Abstract
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by hyperglycemia, insulin resistance, and pancreatic B cell dysfunction. Hyperglycemia can cause several complications, including nephrological, neurological, ophthalmological, and vascular complications. Many modalities, such as medication, physical therapies, and exercise, are developed against vascular disorders. Among all exercise forms, aerobic plus machine-assisted resistance training is widely applied. However, whether this intervention can significantly improve vascular conditions remains controversial. In this study, an electronic search was processed for the Pubmed, Embase, and Cochrane libraries for randomized controlled trials (RCTs) comparing the efficacy of aerobic plus machine-assisted resistance training with no exercise (control) on patients with T2DM. Pulse wave velocity (PWV), the index of arterial stiffness, was chosen as primary outcome. The reliability of the pooled outcome was tested by trial sequential analysis (TSA). Secondary outcomes included systolic blood pressure (SBP) and hemoglobin A1c (HbA1c). Finally, five RCTs with a total of 328 patients were included. Compared with control, aerobic plus machine-assisted resistance training failed to provide significant improvement on PWV (MD −0.54 m/s, 95% CI [−1.69, 0.60], p = 0.35). On the other hand, TSA indicated that this results till needs more verifications. Additionally, this training protocol did not significantly decrease SBP (MD −1.05 mmHg, 95% CI [−3.71, 1.61], p = 0.44), but significantly reduced the level of HbA1c (MD −0.55%, 95% CI [−0.88, −0.22], p = 0.001). In conclusion, this meta-analysis failed to detect a direct benefit of aerobic plus machine-assisted resistance training on vascular condition in T2DM population. Yet the improvement in HbA1c implied a potential of this training method in mitigating vascular damage. More studies are needed to verify the benefit.
1. Introduction
The prevalence of Type 2 Diabetes Mellitus (T2DM) is increasing rapidly worldwide [1]. Characterized by hyperglycemia, insulin resistance, and pancreatic B cell dysfunction, T2DM leads to several severe complications, including but not limited to neuropathy, nephrology, and macrovascular disorders such as cardiovascular diseases [2,3]. Compared to healthy population, the risk of cardiovascular events has a twofold increase [4]. Cardiovascular event is also the leading cause of mortality in patients with T2DM [5]. Therefore, maintaining the function of vessels or retaining the damage of vessels is of vital importance.
Lifestyle modifications, such as diet control, nutrients supply, body weight regulation, and sports exercise, are well-acknowledged to improve the prognosis of T2DM [4,5,6,7]. Sports exercise can be divided into aerobic training, resistance training, or combination. As an easily accessible pattern, aerobic training helps control blood pressure, systemic inflammation, and glycemic level, et al. [8]. Way et al. found aerobic training could improve smooth muscle function, but the improvement of vascular stiffness was still questioned [9]. Alternatively, resistance training can change body composition by increasing the mass of muscle, which is important in controlling blood glucose and ameliorating insulin resistance [10]. When combined with aerobic training, this strategy may improve vascular function in healthy individuals [11]. In 2014, Li et al. found that combined aerobic and resistance training was beneficial for decreasing arterial stiffness in population with or without hypertension [12]. These findings provide the rationality of this combined training for patients with T2DM.
In recent years, clinical trials have been launched to test whether aerobic plus resistance training is beneficial to the vascular complications of T2DM. To better understand where we are now, a systematic review and meta-analysis is organized to verify the effect of aerobic plus machine-assisted resistance training on the vascular condition in patients with T2DM.
2. Materials and Methods
This systematic review was organized according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) checklist [13].
2.1. Search Strategy
On January 2022, the first two authors independently searched on Pubmed, Embase, and Cochrane library. Reference lists of previously published systematic reviews were also reviewed related researches. Key words used were, random *, (vessel * or cardiovascular or vascular), diabet *[title/abstract], and (exercise or training).
2.2. Study Selection
Studies focusing on the comparison of resistance plus aerobic training and no or sham training for improvement of the vascular function in T2DM population were included. The inclusion criteria were (1) randomized controlled trials (RCTs), (2) an intervention consisted of a combination of machine-assisted resistance training and aerobic training, and (3) intervention duration for at least four weeks [14]. The exclusion criteria were (1) non-randomized control trials, (2) animal studies, and (3) RCTs in which the intervention group did not have a combined training protocol, (4) combined protocol in which resistance training protocol was unclear or not machine-assisted, and (5) non-randomized clinical trials, case reports, reference abstracts, or reviews. The first two authors independently screened titles and abstracts of all searched items based on the criteria above. Once the information to make a decision was insufficient, full-text would be retrieved for further judgment. In case of debate, the senior author would decide whether to include the research.
