Immediate-Early Modifications to the Metabolomic Profile of the Perilymph Following an Acoustic Trauma in a Sheep Model
Abstract
1. Introduction
2. Methods
2.1. Experimental Groups and Noise Exposure
2.2. Auditory Measurement
2.3. Preparation of the Samples
2.4. Metabolomic Analysis
2.5. Statistical Analysis
2.6. Pathways Analyses
3. Results
3.1. Animal Model of Acoustic Trauma
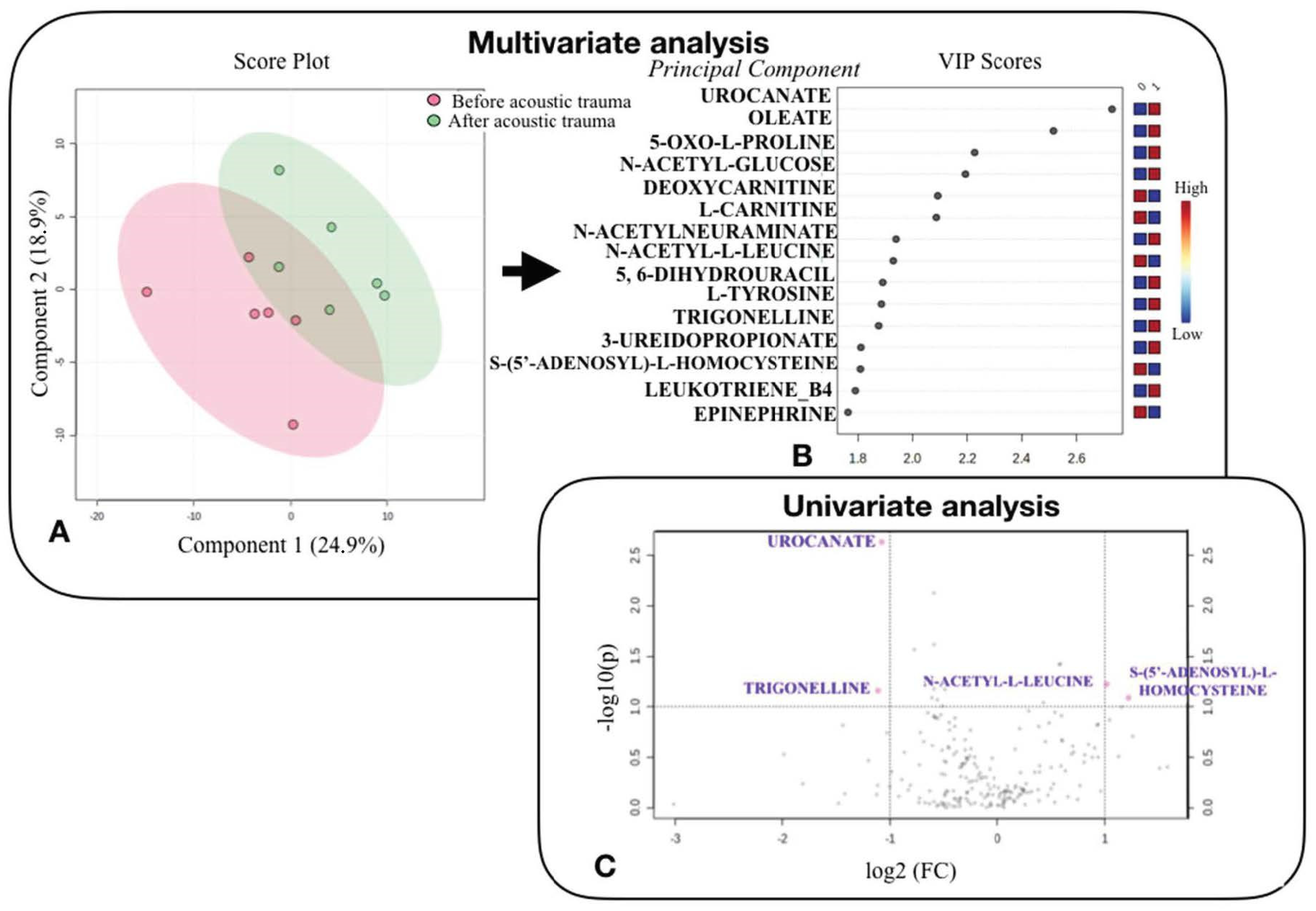
3.2. Effect of Acoustic Trauma on the Perilymph Metabolome
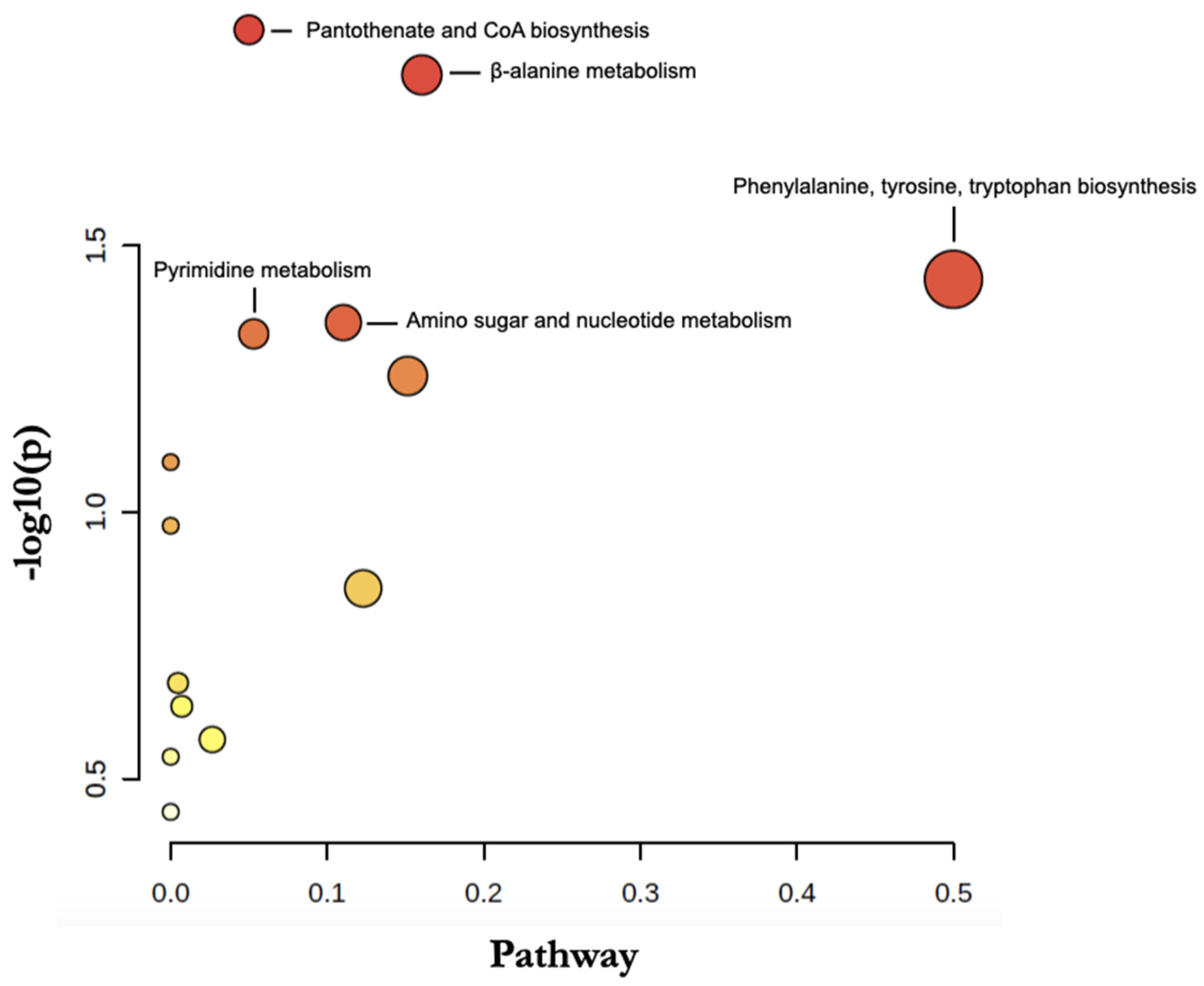
3.3. Analysis of Metabolic Pathways
4. Discussion
4.1. Interest in the Sheep Model
4.2. Research Approach
4.3. Metabolomics as a Powerful Approach to Characterize Perilymph
4.4. Metabolomic Modifications following Acoustic Trauma
4.4.1. Neurotransmission
4.4.2. Oxidative Stress
4.4.3. Mechanical Destruction
4.4.4. Nerve Damage
4.5. Limits to the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Deafness and Hearing Impairment. Available online: http://www.who.int/mediacentre/factsheets/fs300/en/index/html (accessed on 3 June 2022).
