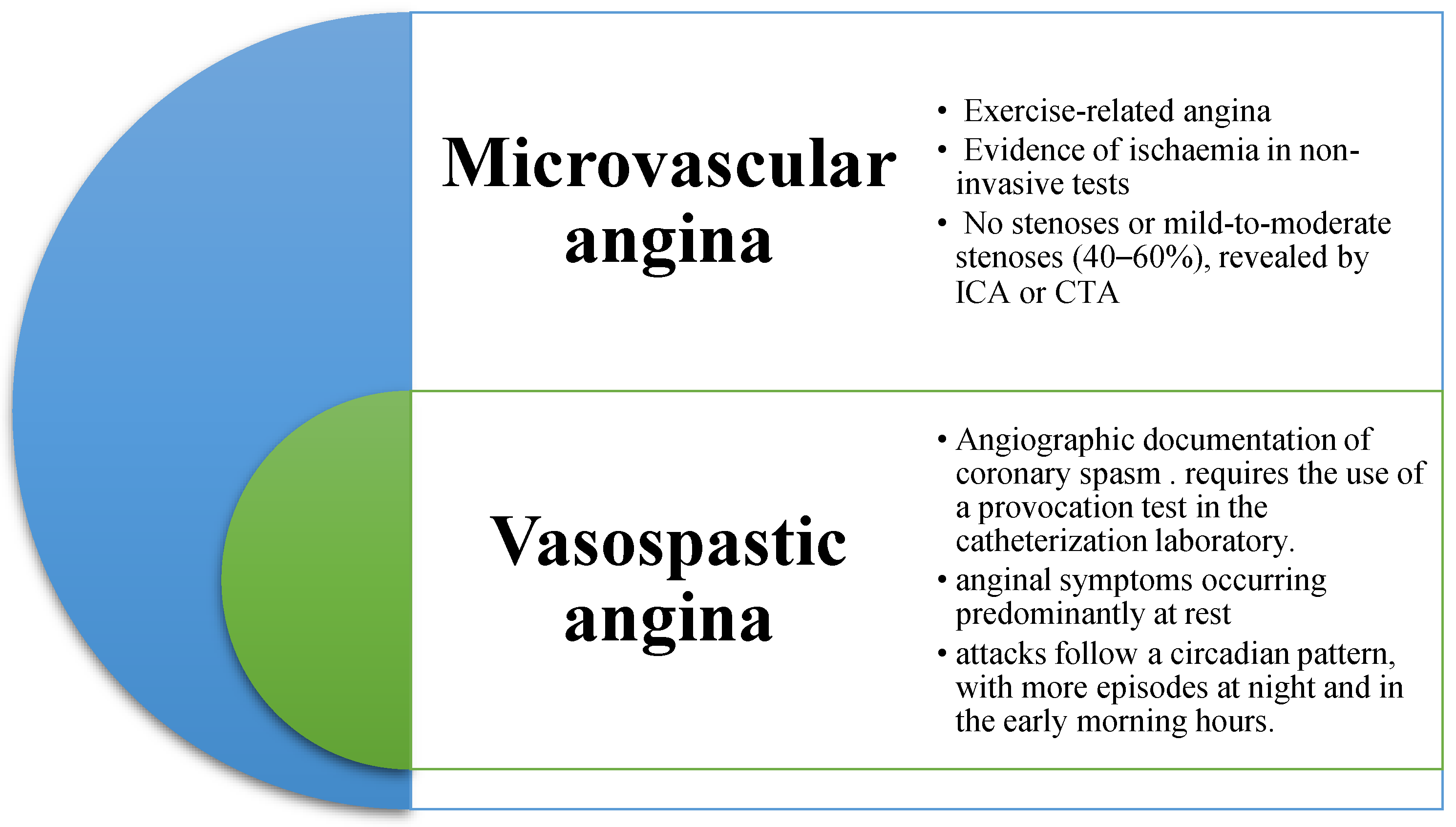
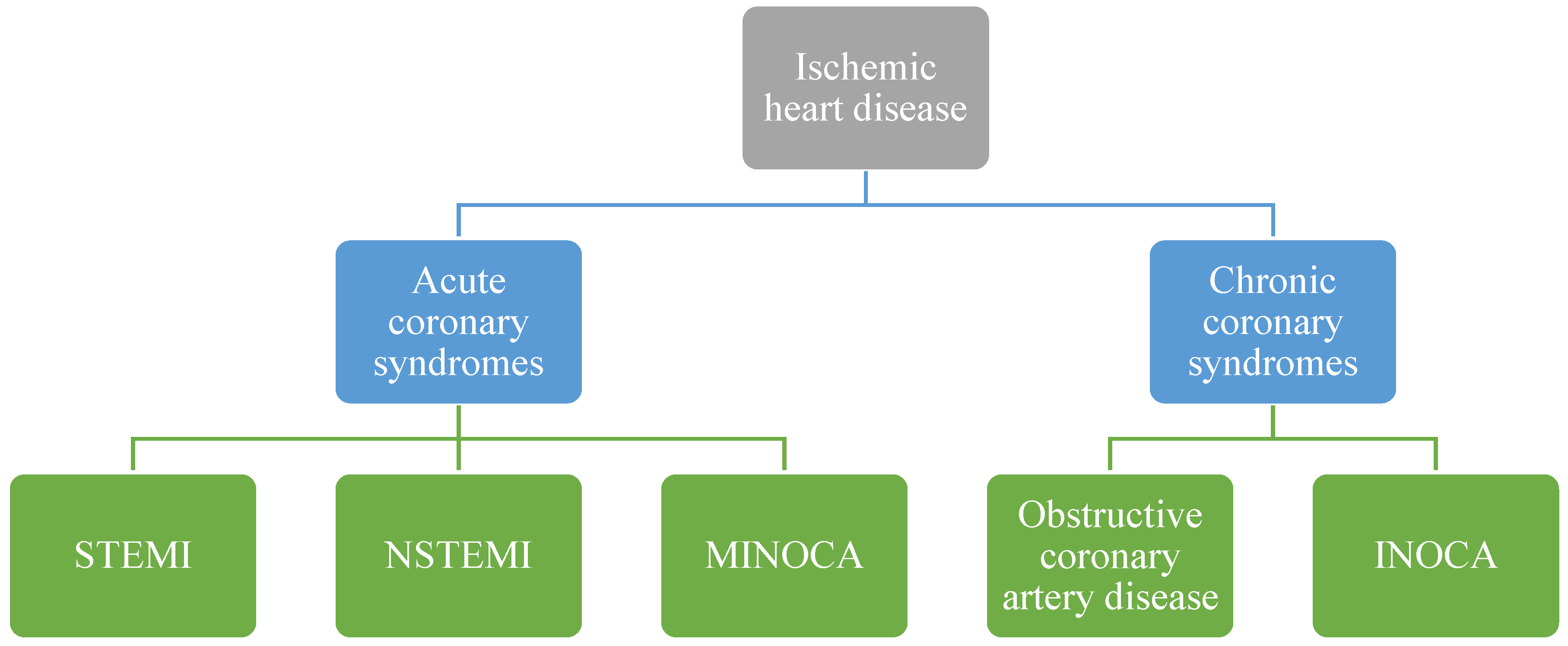
Classification, Diagnosis, and Treatment of Coronary Microvascular Dysfunction
Funding
Conflicts of Interest
References
- Padro, T.; Manfrini, O.; Bugiardini, R.; Canty, J.; Cenko, E.; De Luca, G.; Duncker, D.J.; Eringa, E.C.; Koller, A.; Tousoulis, D.; et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on “coronary microvascular dysfunction in cardiovascular disease”. Cardiovasc. Res. 2020, 116, 741–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sucato, V.; Novo, G.; Saladino, A.; Evola, S.; Galassi, A.R. Coronary microvascular dysfunction. Minerva. Cardioangiol. 2020, 68, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Sucato, V.; Novo, G.; Saladino, A.; Rubino, M.; Caronna, N.; Luparelli, M.; D’Agostino, A.; Novo, S.; Evola, S.; Galassi, A.R. Ischemia in patients with no obstructive coronary artery disease: Classification, diagnosis and treatment of coronary microvascular dysfunction. Coron. Artery Dis. 2020, 31, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Biondi-Zoccai, G.G.; Agostoni, P.; Lipinski, M.J.; Vetrovec, G.W. Recurrent angina after coronary revascularization: A clinical challenge. Eur. Heart J. 2007, 28, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, R.O.; Epstein, S.E. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am. J. Cardiol. 1988, 61, 1338–1343. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; ESC Scientific Document Group; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sucato, V.; Corrado, E.; Manno, G.; Amata, F.; Testa, G.; Novo, G.; Galassi, A.R. Biomarkers of Coronary Microvascular Dysfunction in Patients with Microvascular Angina: A Narrative Review. Angiology 