Renal Toxicities in Cancer Patients Receiving Immune-Checkpoint Inhibitors: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction, Clinical Outcomes, and Quality Assessment
2.3. Statistical Methods
3. Results
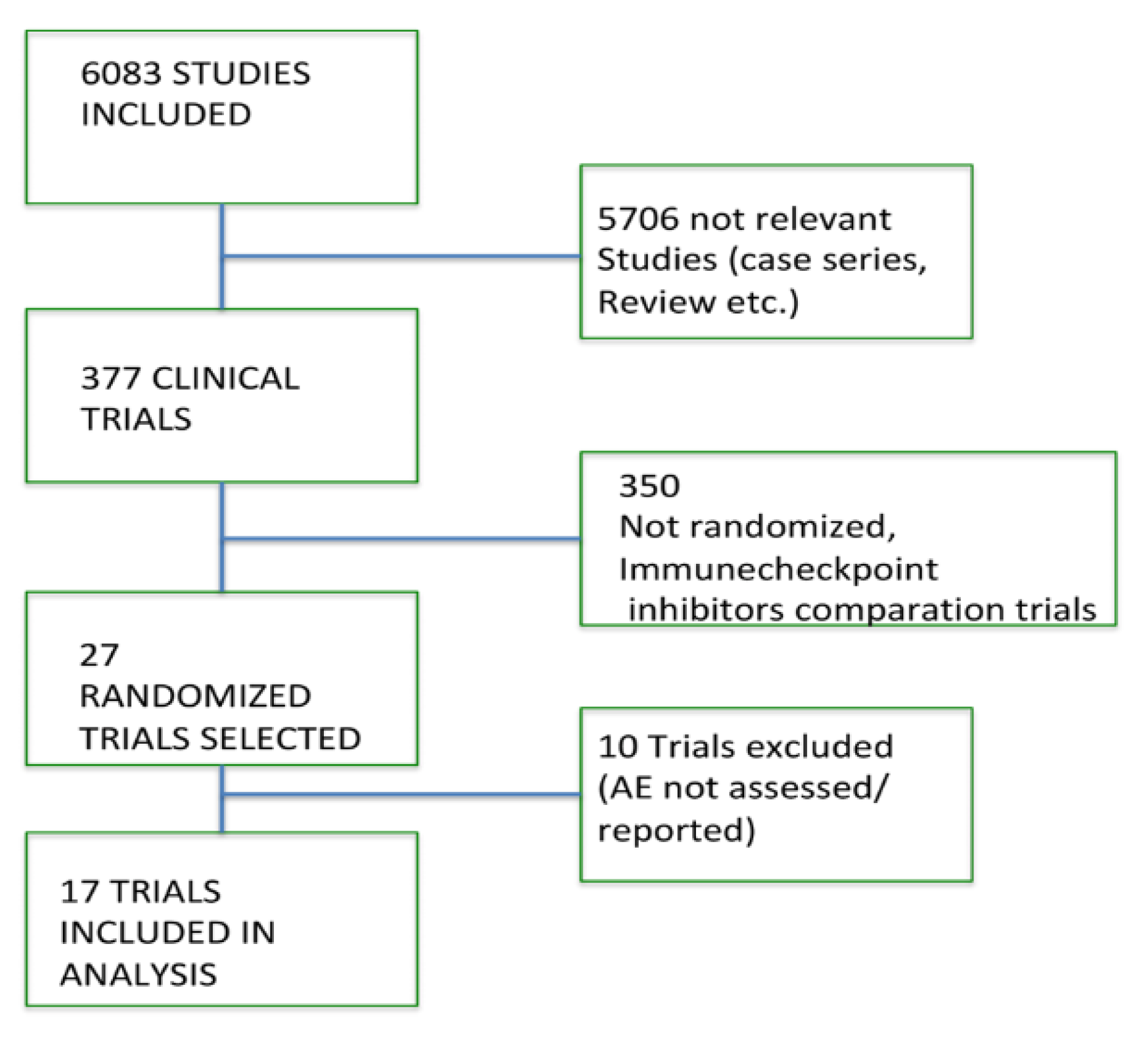
3.1. Search Results
3.2. Quality of Studies
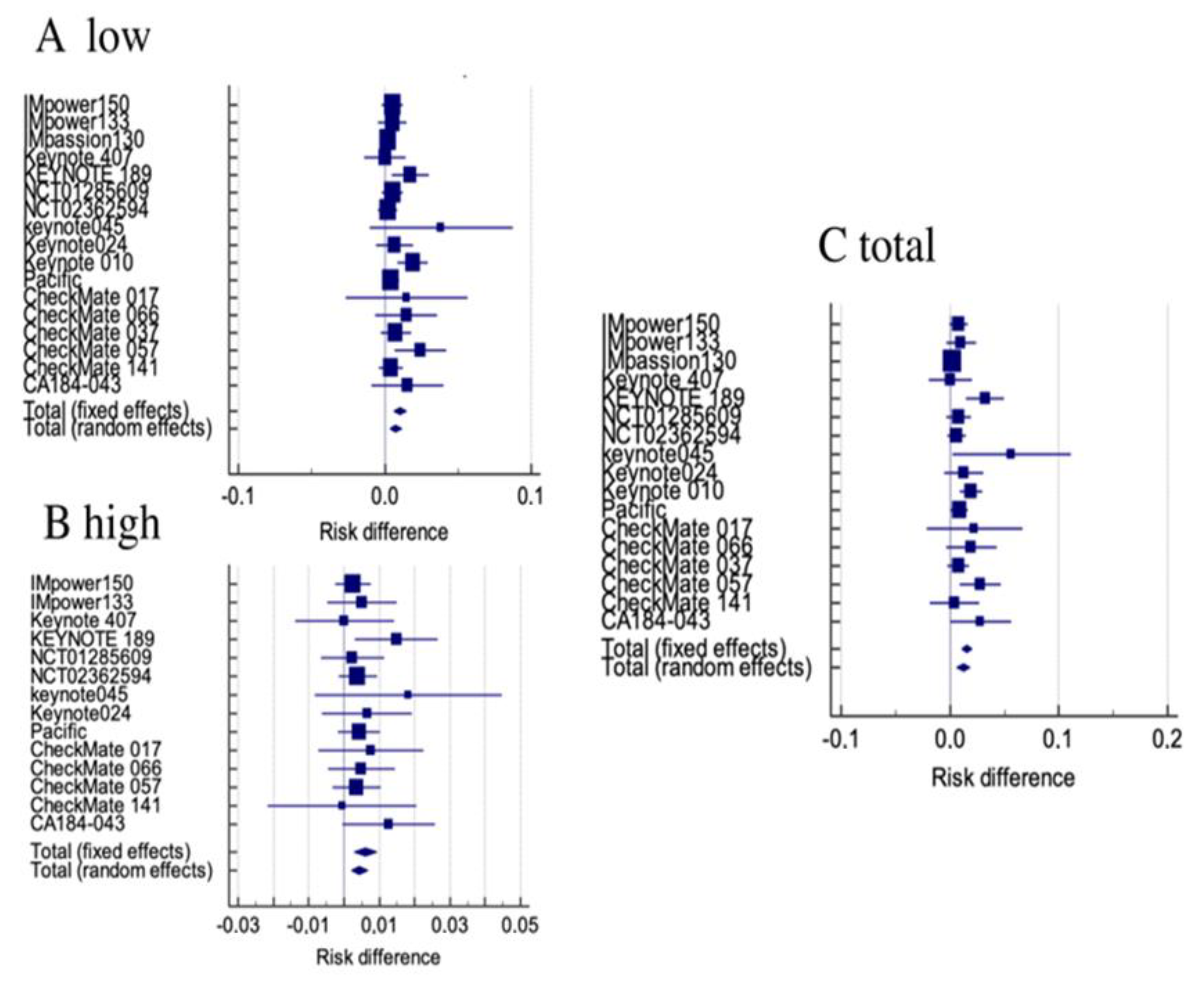
3.3. Renal Toxicity among All Clinical Trials
3.4. Renal Toxicity in Patients Receiving ICIs Alone
3.5. Renal Toxicity in Patients Receiving Combinations of ICIs
3.6. Renal Toxicity in Patients Receiving Anti CTLA-4 Agents
4. Discussion
5. Conclusions
Summary Points
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inno, A.; Metro, G.; Bironzo, P.; Grimaldi, A.M.; Grego, E.; DI Nunno, V.; Picasso, V.; Massari, F.; Gori, S. Pathogenesis, Clinical Manifestations and Management of Immune Checkpoint Inhibitors Toxicity. Tumori J. 2017, 103, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recom-mendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr. Med. Chem. 2018, 25, 1327–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wu, Q.; Zhou, Y.L.; Guo, X.; Ge, J.; Fu, J. Immune-related adverse events from combination immunotherapy in cancer patients: A comprehensive meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2018, 63, 292–298. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, X.-C.; Zhang, C.-G.; Hou, Y.-L.; Yao, Y.-X.; Cao, B.-W. Risk of Immune-Related Pancreatitis in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors: Systematic Assessment with Meta-Analysis. J. Immunol. Res. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Lie, P.; Guo, M.; He, J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis of published data. Int. J. Cancer 2017, 141, 1018–1028. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rahman, O.; Helbling, D.; Schmidt, J.; Petrausch, U.; Giryes, A.; Mehrabi, A.; Schöb, O.; Mannhart, M.; Oweira, H. Treatment-related Death in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. Clin. Oncol. 2016, 29, 218–230. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Fouad, M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: A me-ta-analysis. Ther. Adv. Respir. Dis. 2016, 10, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Rahman, O.; Fouad, M. A network meta-analysis of the risk of immune-related renal toxicity in cancer patients treated with immune checkpoint inhibitors. Immunotherapy 2016, 8, 665–674. [Google Scholar] [CrossRef]