- Kral, A.; O’Donoghue, G.M. Profound deafness in childhood. N. Engl. J. Med. 2010, 363, 1438–1450. [Google Scholar] [CrossRef]
- Huddle, M.G.; Goman, A.M.; Kernizan, F.C.; Folley, D.M.; Price, C.; Frick, K.D.; Lin, F.R. The Economic Impact of Adult Hearing Loss: A Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.; Smith, A.; Concha, M. Global burden of hearing loss in the year 2000. Glob. Burd. Dis. 2000, 18, 1–30. [Google Scholar]
- Blamey, P.; Artieres, F.; Başkent, D.; Bergeron, F.; Beynon, A.; Burke, E.; Dillier, N.; Dowell, R.; Fraysse, B.; Gallégo, S.; et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol. Neurootol. 2013, 18, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Straatman, L.V.; Lea, J. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol. Head Neck Surg. 2017, 46, 41. [Google Scholar] [CrossRef] [PubMed]
- Hamernik, R.P.; Hsueh, K.D. Impulse noise: Some definitions, physical acoustics and other considerations. J. Acoust. Soc. Am. 1991, 90, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G.; Hammill, T.L.; Murphy, W.J. Noise-induced hearing loss and its prevention: Integration of data from animal models and human clinical trials. J. Acoust. Soc. Am. 2019, 146, 4051. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Carta, F.; Lussu, M.; Bandino, F.; Noto, A.; Peppi, M.; Chuchueva, N.; Atzori, L.; Fanos, V.; Puxeddu, R. Metabolomic analysis of urine with Nuclear Magnetic Resonance spectroscopy in patients with idiopathic sudden sensorineural hearing loss: A preliminary study. Auris Nasus Larynx 2017, 44, 381–389. [Google Scholar] [CrossRef]
- Diémé, B.; Mavel, S.; Blasco, H.; Tripi, G.; Bonnet-Brilhault, F.; Malvy, J.; Bocca, C.; Andres, C.R.; Nadal-Desbarats, L.; Emond, P. Metabolomics Study of Urine in Autism Spectrum Disorders Using a Multiplatform Analytical Methodology. J. Proteome Res. 2015, 14, 5273–5282. [Google Scholar] [CrossRef]
- Reis, A.; Dalmolin, S.P.; Dallegrave, E. Animal models for hearing evaluations: A literature review. Revista CEFAC 2017, 19, 417–428. [Google Scholar] [CrossRef]
- Trinh, T.T.; Cohen, C.; Boullaud, L.; Cottier, J.P.; Bakhos, D. Sheep as a large animal model for cochlear implantation. Braz. J. Otorhinolaryngol. 2021, in press. [CrossRef] [PubMed]
- Han, S.; Suzuki-Kerr, H.; Suwantika, M.; Telang, R.S.; Gerneke, D.A.; Anekal, P.V.; Bird, P.; Vlajkovic, S.M.; Thorne, P.R. Characterization of the Sheep Round Window Membrane. J. Assoc. Res. Otolaryngol. 2021, 22, 1–17. [Google Scholar] [CrossRef]
- Soares, H.B.; Lavinsky, L. Histology of sheep temporal bone. Braz. J. Otolhinolaryngol. 2011, 77, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Péus, D.; Dobrev, I.; Prochazka, L.; Thoele, K.; Dalbert, A.; Boss, A.; Newcomb, N.; Probst, R.; Röösli, C.; Sim, J.H.; et al. Sheep as a large animal ear model: Middle-ear ossicular velocities and intracochlear sound pressure. Hear. Res. 2017, 351, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.W.; Heavens, R.P.; Baldwin, B.A. Auditory evoked potentials recorded from conscious sheep. Brain Res. Bull. 1985, 15, 453–458. [Google Scholar] [CrossRef]
