| Lumbar spine (gHA/cm2) | 0.66 ± 0.07 | 0.65 ± 0.05 | 0 | 0.782 |
| DmAb (n = 10) | p | |||
|---|---|---|---|---|
| Basal | 12 Months | % Change | ||
| Femoral neck (gHA/cm2) | 0.60 ± 0.07 | 0.61 ± 0.07 | 1.6 | 0.126 |
| Hip (gHA/cm2) | 0.83 ± 0.11 | 0.82 ± 0.17 | 0 | 0.515 |
| Lumbar spine (gHA/cm2) | 0.70 ± 0.12 | 0.74 ± 0.12 | 5.7 | 0.363 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giner, M.; Vázquez-Gámez, M.A.; Miranda, M.J.; Bocio-Nuñez, J.; Olmo-Montes, F.J.; Rico, M.A.; Colmenero, M.A.; Montoya-García, M.-J. Circulating Osteogenic Progenitor Cells Enhanced with Teriparatide or Denosumab Treatment. J. Clin. Med. 2022, 11, 4749. https://doi.org/10.3390/jcm11164749
Giner M, Vázquez-Gámez MA, Miranda MJ, Bocio-Nuñez J, Olmo-Montes FJ, Rico MA, Colmenero MA, Montoya-García M-J. Circulating Osteogenic Progenitor Cells Enhanced with Teriparatide or Denosumab Treatment. Journal of Clinical Medicine. 2022; 11(16):4749. https://doi.org/10.3390/jcm11164749
Chicago/Turabian StyleGiner, Mercè, María Angeles Vázquez-Gámez, María José Miranda, Jesús Bocio-Nuñez, Francisco Jesús Olmo-Montes, Miguel Angel Rico, Miguel Angel Colmenero, and María-José Montoya-García. 2022. "Circulating Osteogenic Progenitor Cells Enhanced with Teriparatide or Denosumab Treatment" Journal of Clinical Medicine 11, no. 16: 4749. https://doi.org/10.3390/jcm11164749
APA StyleGiner, M., Vázquez-Gámez, M. A., Miranda, M. J., Bocio-Nuñez, J., Olmo-Montes, F. J., Rico, M. A., Colmenero, M. A., & Montoya-García, M.-J. (2022). Circulating Osteogenic Progenitor Cells Enhanced with Teriparatide or Denosumab Treatment. Journal of Clinical Medicine, 11(16), 4749. https://doi.org/10.3390/jcm11164749






