Cortical and Subcortical Alterations and Clinical Correlates after Traumatic Brain Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive Functional Assessment and Neuropsychological Assessment
2.3. Image Acquisition
2.4. T1 MRI Data Processing and Analysis
2.5. Diffusion Tensor Imaging(DTI) Data Preprocessing and Analysis
2.6. Correlation Analysis and Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
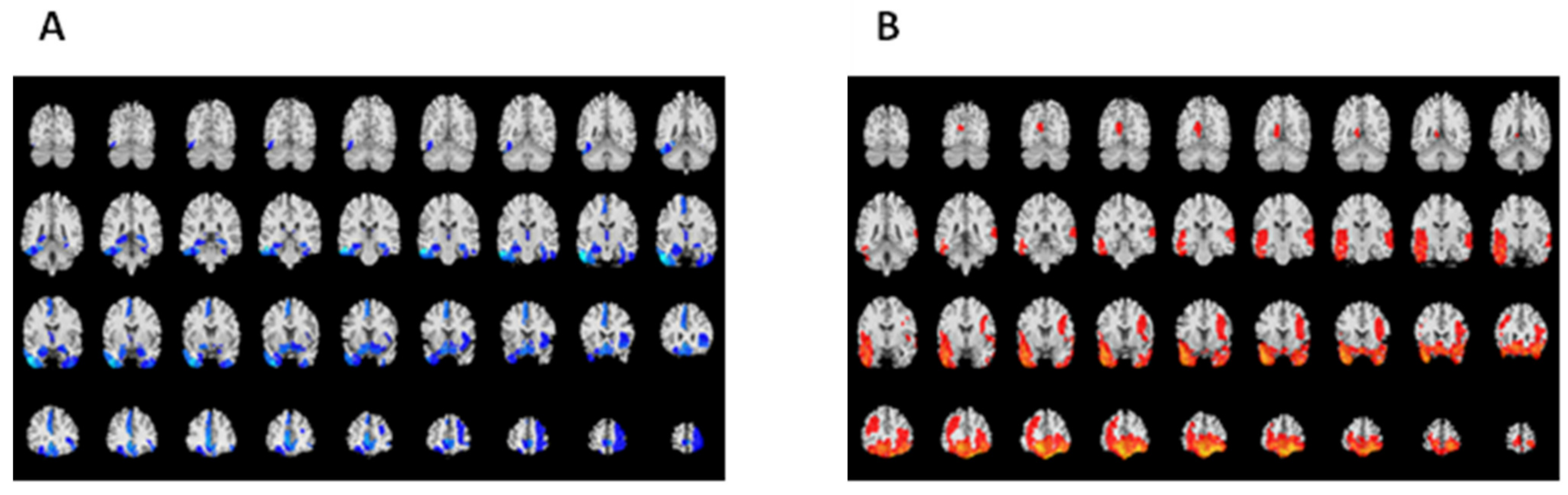
3.2. Reduced GM Volumes in Patients with Traumatic Brain Injury
3.3. Correlations between GM Volume and Clinical Parameters in Patients with Traumatic Brain Injury
3.4. WM Microstructure Alterations in Patients with Traumatic Brain Injury
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bombardier, C.H.; Fann, J.R.; Temkin, N.R.; Esselman, P.C.; Barber, J.; Dikmen, S.S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010, 303, 1938–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponsford, J.; Alway, Y.; Gould, K.R. Epidemiology and Natural History of Psychiatric Disorders After TBI. J. Neuropsychiatry Clin. Neurosci. 2018, 30, 262–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, N.S.; Sharp, D.J. Understanding neurodegeneration after traumatic brain injury: From mechanisms to clinical trials in dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1221–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazel, S.; Wolf, A.; Pillas, D.; Lichtenstein, P.; Långström, N. Suicide, fatal injuries, and other causes of premature mortality in patients with traumatic brain injury: A 41-year Swedish population study. JAMA Psychiatry 2014, 71, 326–333. [Google Scholar] [CrossRef]
- Alway, Y.; Gould, K.R.; Johnston, L.; McKenzie, D.; Ponsford, J. A prospective examination of Axis I psychiatric disorders in the first 5 years following moderate to severe traumatic brain injury. Psychol. Med. 2016, 46, 1331–1341. [Google Scholar] [CrossRef]
- Singh, R.; Mason, S.; Lecky, F.; Dawson, J. Comparison of early and late depression after TBI; (the SHEFBIT study). Brain Inj. 2019, 33, 584–591. [Google Scholar] [CrossRef]
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent advances in traumatic brain injury. J. Neurol. 2019, 266, 2878–2889. [Google Scholar] [CrossRef] [Green Version]
- Gentry, L.R.; Godersky, J.C.; Thompson, B. MR imaging of head trauma: Review of the distribution and radiopathologic features of traumatic lesions. AJR Am. J. Roentgenol. 1988, 150, 663–672. [Google Scholar] [CrossRef] [Green Version]
- McAllister, T.W. Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 2011, 13, 287–300. [Google Scholar] [CrossRef]
- Mesfin, F.B.; Gupta, N.; Hays Shapshak, A.; Taylor, R.S. Diffuse axonal injury. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Stuss, D.T. Traumatic brain injury: Relation to executive dysfunction and the frontal lobes. Curr. Opin. Neurol. 2011, 24, 584–589. [Google Scholar] [CrossRef]