2.3. Data Extraction
The same authors independently extracted data from eligible studies including name of first author, published year, inclusion and exclusion criteria, number of patients included, training protocols, and items of measurements, as well as conclusions. The difference of changes of central pulse wave velocity (PWV) between two groups was selected as the primary outcome, since PWV is not only an indicator arterial stiffness but also an independent predictor of cardiovascular risk [15]. Secondary outcomes included the difference of changes of systolic blood pressure (SBP) and hemoglobin A1c (HbA1c) between two group. The former reflects vasculature plasticity [16], while the latter is used for evaluating blood-glucose control over a period of time and to predict the occurrence of long-term complications due to diabetes [17].
2.4. Data Analysis
The random-effects model was applied for each comparison since patient conditions, exercise duration and modes, as well as other factors were inconsistent across RCTs. Difference in primary and secondary outcomes were measured by mean difference (MD) and 95% confidence interval (CI). For researches in which the standard deviation (SD) of pre-intervention and post-intervention difference was not reported, a correlation of 0.5 was used for dispersion estimation [18]. For researches with multiple eligible intervention groups, the control group was split equally based on the number of intervention groups, and two or more comparison pairs were input [19]. Heterogeneity was assessed by Q statistic and I2 statistic. I2 statistic larger than 50% were considered to have significant heterogeneity [20]. When significant heterogeneity was noticed regarding primary outcome, sensitivity analysis was conducted. One study was omitted in each turn to locate the potential source of heterogeneity. Since the number of RCTs included did not reach ten, publication bias was not detected [21]. Two-tail p value < 0.05 was considered statistically significant. Analyses were performed using Review Manager, Version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen, Denmark).
2.5. Quality Assessment
The Cochrane’s risk of bias tool was used by the first two reviewers independently assess the quality of included studies [22]. Value of low, unclear or high risk of bias was assigned to the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Disagreement was solved by discussion. The degree of inter-reviewer agreement was measured by κ value. A κ from 0.40 to 0.59 was regarded as fair, 0.60 to 0.74 as good, 0.75 or more as excellent [22].
The quality of evidence for primary outcome was rated by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. The level of evidence was entitled as high, moderate, low, or very low, according to five domains: high risk of bias, imprecision, indirectness, heterogeneity, and publication bias [23,24,25,26,27]. Considering the limited number of studies included, publication bias could not be assessed. Instead, evidence was downgraded when heterogeneity exceeded 40% [25].
2.6. Trial Sequential Analysis
Given sparse data and repeated significance testing, the risk of type I error might be elevated by cumulative meta-analyses [28,29,30,31]. To control this potential risk, trial sequential analysis (TSA) was launched (TSA software version 0.9 Beta; Copenhagen Trial Unit, Copenhagen, Denmark) for all measurements by empirical method for the estimation of the required information size. The diversity-adjusted required information size (DIS) and the eventual breach of the cumulative Z-curve of relevant trial sequential monitoring boundaries was obtained to calculate the required information size together with a threshold for a statistically significant treatment effect [32]. An overall 5% risk of a type I error was maintained with a power of 80% [32].
3. Results
A total of 4838 titles were identified after electronic screening in three databases. After reading titles and abstracts, the full-text of seven titles were retrieved for further exclusion. The resistance training in the trial reported by Okada et al. [33] was not machine-assisted, therefore was excluded. Two studies shared the same patient cohort, so the latter one, which was a secondary analysis of the original population, was excluded [34]. One eligible study [35] was identified from a systematic review [14], and was included (Figure 1).
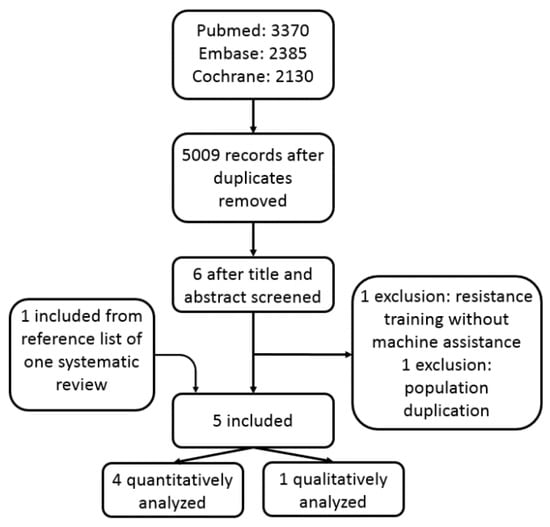
Figure 1.
The flowchart of study inclusion.