- Cosmai, L.; Porta, C.; Gallieni, M.; Perazella, M.A. Onco-nephrology: A decalogue. Nephrol. Dial. Transplant. 2016, 31, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systemic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–431. [Google Scholar] [CrossRef] [Green Version]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Altman, D.G.; Machin, D.; Bryant, T.N.; Gardner, M.J. Statistics with Confidence, 2nd ed.; British Medical Association (BMA): London, UK, 2000; pp. 45–56. [Google Scholar]
- Yates, F. Contingency table involving small numbers and the χ2 test. J. R. Stat. Soc. 1934, 1, 217–235. [Google Scholar]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Govindan, R.; Szczesna, A.; Ahn, M.-J.; Schneider, C.-P.; Mella, P.F.G.; Barlesi, F.; Han, B.; Ganea, D.E.; Von Pawel, J.; Vladimirov, V.; et al. Phase III Trial of Ipilimumab Combined with Paclitaxel and Carboplatin in Advanced Squamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2017, 35, 3449–3457. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [Green Version]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castra-tion-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef] [Green Version]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirali, A.C.; Perazella, M.A.; Gettinger, S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am. J. Kidney Dis. 2016, 68, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Mamlouk, O.; Selamet, U.; Machado, S.; Abdelrahim, M.; Glass, W.F.; Tchakarov, A.; Gaber, L.; Lahoti, A.; Workeneh, B.; Chen, S.; et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J. Immunother. Cancer 2019, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, F.B.; Kibbelaar, Z.A.; Glezerman, I.G.; Abudayyeh, A.; Mamlouk, O.; Motwani, S.S.; Murakami, N.; Herrmann, S.M.; Manohar, S.; Shirali, A.C.; et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor–Associated AKI: A Multicenter Study. J. Am. Soc. Nephrol. 2020, 31, 435–446. [Google Scholar] [CrossRef]
- Seethapathy, H.; Street, S.; Strohbehn, I.; Lee, M.; Zhao, S.H.; Rusibamayila, N.; Chute, D.F.; Gao, X.; Michaelson, M.D.; Rahma, O.E.; et al. Immune-related adverse events and kidney function decline in patients with genitourinary cancers treated with immune checkpoint inhibitors. Eur. J. Cancer 2021, 157, 50–58. [Google Scholar] [CrossRef]