- Bigler, E.D. Distinguished Neuropsychologist Award Lecture 1999. The lesion(s) in traumatic brain injury: Implications for clinical neuropsychology. Arch. Clin. Neuropsychol. 2001, 16, 95–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misquitta, K.; Dadar, M.; Tarazi, A.; Hussain, M.W.; Alatwi, M.K.; Ebraheem, A.; Multani, N.; Khodadadi, M.; Goswami, R.; Wennberg, R.; et al. The relationship between brain atrophy and cognitive-behavioural symptoms in retired Canadian football players with multiple concussions. Neuroimage Clin. 2018, 19, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Lutkenhoff, E.S.; Wright, M.J.; Shrestha, V.; Real, C.; McArthur, D.L.; Buitrago-Blanco, M.; Vespa, P.M.; Monti, M.M. The subcortical basis of outcome and cognitive impairment in TBI: A longitudinal cohort study. Neurology 2020, 95, e2398–e2408. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and WM degeneration persist for years after a single traumatic brain injury. Brain 2013, 136 Pt 1, 28–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Mahmood, A.; Chopp, M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 2018, 21, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Lauer, J.; Moreno-López, L.; Manktelow, A.; Carroll, E.L.; Outtrim, J.G.; Coles, J.P.; Newcombe, V.F.; Sahakian, B.J.; Menon, D.K.; Stamatakis, E.A. Neural correlates of visual memory in patients with diffuse axonal injury. Brain Inj. 2017, 31, 1513–1520. [Google Scholar] [CrossRef]
- Spitz, G.; Bigler, E.D.; Abildskov, T.; Maller, J.J.; O’Sullivan, R.; Ponsford, J.L. Regional cortical volume and cognitive functioning following traumatic brain injury. Brain Cogn. 2013, 83, 34–44. [Google Scholar] [CrossRef]
- Mori, S.; Oishi, K.; Jiang, H.; Jiang, L.; Li, X.; Akhter, K.; Hua, K.; Faria, A.V.; Mahmood, A.; Woods, R. Stereotaxic WM atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008, 40, 570–582. [Google Scholar] [CrossRef] [Green Version]
- Marin, J.R.; Weaver, M.D.; Yealy, D.M.; Mannix, R.C. Trends in visits for traumatic brain injury to emergency departments in the United States. JAMA 2014, 311, 1917–1919. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Li, F.; Ma, Y.; Chen, H.; Wang, P.; Peng, M.; Chen, Y.C.; Yin, X. Functional connectivity disruption of the substantia nigra associated with cognitive impairment in acute mild traumatic brain injury. Eur. J. Radiol. 2019, 114, 69–75. [Google Scholar] [CrossRef]
- Kanthimathinathan, H.K.; Mehta, H.; Scholefield, B.R.; Morris, K.P. Traumatic Brain Injury Practice Guidelines: Variability in U.K. PICUs. Pediatr. Crit. Care Med. 2021, 22, e270–e274. [Google Scholar] [CrossRef] [PubMed]