Five RCTs with a total of 328 patients were included, of which four RCTs were quantitatively analyzed [35,36,37,38], while one was descriptively analyzed [39]. Basic characteristics of these studies were listed in Table 1. These researches were published from 2001 to 2019. A total of 176 patients with T2DM were allocated to aerobic plus resistance training group. The age range of the patients included in the systematic review was from about 40 to over 60 years old. In one study, only male participants were enrolled [35]. Detailed intervention, follow-up duration, and conclusions were listed in Table 2. The aerobic training consisted of cycle ergometry, walking, or treadmill et al., while resistance training focused mainly on trunk and extremities on machines. All training processes use a heart rate detector to determine the quality and quantity of exercise. Started from the beginning of exercise, two RCTs had a follow-up of 52 weeks [35,38], two had a follow-up of 26 weeks [36,37], and one had 16 weeks [39].

Table 1.
Basic characteristics of included studies.

Table 2.
Measurements, exercise protocols, follow-up duration, and findings.
Risk of bias of included studies was shown in Figure 2. Most of the studies did not mention the detail of randomization or allocation concealment. Considering the nature of exercise process, it was impossible to keep patients blinded to interventions. No studies had incomplete outcome data or selective reporting. Regarding other biases, two of the five studies had sample size calculation prior to patient enrollment, and therefore was ranked as low risk [36,38]. The κ value was 0.82, indicating an excellent consistency between two reviewers.

Figure 2.
Risk of bias of each included study [35,36,37,38,39]. +: low risk; -: high risk; ?: unclear risk.
3.1. Primary Outcome
Compared with control, aerobic plus resistance training did not significantly improve PWV of patients with T2DM (MD −0.54 m/s, 95% CI [−1.69, 0.60], p = 0.35, three studies included [35,37,38]). The heterogeneity was not remarkable (I2 = 0%, p = 0.86) (Figure 3). Considering that study design might introduce bias and some data were calculated based on estimation, the level of evidence was low. However, this insignificance was not supported by TSA, which indicated the current outcome might be a result of limited sample size (Figure 4).

Figure 3.
The pooled result of the difference of changes in pulse wave velocity between two groups [35,37,38].

Figure 4.
The result of TSA for PWV. TSA showed that the pooled results did not (z-curve, blue curve) crossed the conventional boundary of benefit (brown line) or the trial sequential monitoring boundary for benefit (upper red line), and did not reach the required sample size based on TSA (n = 3681) [35,37,38].
3.2. Secondary Outcomes
Compared with control, aerobic plus resistance training did not significantly improve SBP of patients with T2DM (MD −1.05 mmHg, 95% CI [−3.71, 1.61], p = 0.44, four studies included [35,36,37,38]). The heterogeneity was not significant (I2 = 0%, p = 0.87) (Figure 5).
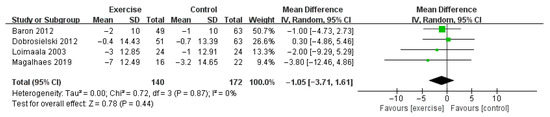
Figure 5.
The pooled result of the difference of changes in systolic blood pressure between two groups [35,36,37,38].
On the other hand, aerobic plus resistance training significantly decreasedHbA1c of patients with T2DM (MD −0.55%, 95% CI [−0.88, −0.22], p = 0.001, three studies included [34,36,37]). The heterogeneity was insignificant (I2 = 0%, p = 0.72) (Figure 6). This outcome was in consistent with the records of Maiorana et al. [39] that, at the final follow-up, the level of HbA1c was 7.9 ± 0.3% in patients with exercise, significantly lower than those without exercise (8.5 ± 0.4%).

Figure 6.
The pooled result of the difference of changes in hemoglobin A1c between two groups [35,36,37].
4. Discussion
Numerous meta-analyses have discussed the benefit of various types of exercise training on T2DM in glycemic control, psychosocial performance, the level of inflammatory cytokines, et al. [40,41,42]. In the current study, we primarily focused on the effect of aerobic plus machine-assisted resistance training on vascular function in patients with T2DM. Based on the data available, we failed to detect a statistical significance of this combined training method for vascular condition, as indicated by PWV or SBP. On the other hand, TSA suggested that this insignificant difference might be attributed to a relatively small sample size. Moreover, aerobic plus machine-assisted resistance training significantly reduced the level of HbA1c, which is associated with cardiovascular risk. Therefore, it is currently not appropriate to negate the benefit of this training method on vascular health in T2DM population.