- Ji, L.; Lee, H.J.; Wan, G.; Wang, G.P.; Zhang, L.; Sajjakulnukit, P.; Schacht, J.; Lyssiotis, C.A.; Corfas, G. Auditory metabolomics, an approach to identify acute molecular effects of noise trauma. Sci. Rep. 2019, 9, 9273. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Yamashita, D.; Irino, Y.; Kitamoto, J.; Fukuda, Y.; Inokuchi, G.; Hasegawa, S.; Otsuki, N.; Yoshida, M.; Nibu, K.I. Metabolomic profiling in inner ear fluid by gas chromatography/mass spectrometry in guinea pig cochlea. Neurosci. Lett. 2015, 606, 188–193. [Google Scholar] [CrossRef]
- Valero, M.D.; Burton, J.A.; Hauser, S.N.; Hackett, T.A.; Ramachandran, R.; Liberman, M.C. Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta). Hear. Res. 2017, 353, 213–223. [Google Scholar] [CrossRef]
- Mavel, S.; Lefèvre, A.; Bakhos, D.; Dufour-Rainfray, D.; Blasco, H.; Emond, P. Validation of metabolomics analysis of human perilymph fluid using liquid chromatography-mass spectroscopy. Hear. Res. 2018, 367, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.T.; Blasco, H.; Emond, P.; Andres, C.; Lefevre, A.; Lescanne, E.; Bakhos, D. Relationship between metabolomics profile of perilymph in Cochlear-Implanted patients and duration of hearing loss. Metabolites 2019, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Pirttilä, K.; Videhult Pierre, P.; Haglöf, J.; Engskog, M.; Hedeland, M.; Laurell, G.; Arvidsson, T.; Pettersson, C. An LCMS-based Untargeted Metabolomics Protocol for Cochlear Perilymph: Highlighting Metabolic Effects of Hydrogen Gas on the Inner Ear of Noise Exposed Guinea Pigs. Metabolomics 2019, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Fransson, A.E.; Kisiel, M.; Pirttilä, K.; Pettersson, C.; Videhult Pierre, P.; Laurell, G.F. Hydrogen Inhalation Protects against Ototoxicity Induced by Intravenous Cisplatin in the Guinea Pig. Front. Cell Neurosci. 2017, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S; discussion 1548S. [Google Scholar] [CrossRef] [PubMed]
- Mroz, E.A.; Sewell, W.F. Pharmacological alterations of the activity of afferent fibers innervating hair cells. Hear. Res. 1989, 38, 141–162. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. Histidine Metabolism and Function. J. Nutr. 2020, 150 (Suppl. S1), 2570S–2575S. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Norval, M. The Multiple Roles of Urocanic Acid in Health and Disease. J. Invest. Dermatol. 2021, 141, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Tahiliani, A.G.; Beinlich, C.J. Pantothenic acid in health and disease. Vitam. Horm. 1991, 46, 165–228. [Google Scholar] [CrossRef]
- Ma, T.; Liu, T.; Xie, P.; Jiang, S.; Yi, W.; Dai, P.; Guo, X. UPLC-MS-based urine nontargeted metabolic profiling identifies dysregulation of pantothenate and CoA biosynthesis pathway in diabetic kidney disease. Life Sci. 2020, 258, 118160. [Google Scholar] [CrossRef] [PubMed]
- Partearroyo, T.; Vallecillo, N.; Pajares, M.A.; Varela-Moreiras, G.; Varela-Nieto, I. Cochlear Homocysteine Metabolism at the Crossroad of Nutrition and Sensorineural Hearing Loss. Front. Mol. Neurosci. 2017, 10, 107. [Google Scholar] [CrossRef]
- Drescher, M.J.; Drescher, D.G. N-acetylhistidine, glutamate, and beta-alanine are concentrated in a receptor cell layer of the trout inner ear. J. Neurochem. 1991, 56, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, R.; Rodriguez, I.; Nam, Y.H.; Hong, B.N.; Kang, T.H. Trigonelline promotes auditory function through nerve growth factor signaling on diabetic animal models. Phytomedicine 2017, 36, 128–136. [Google Scholar] [CrossRef]