- Gutierre, M.U.; Telles, J.P.M.; Welling, L.C.; Rabelo, N.N.; Teixeira, M.J.; Figueiredo, E.G. Biomarkers for traumatic brain injury: A short review. Neurosurg. Rev. 2021, 44, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois-Tardif, S.; de Beaumont, L.; Rivera, J.C.; Chemtob, S.; Weil, A.G. Role of innate inflammation in traumatic brain injury. Neurol. Sci. 2021, 42, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Livny, A.; Biegon, A.; Kushnir, T.; Harnof, S.; Hoffmann, C.; Fruchter, E.; Weiser, M. Cognitive Deficits Post-Traumatic Brain Injury and Their Association with Injury Severity and GM Volumes. J. Neurotrauma 2017, 34, 1466–1472. [Google Scholar] [CrossRef]
- Wallace, E.J.; Mathias, J.L.; Ward, L. The relationship between diffusion tensor imaging findings and cognitive outcomes following adult traumatic brain injury: A meta-analysis. Neurosci. Biobehav. Rev. 2018, 92, 93–103. [Google Scholar] [CrossRef]
- Grassi, D.C.; Conceição, D.M.D.; Leite, C.D.C.; Andrade, C.S. Current contribution of diffusion tensor imaging in the evaluation of diffuse axonal injury. Arq. Neuropsiquiatr. 2018, 76, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Wallace, E.J.; Mathias, J.L.; Ward, L. Diffusion tensor imaging changes following mild, moderate and severe adult traumatic brain injury: A meta-analysis. Brain Imaging Behav. 2018, 12, 1607–1621. [Google Scholar] [CrossRef]
- D’Souza, M.M.; Kumar, M.; Choudhary, A.; Kaur, P.; Kumar, P.; Rana, P.; Trivedi, R.; Sekhri, T.; Singh, A.K. Alterations of connectivity patterns in functional brain networks in patients with mild traumatic brain injury: A longitudinal resting-state functional magnetic resonance imaging study. Neuroradiol. J. 2020, 33, 186–197. [Google Scholar] [CrossRef]
- Sporns, O. Graph theory methods: Applications in brain networks. Dialogues Clin. Neurosci. 2018, 20, 111–121. [Google Scholar] [CrossRef]
- Duboc, V.; Dufourcq, P.; Blader, P.; Roussigné, M. Asymmetry of the Brain: Development and Implications. Annu. Rev. Genet. 2015, 49, 647–672. [Google Scholar] [CrossRef]
- Minkova, L.; Habich, A.; Peter, J.; Kaller, C.P.; Eickhoff, S.B.; Klöppel, S. GM asymmetries in aging and neurodegeneration: A review and meta-analysis. Hum. Brain Mapp. 2017, 38, 5890–5904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Zhong, Z.; Merkitch, D.; Karaman, M.M.; Zhang, J.; Sui, Y.; Goldman, J.G.; Zhou, X.J. High-Spatial-Resolution Diffusion MRI in Parkinson Disease: Lateral Asymmetry of the Substantia Nigra. Radiology 2019, 291, 149–157. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Evans, A. Graph theoretical modeling of brain connectivity. Curr. Opin. Neurol. 2010, 23, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manley, G.T.; Mac Donald, C.L.; Markowitz, A.J.; Stephenson, D.; Robbins, A.; Gardner, R.C.; Winkler, E.; Bodien, Y.G.; Taylor, S.R.; Yue, J.K.; et al. The Traumatic Brain Injury Endpoints Development (TED) Initiative: Progress on a Public-Private Regulatory Collaboration to Accelerate Diagnosis and Treatment of Traumatic Brain Injury. J. Neurotrauma 2017, 34, 2721–2730. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.H.; Wi, S.; Kim, M.; Pyo, S.; Shin, Y.K.; Oh, K.J.; Han, K.; Kim, Y.W.; Cho, S.R. Factors affecting cognition and emotion in patients with traumatic brain injury. NeuroRehabilitation 2020, 46, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Pandit, A.S.; Expert, P.; Lambiotte, R.; Bonnelle, V.; Leech, R.; Turkheimer, F.E.; Sharp, D.J. Traumatic brain injury impairs small-world topology. Neurology 2013, 80, 1826–1833. [Google Scholar] [CrossRef] [Green Version]
- Fagerholm, E.D.; Hellyer, P.J.; Scott, G.; Leech, R.; Sharp, D.J. Disconnection of network hubs and cognitive impairment after traumatic brain injury. Brain 2015, 138 Pt 6, 1696–1709. [Google Scholar] [CrossRef] [Green Version]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Palacios, E.M.; Owen, J.P.; Yuh, E.L.; Wang, M.B.; Vassar, M.J.; Ferguson, A.R.; Diaz-Arrastia, R.; Giacino, J.T.; Okonkwo, D.O.; Robertson, C.S.; et al. TRACK-TBI Investigators. The evolution of WM microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Sci. Adv. 2020, 6, eaaz6892. [Google Scholar] [CrossRef]

| TBI (n = 32) | Healthy Controls (n = 23) | p Value | |
|---|---|---|---|
| Age, mean (SD), y | 35.59 (10.64) | 33.35 (9.42) | 0.422 |
| Male, No. (%) | 22 (68.35%) | 16 (69.57%) | 0.949 |
| Educational lever, mean (SD), y | 9.22 (4.16) | 9.57 (3.89) | 0.756 |
| Time since injury, mean (SD), m | 8.41 (7.02) | NA | NA |
| GCS, No. (%) | |||
| 13–15 | 22 (68.75%) | NA | NA |
| 9–12 | 6 (12.89%) | NA | NA |
| 3–8 | 4 (12.50%) | NA | NA |
| MMSE, mean (SD) | 27.281 (2.57) | 29.26 (0.92) | 0.001 |
| HADS anxiety, mean (SD) | 8.63 (4.65) | 4.22 (2.43) | <0.001 |
| HADS depression, mean (SD) | 7.97 (5.96) | 3.09 (2.25) | <0.001 |
| Memory, mean (SD) | 40.31 (13.54) | 52.04 (7.10) | <0.001 |
| Regions # | TBI (n = 32) Mean (±SD), mm3 | HC (n = 23) Mean (±SD), mm3 | % of Volumetric Decreases | p Value |
|---|---|---|---|---|
| SFG_L_7_1 | 679.38 ± 95.23 | 763.34 ± 80.17 | 11.00% | 0.019 |
| SFG_L_7_5 | 688.43 ± 90.55 | 762.78 ± 79.24 | 9.75% | 0.033 |
| SFG_L_7_6 | 617.15 ± 104.01 | 704.67 ± 91.78 | 12.42% | 0.033 |
| MFG_R_7_3 | 770.74 ± 175.19 | 914.94 ± 132.80 | 15.76% | 0.041 |
| MFG_R_7_7 | 724.51 ± 155.40 | 851.19 ± 112.29 | 14.88% | 0.045 |
| IFG_R_6_5 | 522.33 ± 75.282 | 601.15 ± 101.43 | 13.11% | 0.034 |
| OrG_L_6_1 | 415.58 ± 88.91 | 487.35 ± 65.21 | 14.73% | 0.048 |
| OrG_R_6_1 | 549.00 ± 129.35 | 677.97 ± 98.88 | 19.02% | 0.017 |
| OrG_L_6_3 | 760.58 ± 144.75 | 886.59 ± 110.28 | 14.21% | 0.034 |
| OrG_L_6_5 | 907.44 ± 148.74 | 1056.28 ± 137.44 | 14.09% | 0.017 |
| OrG_R_6_5 | 755.51 ± 134.16 | 879.01 ± 112.78 | 14.05% | 0.033 |