2022, 73, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L.; Camici, P.G.; Chilian, W.M.; Clayton, J.A.; Cooper, L.S.; Crea, F.; Di Carli, M.; et al. Ischemia and no obstructive coronary artery disease (INOCA): Developing evidence-based therapies research agenda for the next decade. Circulation 2017, 135, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Sucato, V.; Testa, G.; Puglisi, S.; Evola, S.; Galassi, A.R.; Novo, G. Myocardial infarction with non-obstructive coronary arteries (MINOCA): Intracoronary imaging-based diagnosis and management. J. Cardiol. 2021, 77, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Sucato, V.; Novo, S.; Rubino, M.; D’Agostino, A.; Evola, S.; Novo, G. Prognosis in patients with microvascular angina: A clinical follow-up. J. Cardiovasc. Med. 2019, 20, 794–795. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sucato, V.; Madaudo, C.; Galassi, A.R. Classification, Diagnosis, and Treatment of Coronary Microvascular Dysfunction. J. Clin. Med. 2022, 11, 4610. https://doi.org/10.3390/jcm11154610
Sucato V, Madaudo C, Galassi AR. Classification, Diagnosis, and Treatment of Coronary Microvascular Dysfunction. Journal of Clinical Medicine. 2022; 11(15):4610. https://doi.org/10.3390/jcm11154610
Chicago/Turabian StyleSucato, Vincenzo, Cristina Madaudo, and Alfredo Ruggero Galassi. 2022. "Classification, Diagnosis, and Treatment of Coronary Microvascular Dysfunction" Journal of Clinical Medicine 11, no. 15: 4610. https://doi.org/10.3390/jcm11154610
APA StyleSucato, V., Madaudo, C., & Galassi, A. R. (2022). Classification, Diagnosis, and Treatment of Coronary Microvascular Dysfunction. Journal of Clinical Medicine, 11(15), 4610. https://doi.org/10.3390/jcm11154610