- Magee, D.E.; Hird, A.E.; Klaassen, Z.; Sridhar, S.S.; Nam, R.K.; Wallis, C.J.D.; Kulkarni, G.S. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: A systematic review and met-analysis of randomized clinical trials. Ann. Oncol. 2020, 31, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Cheun, H.; Kim, M.; Lee, H.; Oh, K.-H.; Keam, B. Safety and efficacy of immune checkpoint inhibitors for end-stage renal disease patients undergoing dialysis: A retrospective case series and literature review. Investig. New Drugs 2019, 37, 579–583. [Google Scholar] [CrossRef]
- Ong, M.; Ibrahim, A.M.; Bourassa-Blanchette, S.; Canil, C.; Fairhead, T.; Knoll, G. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J. Immunother. Cancer 2016, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Ansari, J.; Ali, M.; Farrag, A.; Ali, A.; Alhamad, A. Efficacy of Nivolumab in a Patient with Metastatic Renal Cell Carcinoma and End-Stage Renal Disease on Dialysis: Case Report and Literature Review. Case Rep. Immunol. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- Vitale, M.G.; Baldessari, C.; Milella, M.; Buti, S.; Militello, A.M.; Di Girolamo, S.; Fornarini, G.; Perri, G.; Basso, U.; Maruzzo, M.; et al. Immunotherapy in Dialysis-Dependent Cancer Patients: Our Experience in Patients with Metastatic Renal Cell Carcinoma and a Review of the Literature. Clin. Genitourin. Cancer 2019, 17, e903–e908. [Google Scholar] [CrossRef] [PubMed]

| Study Selected | Phase | First Name | Disease | Setting | Experimental Arm n/Safety Population | Comparator Arm n/Safety Population | Jaded Score |
|---|---|---|---|---|---|---|---|
| IMpower150 [14] | III | Socinski MA | Non squamous NSCLC | First line | Atezolizumab + Bevacizumab + Carboplatin + Paclitaxel 800/393 | Bevacizumab + Carboplatin + Paclitaxel 400/394 | 3 |
| IMpower133 [15] | III | Horn L | SCLC | First line | Atezolizumab + Carboplatin + Etoposide 201/198 | Carboplatin + Etoposide 202/196 | 5 |
| IMpassion130 [16] | III | Schmid P | Breast cancer | First line | Atezolizumab + Nab Paclitaxel 452/451 | Placebo + Nab paclitaxel 451/438 | 5 |
| Keynote 407 [17] | III | Paz-Ares L | Squamous NSCLC | First line | Pembrolizumab + Carboplatin + Nab paclitaxel or paclitaxel 278/278 278 | Placebo + Carboplatin + Nab paclitaxel or paclitaxel 281/280 281 | 5 |
| Keynote 189 [18] | III | Gandhi L | non squamous NSCLC | First line | Pembrolizumab + Pemetrexed + Platinum compounds 410/405 | Placebo + Pemetrexed + Platinum compounds 206/202 | 5 |