| OrG_R_6_6 | 412.28 ± 67.12 | 472.76 ± 56.05 | 12.79% | 0.034 |
| STG_L_6_1 | 632.45 ± 129.23 | 727.36 ± 90.08 | 13.05% | 0.048 |
| MTG_L_4_2 | 692.60 ± 151.93 | 835.50 ± 126.73 | 17.10% | 0.017 |
| ITG_L_7_1 | 252.28 ± 45.31 | 300.55 ± 45.66 | 16.06% | 0.017 |
| ITG_L_7_3 | 461.55 ± 84.69 | 568.62 ± 81.82 | 18.83% | 0.005 |
| ITG_R_7_3 | 410.09 ± 73.29 | 474.31 ± 55.33 | 13.54% | 0.034 |
| ITG_L_7_4 | 431.60 ± 89.55 | 551.51 ± 82.59 | 21.74% | <0.001 |
| ITG_R_7_4 | 480.15 ± 80.21 | 553.43 ± 82.71 | 13.24% | 0.035 |
| ITG_L_7_7 | 497.54 ± 89.74 | 586.11 ± 92.01 | 15.11% | 0.017 |
| FuG_L_3_1 | 997.68 ± 149.18 | 1154.11 ± 146.99 | 13.55% | 0.017 |
| FuG_R_3_1 | 1111.18 ± 164.05 | 1244.75 ± 145.85 | 10.73% | 0.047 |
| FuG_L_3_3 | 948.99 ± 136.76 | 1061.95 ± 148.23 | 10.64% | 0.050 |
| PhG_L_6_5 | 115.27 ± 17.03 | 130.30 ± 18.02 | 11.53% | 0.039 |
| INS_R_6_2 | 246.82 ± 31.88 | 276.64 ± 37.96 | 10.78% | 0.035 |
| INS_R_6_3 | 285.86 ± 41.35 | 323.85 ± 54.10 | 11.73% | 0.050 |
| CG_L_7_3 | 470.88 ± 88.78 | 554.36 ± 66.22 | 15.06% | 0.017 |
| CG_L_7_7 | 633.52 ± 147.04 | 794.16 ± 118.78 | 20.23% | 0.006 |
| Amyg_L_2_1 | 185.75 ± 21.72 | 207.61 ± 26.65 | 10.53% | 0.034 |
| Amyg_R_2_1 | 267.19 ± 32.58 | 297.09 ± 38.82 | 10.07% | 0.049 |
| Amyg_L_2_2 | 93.16 ± 10.40 | 103.25 ± 12.28 | 9.77% | 0.033 |
| Amyg_R_2_2 | 140.75 ± 15.64 | 156.67 ± 18.30 | 10.16% | 0.028 |
| Hipp_L_2_1 | 666.67 ± 80.30 | 737.97 ± 80.84 | 9.66% | 0.034 |
| Hipp_L_2_2 | 485.14 ± 67.36 | 541.11 ± 64.92 | 10.34% | 0.050 |
| Hipp_R_2_2 | 566.44 ± 75.22 | 633.31 ± 60.04 | 10.56% | 0.034 |
| BG_L_6_3 | 368.88 ± 49.71 | 411.61 ± 48.00 | 10.38% | 0.033 |
| BG_R_6_3 | 456.63 ± 64.02 | 507.36 ± 57.34 | 10.00% | 0.050 |
| Tha_L_8_4 | 174.26 ± 30.13 | 198.40 ± 24.08 | 12.17% | 0.034 |
| Tha_R_8_4 | 185.47 ± 33.66 | 213.33 ± 28.92 | 13.06% | 0.034 |
| Regions # | MMSE | Memory | HADS-A | HADS-D | ||||
|---|---|---|---|---|---|---|---|---|
| r_Value | p_Value | r_Value | p_Value | r_Value | p_Value | r_Value | p_Value | |
| SFG_L_7_1 | NS | NS | NS | NS | NS | NS | 0.36 | 0.04 |
| SFG_L_7_5 | NS | NS | NS | NS | 0.37 | 0.03 | NS | NS |
| MFG_R_7_3 | NS | NS | −0.34 | 0.05 | NS | NS | NS | NS |
| MTG_L_4_2 | 0.45 | 0.01 | NS | NS | NS | NS | NS | NS |
| ITG_L_7_1 | 0.37 | 0.03 | NS | NS | NS | NS | NS | NS |
| ITG_L_7_4 | 0.49 | <0.01 | NS | NS | NS | NS | NS | NS |
| ITG_L_7_7 | 0.36 | 0.04 | NS | NS | NS | NS | NS | NS |
| FuG_L_3_1 | 0.45 | 0.01 | NS | NS | NS | NS | NS | NS |
| FuG_L_3_3 | 0.58 | <0.01 | NS | NS | NS | NS | NS | NS |
| INS_R_6_2 | NS | NS | −0.45 | 0.01 | NS | NS | NS | NS |
| CG_L_7_3 | 0.40 | 0.02 | NS | NS | NS | NS | NS | NS |
| Hipp_L_2_2 | 0.55 | <0.01 | NS | NS | 0.38 | 0.03 | NS | NS |
| Hipp_R_2_2 | 0.44 | 0.01 | NS | NS | NS | NS | NS | NS |
| Tha_L_8_4 | 0.49 | <0.01 | 0.51 | <0.01 | NS | NS | NS | NS |