- Yoshinari, O.; Takenake, A.; Igarashi, K. Trigonelline ameliorates oxidative stress in type 2 diabetic Goto-Kakizaki rats. J. Med. Food 2013, 16, 34–41. [Google Scholar] [CrossRef]
- Günther, L.; Beck, R.; Xiong, G.; Potschka, H.; Jahn, K.; Bartenstein, P.; Brandt, T.; Dutia, M.; Dieterich, M.; Strupp, M.; et al. N-acetyl-L-leucine accelerates vestibular compensation after unilateral labyrinthectomy by action in the cerebellum and thalamus. PLoS ONE 2015, 10, e0120891. [Google Scholar] [CrossRef]
- Xiao, Y.; Wen, J.; Bai, Y.; Duan, N.; Jing, G.X. Different effects of propofol and isoflurane on cochlear blood flow and hearing function in Guinea pigs. PLoS ONE 2014, 9, e96861. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Sheep | Breed | Sex | Age (Months) | Weight (kg) | Duration of Anesthesia (Min) | Stimulation Sampling Time (Min) |
|---|---|---|---|---|---|---|
| 1 | IDF | F | 27.5 | 64 | 240 | 45 |
| 2 | IDF | F | 27.8 | 52 | 240 | 80 |
| 3 | IDF | F | 28.4 | 72 | 180 | 60 |
| 4 | IDF | F | 30.0 | 82 | 180 | 50 |
| 5 | IDF | F | 35.5 | 63 | 210 | 60 |
| 6 | IDF | F | 29.9 | 72 | 180 | 45 |
| Mean | 29.9 | 67.5 | 205 | 56.7 | ||
| SD | 2.97 | 10.23 | 29.5 | 13.3 |
| NH Model | NIHL Model | ||||
|---|---|---|---|---|---|
| Sheep N° | Side | Threshold before Sampling (dB) | Side | Threshold before Sampling (dB) | Threshold after Acoustic Trauma (dB) |
| 1 | L | 30 | R | 30 | 60 |
| 2 | L | 30 | R | 30 | 50 |
| 3 | R | 30 | L | 30 | 60 |
| 4 | R | 30 | L | 30 | 60 |
| 5 | R | 30 | L | 20 | 60 |
| 6 | L | 20 | R | 20 | 60 |
| Mean | - | 28.3 | 26.7 | 58.3 | |
| SD | - | 4.1 | 5.2 | 4.1 | |
| Pathway | p-Value | p-FDR | Impact |
|---|---|---|---|
| Pantothenate and CoA biosynthesis | 0.012 | 0.64 | 0.05 |
| β-alanine metabolism | 0.015 | 0.64 | 0.16 |
| Phenylalanine, tyrosine, tryptophan biosynthesis | 0.037 | 0.78 | 0.50 |
| Amino sugar and nucleotide metabolism | 0.044 | 0.78 | 0.11 |
| Pyrimidine metabolism | 0.046 | 0.78 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boullaud, L.; Blasco, H.; Caillaud, E.; Emond, P.; Bakhos, D. Immediate-Early Modifications to the Metabolomic Profile of the Perilymph Following an Acoustic Trauma in a Sheep Model. J. Clin. Med. 2022, 11, 4668. https://doi.org/10.3390/jcm11164668
Boullaud L, Blasco H, Caillaud E, Emond P, Bakhos D. Immediate-Early Modifications to the Metabolomic Profile of the Perilymph Following an Acoustic Trauma in a Sheep Model. Journal of Clinical Medicine. 2022; 11(16):4668. https://doi.org/10.3390/jcm11164668
Chicago/Turabian StyleBoullaud, Luc, Hélène Blasco, Eliott Caillaud, Patrick Emond, and David Bakhos. 2022. "Immediate-Early Modifications to the Metabolomic Profile of the Perilymph Following an Acoustic Trauma in a Sheep Model" Journal of Clinical Medicine 11, no. 16: 4668. https://doi.org/10.3390/jcm11164668
APA StyleBoullaud, L., Blasco, H., Caillaud, E., Emond, P., & Bakhos, D. (2022). Immediate-Early Modifications to the Metabolomic Profile of the Perilymph Following an Acoustic Trauma in a Sheep Model. Journal of Clinical Medicine, 11(16), 4668. https://doi.org/10.3390/jcm11164668