| NCT01285609 [19] | III | Govindan R | squamous NSCLC | First line | Ipilimumab + Paclitaxel 388/388 | Placebo + Paclitaxel 361/361 | 5 |
| NCT02362594 [20] | III | Eggermont AMN | melanoma | adjuvant | Pembrolizumab 514/509 | Placebo 505/502 | 5 |
| Keynote045 [21] | III | Bellmunt J | urothelial carcinoma | Previously treated | Pembrolizumab 270/266 | Docetaxel or Vinflunine or Paclitaxel 272/255 | 3 |
| Keynote024 [22] | III | Reck M | NSCLC | First line | Pembrolizumab 1,547,154 | Platinum based therapy 151/150 | 3 |
| Keynote 010 [23] | II/III | Herbst RS | NSCLC | Previously treated | Pembrolizumab 690/682 | Docetaxel 343/309 | 3 |
| Pacific [24] | III | Antonia SJ | NSCLC | maintenance | Durvalumab 476/475 | Placebo 237/234 | 5 |
| CheckMate 017 [25] | III | Brahmer J | squamous NSCLC | Previously treated | Nivolumab 135/131 | Docetaxel 137/129 | 3 |
| CheckMate 066 [26] | III | Robert C | melanoma | First line | Nivolumab + Dacarbazine 210/206 | Placebo + Dacarbazine 208/205 | 4 |
| CheckMate 037 [27] | III | Weber JS | melanoma | Previously treated | Nivolumab 272/268 | Dacarbazine or Paclitaxel + Carboplatin 133/102 | 3 |
| CheckMate 057 [28] | III | Borghaei H | non squamous NSCLC | Previously treated | Nivolumab 292/287 | Docetaxel 290/268 | 3 |
| CheckMate 141 [29] | III | Ferris RL | squamous head and neck | Previously treated | Nivolumab 240/236 | Docetaxel or methotrexate or Cetuximab 121/111 | 3 |
| CA184-043 [30] | III | Kwan ED | mCRPC | Previously treated | Ipilimumab 399/393 | Placebo 400/396 | 5 |
| Study | G1-2 RD | G1-2 RR | G3-5 RD | G3-5 RR | Gtot RD | Gtot RR |
|---|---|---|---|---|---|---|
| IMpower150 [14] | 0.00509 (−0.00195 to 0.0121) | 5.013 (0.241 to 104.082) | 0.00254 (−0.00244 to 0.00753) | 3.008 (0.123 to 73.609) | 0.00763 (−0.000972 to 0.0162) | 7.018 (0.364 to 135.422) |
| IMpower133 [15] | 0.00505 (−0.00482 to 0.0149) | 2.970 (0.122 to 72.465) | 0.00505 (−0.00482 to 0.0149) | 2.970 (0.122 to 72.465) | 0.0101 (−0.00383 to 0.0240) | 4.950 (0.239 to 102.448) |
| IMpassion130 [16] | 0.00221 (−0.00212 to 0.00654) | 2.907 (0.119 to 71.179) | NE | NE | 0.00221 (−0.00212 to 0.00654) | 2.907 (0.119 to 71.179) |
| Keynote 407 [17] | 0.0000514 (−0.0139 to 0.0141) | 1.007 (0.143 to 7.100) | 0.0000514 (−0.0139 to 0.0141) | 1.007 (0.143 to 7.100) | 0.000103 (−0.0196 to 0.0198) | 1.007 (0.254 to 3.987) |