| Regions # | Fractional Anisotropy | Mean Diffusivity | ||||
|---|---|---|---|---|---|---|
| TBI (n = 32) Mean (±SD) | HC (n = 23) Mean (±SD) | p-Value | TBI (n = 32) Mean (±SD) | HC (n = 23) Mean (±SD) | p-Value | |
| Forceps.major | 0.68 ± 0.02 | 0.70 ± 0.02 | 0.03 | NS | NS | NS |
| Forceps.minor | 0.53 ± 0.03 | 0.56 ± 0.03 | 0.01 | 0.00077 ± 0.000036 | 0.00073 ± 0.000043 | 0.04 |
| Inferior.fronto-occipital.fasciculus.L | 0.51 ± 0.03 | 0.53 ± 0.03 | 0.04 | NS | NS | NS |
| Superior.longitudinal.fasciculus.L | 0.48 ± 0.03 | 0.49 ± 0.03 | 0.04 | NS | NS | NS |
| Uncinate.fasciculus.L | 0.48 ± 0.05 | 0.52 ± 0.03 | 0.03 | NS | NS | NS |
| Uncinate.fasciculus.R | 0.52 ± 0.05 | 0.55 ± 0.03 | 0.04 | NS | NS | NS |
| Left Uncinate Fasciculus | Left Inferior Fronto-Occipital Fasciculus | |||
|---|---|---|---|---|
| Regions # | r | p | r | p |
| STG_L_6_1 | 0.358 | 0.041 | NS | NS |
| STG_L_6_2 | −0.439 | 0.011 | −0.399 | 0.021 |
| STG_L_6_5 | 0.435 | 0.011 | NS | NS |
| STG_L_6_6 | 0.347 | 0.048 | NS | NS |
| MTG_L_4_1 | 0.391 | 0.025 | NS | NS |
| MTG_L_4_2 | 0.555 | <0.001 | NS | NS |
| MTG_L_4_3 | 0.491 | 0.004 | 0.427 | 0.013 |
| ITG_L_7_1 | 0.504 | 0.003 | 0.573 | <0.001 |
| ITG_L_7_3 | 0.398 | 0.022 | NS | NS |
| ITG_L_7_4 | 0.397 | 0.022 | NS | NS |
| ITG_L_7_5 | 0.403 | 0.020 | NS | NS |
| ITG_L_7_6 | 0.542 | 0.001 | 0.530 | 0.002 |
| FuG_L_3_1 | 0.471 | 0.006 | NS | NS |
| FuG_L_3_3 | NS | NS | 0.379 | 0.029 |
| PhG_L_6_1 | 0.422 | 0.014 | NS | NS |
| PhG_L_6_2 | NS | NS | 0.387 | 0.026 |
| PhG_L_6_4 | 0.464 | 0.007 | 0.359 | 0.040 |
| PhG_L_6_5 | 0.598 | <0.001 | NS | NS |
| Hipp_L_2_1 | 0.559 | <0.001 | NS | NS |
| Hipp_L_2_1 | 0.512 | 0.002 | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Q.; Wang, L.; Zhao, Y.; Tong, W.; Wang, J.; Li, G.; Cheng, W.; Gao, L.; Dong, Y. Cortical and Subcortical Alterations and Clinical Correlates after Traumatic Brain Injury. J. Clin. Med. 2022, 11, 4421. https://doi.org/10.3390/jcm11154421
Xue Q, Wang L, Zhao Y, Tong W, Wang J, Li G, Cheng W, Gao L, Dong Y. Cortical and Subcortical Alterations and Clinical Correlates after Traumatic Brain Injury. Journal of Clinical Medicine. 2022; 11(15):4421. https://doi.org/10.3390/jcm11154421
Chicago/Turabian StyleXue, Qiang, Linbo Wang, Yuanyu Zhao, Wusong Tong, Jiancun Wang, Gaoyi Li, Wei Cheng, Liang Gao, and Yan Dong. 2022. "Cortical and Subcortical Alterations and Clinical Correlates after Traumatic Brain Injury" Journal of Clinical Medicine 11, no. 15: 4421. https://doi.org/10.3390/jcm11154421
APA StyleXue, Q., Wang, L., Zhao, Y., Tong, W., Wang, J., Li, G., Cheng, W., Gao, L., & Dong, Y. (2022). Cortical and Subcortical Alterations and Clinical Correlates after Traumatic Brain Injury. Journal of Clinical Medicine, 11(15), 4421. https://doi.org/10.3390/jcm11154421