| Keynote 189 [18] | 0.0173 (0.00459 to 0.0300) | 7.500 (0.430 to 130.674) | 0.0148 (0.00305 to 0.0266) | 6.500 (0.368 to 114.819) | 0.0321 (0.0149 to 0.0493) | 13.500 (0.807 to 225.965) |
| NCT01285609 [19] | 0.00515 (−0.00197 to 0.0123) | 4.653 (0.224 to 96.597) | 0.00238 (−0.00657 to 0.0113) | 1.861 (0.169 to 20.435) | 0.00754 (−0.00388 to 0.0190) | 3.722 (0.418 to 33.143) |
| NCT02362594 [20] | 0.00194 (−0.00475 to 0.00863) | 1.972 (0.179 to 21.685) | 0.00393 (−0.00151 to 0.00936) | 4.931 (0.237 to 102.467) | 0.00587 (−0.00274 to 0.0145) | 3.945 (0.442 to 35.175) |
| Keynote045 [21] | 0.0384 (−0.0105 to 0.0873) | 1.544 (0.880 to 2.711) | 0.0181 (−0.00840 to 0.0447) | 2.157 (0.673 to 6.917) | 0.0566 (0.00221 to 0.111) | 1.656 (1.008 to 2.720) |
| Keynote024 [22] | 0.00649 (−0.00619 to 0.0192) | 2.923 (0.120 to 71.185) | 0.00649 (−0.00619 to 0.0192) | 2.923 (0.120 to 71.185) | 0.0130 (−0.00489 to 0.0309) | 4.871 (0.236 to 100.628) |
| Keynote 010 [23] | 0.0191 (0.00880 to 0.0293) | 12.255 (0.731 to 205.500) | NE | NE | 0.0191 (0.00880 to 0.0293) | 12.255 (0.731 to 205.500) |
| Pacific [24] | 0.00421 (−0.00161 to 0.0100) | 2.468 (0.119 to 51.213) | 0.00421 (−0.00161 to 0.0100) | 2.468 (0.119 to 51.213) | 0.00842 (0.000203 to 0.0166) | 4.443 (0.240 to 82.188) |
| CheckMate 017 [25] | 0.0149 (−0.0270 to 0.0568) | 1.641 (0.400 to 6.726) | 0.00763 (−0.00727 to 0.0225) | 2.955 (0.121 to 71.868) | 0.0225 (−0.0217 to 0.0668) | 1.969 (0.503 to 7.708) |
| CheckMate 066 [26] | 0.0145 (−0.00658 to 0.0357) | 3.981 (0.449 to 35.312) | 0.00485 (−0.00464 to 0.0143) | 2.986 (0.122 to 72.866) | 0.0194 (−0.00368 to 0.0425) | 4.976 (0.586 to 42.221) |
| CheckMate 037 [27] | 0.00746 (−0.00284 to 0.0178) | 1.914 (0.0927 to 39.542) | NE | NE | 0.00746 (−0.00284 to 0.0178) | 1.914 (0.0927 to 39.542) |
| CheckMate 057 [28] | 0.0244 (0.00654 to 0.0422) | 14.010 (0.804 to 244.139) | 0.00348 (−0.00333 to 0.0103) | 2.802 (0.115 to 68.492) | 0.0279 (0.00883 to 0.0469) | 15.878 (0.921 to 273.783) |
| CheckMate 141 [29] | 0.00424 (−0.00405 to 0.0125) | 1.418 (0.0582 to 34.530) | −0.000534 (−0.0216 to 0.0206) | 0.941 (0.0862 to 10.265)) | 0.00370 (−0.0190 to 0.0264) | 1.411 (0.148 to 13.413) |
| CA184-043 [30] | 0.0155 (−0.00945 to 0.0404) | 1.612 (0.741 to 3.509) | 0.0127 (−0.000350 to 0.0258) | 6.046 (0.731 to 49.989) | 0.0282 (0.000299 to 0.0561) | 2.015 (0.991 to 4.100) |
| Tot fixed | 0.0105 (0.00629 to 0.0146) p < 0.001 | 2.185 (1.515 to 3.152) p < 0.001 | 0.00610 (0.00292 to 0.00929) p < 0.001 | 2.610 (1.409 to 4.833) p = 0.002 | 0.0153 (0.0105 to 0.0201) p < 0.001 | 2.473 (1.782 to 3.431) p < 0.001 |
| Tot Randomized | 0.00746 (0.00337 to 0.0115) p < 0.001 | 1.893 (1.303 to 2.751) p = 0.001 | 0.00444 (0.00216 to 0.00673) p < 0.001 | 2.413 (1.282 to 4.543) p = 0.006 | 0.0122 (0.00601 to 0.0185) p < 0.001 | 2.107 (1.509 to 2.942) p < 0.001 |
| IMMUNECHECKPOINT INHIBITOR ALONE | ||||||
| RD Tot fixed | 0.0138 (0.00694 to 0.0206) p < 0.001 | 2.007 (1.349 to 2.985) p = 0.001 | 0.00729 (0.00256 to 0.0120) p = 0.002 | 2.698 (1.262 to 5.769) p = 0.010 | 0.0195 (0.0119 to 0.0272) p < 0.001 | 2.257 (1.576 to 3.232) p < 0.001 |
| RD Tot Randomized | 0.00975 (0.00245 to 0.0170) p = 0.009 | 1.760 (1.176 to 2.634) p = 0.006 | 0.00481 (0.00173 to 0.00790) p = 0.002 | 2.551 (1.170 to 5.561) p = 0.018 | 0.0141 (0.00558 to 0.0226) p = 0.001 | 1.995 (1.387 to 2.870) p < 0.001 |
| COMBINATION | ||||||
| RD Tot fixed | 0.00627 (0.00246 to 0.0101) p = 0.001 | 3.318 (1.265 to 8.702) p = 0.015 | 0.00459 (0.000563 to 0.00862) p = 0.025 | 2.454 (0.856 to 7.033) p = 0.095 | 0.00992 (0.00496 to 0.0149) p < 0.001 | 3.576 (1.605 to 7.9689 p = 0.002 |
| RD Tot Randomized | 0.00501 (0.00129 to 0.00872) p = 0.008 | 2.955 (1.091 to 8.002) p = 0.033 | 0.00399 (0.000592 to 0.00739) p = 0.021 | 2.167 (0.733 to 6.406) p = 0.162 | 0.00979 (0.00165 to 0.0179) p = 0.018 | 2.828 (1.216 to 6.579) p = 0.016 |
| CTLA-4 | ||||||
| Tot Fixed | 0.00746 (0.00150 to 0.0134) p = 0.014 | 1.763 (0.834 to 3.723) p = 0.137 | 0.00907 (−0.00206 to 0.0202) p = 0.110 | 3.912 (0.840 to 18.221) p = 0.082 | 0.0181 (0.00278 to 0.0335) p = 0.021 | 2.163 (1.103 to 4.242) p = 0.025 |
| Tot Randomized | 0.00565 (0.0000706 to 0.0112) p = 0.47 | 1.721 (0.810 to 3.656) p = 0.158 | 0.00879 (−0.00310 to 0.0207) p = 0.147 | 3.611 (0.740 to 17.613) p = 0.112 | 0.0159 (−0.0130 to 0.0449) p = 0.280 | 2.137 (1.087 to 4.199) p = 0.028 |
| Population | Q | DF | Significance Level | I2 | 95% I2 |
|---|---|---|---|---|---|
| RD analysis | |||||
| Overall G1-2 | 42.9206 | 16 | p = 0.0003 | 62.72% | 37.10 to 77.91 |
| Immunecheckpoint Inhibitor Alone G1-2 | 33.4909 | 9 | p = 0.0001 | 73.13% | 49.33 to 85.75 |
| Combination G1-2 | 7.8762 | 6 | p = 0.24 | 23.82% | 0.00 to 66.37 |
| CTLA-4 G1-2 | 3.7443 | 1 | p = 0.0530 | 73.29% | 0.00 to 93.98 |
| Overall G 3-5 | 9.3461 | 13 | p = 0.7463 | 0.00% | 0.00 to 37.57 |
| Immunecheckpoint Inhibitors Alone G3-4 | 5.5952 | 7 | p = 0.58 | 0.00% | 0.00 to 59.86 |
| Combination G3-5 | 4.1982 | 5 | p = 0.52 | 0% | 0.00 to 70.65 |
| CTLA-4 G3-5 | 1.0972 | 1 | p = 0.2949 | 8.86% | 8.86 to 8.86 |
| Overall Tox | 62.5155 | 16 | p < 0.0001 | 74.41% | 58.81 to 84.10 |
| Immunecheckpoint Inhibitors Alone Overall | 27.3042 | 9 | p = 0.0012 | 67.04% | 35.85–83.06% |
| Combination Tox | 20.6177 | 6 | p = 0.0021 | 70.90% | 36.57 to 86.65 |
| CTLA-4 overall Tox | 3.8129 | 1 | p = 0.0509 | 73.77% | 0.00 to 94.08 |
| RR analysis | |||||
| Overall G1-2 | 7.5923 | 16 | p = 0.96 | 0% | 0 to 0 |
| Immunecheckpoint Inhibitors Alone G1-2 | 4.6905 | 9 | p = 0.86 | 0% | 0.00 to 28.17 |
| Combination G1-2 | 1.9013 | 6 | p = 0.9286 | 0% | 0.00 to 9.40 |
| CTLA-4 G1-2 | 0.4440 | 1 | p = 0.50 | 0% | 0 to 0 |
| Overall G 3-5 | 2.9917 | 13 | p = 0.99 | 0% | 0 to 0 |
| Immunecheckpoint Inhibitors Alone G3-5 | 1.6102 | 7 | p = 0.97 | 0% | 0 to 0 |
| Combination G3-5 | 4.4643 | 6 | p = 0.61 | 0% | 0.00 to 61.41 |
| CTLA-4 G3-5 | 0.5325 | 1 | p = 0.46 | 0% | 0.00 to 0.00 |
| Overall Tox | 10.8636 | 16 | p = 0.81 | 0% | 0.00 to 28.04 |
| Immunecheckpoint Inhibitors Alone Overall | 5.7022 | 9 | p = 0.76 | 0% | 0.00 to 40.91 |
| Combination Tox | 1.3359 | 5 | p = 0.93 | 0% | 0.00 to 7.76 |
| CTLA-4 overall Tox | 0.2747 | 1 | p = 0.60 | 0% | 0.00 to 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righini, M.; Mollica, V.; Rizzo, A.; La Manna, G.; Massari, F. Renal Toxicities in Cancer Patients Receiving Immune-Checkpoint Inhibitors: A Meta-Analysis. J. Clin. Med. 2022, 11, 4373. https://doi.org/10.3390/jcm11154373
Righini M, Mollica V, Rizzo A, La Manna G, Massari F. Renal Toxicities in Cancer Patients Receiving Immune-Checkpoint Inhibitors: A Meta-Analysis. Journal of Clinical Medicine. 2022; 11(15):4373. https://doi.org/10.3390/jcm11154373
Chicago/Turabian StyleRighini, Matteo, Veronica Mollica, Alessandro Rizzo, Gaetano La Manna, and Francesco Massari. 2022. "Renal Toxicities in Cancer Patients Receiving Immune-Checkpoint Inhibitors: A Meta-Analysis" Journal of Clinical Medicine 11, no. 15: 4373. https://doi.org/10.3390/jcm11154373
APA StyleRighini, M., Mollica, V., Rizzo, A., La Manna, G., & Massari, F. (2022). Renal Toxicities in Cancer Patients Receiving Immune-Checkpoint Inhibitors: A Meta-Analysis. Journal of Clinical Medicine, 11(15), 4373. https://doi.org/10.3390/jcm11154373