Resistance training can be either machine-assisted [38,39], elastic band-assisted [43], or even free weight [44]. Some studies used elastic bands in resistance training section, but did not notice an improvement in flow-mediated dilation or endothelium-independent vasodilation in T2DM population [43,45]. On the other hand, aerobic plus free weight-based resistance training could significantly decrease carotid intima-media thickness and arterial stiffness [44,46]. Considering that the uncertainty in body weight or the elasticity of bands may act as confounders, we focused specifically on machine-assisted resistance training.
As the widely used structural and functional index for measuring arterial stiffness [47], PWV is usually faster in T2DM population [48], indicating vascular stiffening and high cardiovascular risk [49]. Surprisingly, the present data did not support the application of this exercise protocol for improving vascular condition in T2DM population. This may be explained by two factors. First, as aforementioned, those receiving free weight exercise have improved vascular condition, so one can speculate that different resistance training protocol may yield different outcomes. Second, by conducting TSA, we noted that the required sample size was not reached, therefore more clinical trials are still needed.
Next, we compared the change in hemodynamic index, SBP. As an reflection of the plasticity in vasculature [16], SBP is always higher in stiffened vessel [50]. We found that the change of SBP following aerobic plus resistance training was comparable to that in control group, indicating that this training protocol may not be able to improve vascular function in T2DM population.
However, in agreement with a recent meta-analysis [42], we noticed a significant decrease of HbA1c in T2DM population with exercise, implying that aerobic plus machine-assisted resistance training could help control blood glucose. In hyperglycemia-induced complications of T2DM, especially vascular dysfunction, oxidative stress plays a pivotal role [51]. Oxidative damage caused by excessive reactive oxygen species can lead to endothelial damage via several signaling pathways, aggravating vascular stiffness and impairing vasorelaxation [52,53]. To prevent or retard the progression of complications, long-term control of blood glucose is of vital importance [54], the benefit of aerobic plus machine-assisted resistance training on blood glucose control was an indirect evidence that this exercise protocol could be meaningful for controlling vascular complications. This was in accordance with previous researches that high HbA1c was associated vascular risk and could be predictive of vascular events [55,56].
Previously, several systematic reviews and meta-analyses studied the influence of exercise on vascular function in T2DM. By measuring brachial artery flow-mediated dilation, Lee found that exercise as a whole, regardless of the pattern, significantly improved vascular endothelial function [57]. Dos et al. noticed that aerobic plus resistance training could improve vascular function in T2DM [14]. However, some of the included studies were not RCTs, which may act as the origin of divergence compared with ours.
The current findings should be interpreted with caution. First, some data we input were based on estimation, which might introduce impreciseness. The sample size was also not statistically sufficient, as indicated by TSA. In addition, the relatively short follow-up duration may contribute to the insignificant difference of PWV or SBP. Contrarily, we found that HbA1c was improved by training. Since HbA1c is related to better glucose metabolism, which indicates greater redox balance, one can expect a better vascular system [58]. Next, the evaluation of vascular condition should be multi-dimensional. A comprehensive understanding of vascular status in T2DM patients with exercise can be conducted in the future. Thirdly, albeit an exact protocol of exercise in each study, confounders were unavoidable. The age ranges from 40 to over 60, the follow-up duration ranges from 16 to 52 weeks, and even one study only recruited male patients. To reveal the effect of aerobic plus resistance training on vascular health, a longer follow-up period in a group of patients with closer age range is necessary. Finally, HbA1c does not indicate the variation of the glycemic profile, which is also a risk factor for cardiovascular events in T2DM population [59]. The effect of exercise on glycemic variability can be detected in further trials.
In conclusion, the outcome of the current meta-analysis was not supportive of the benefit of aerobic plus machine-assisted resistance training on vascular condition in T2DM population. However, this finding could be a result of small sample size. Considering that there was a significant improvement of HbA1c after training, this method may still have the potential of maintaining vascular health. More studies with longer follow-up duration are required to verify this potential.
Author Contributions
Conceptualization, Z.L.; methodology, H.Z.; software, writing-original draft preparation: X.G.; software: S.G.; investigation, X.G. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
Chenguang Plan of Yangpu Hospital, Tongji University (2020-14). Fund for psychosomatic medicine, key discipline of district level of Shanghai Yangpu District mental health center ([2019] No. 108).
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418, Erratum in Diabetes Care 2017, 40, 1285. [Google Scholar] [CrossRef] [PubMed]
- Ismail-Beigi, F.; Craven, T.; Banerji, M.A.; Basile, J.; Calles, J.; Cohen, R.M.; Cuddihy, R.; Cushman, W.C.; Genuth, S.; Grimm, R.H., Jr.; et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 2010, 376, 419–430. [Google Scholar] [CrossRef]
- Yun, J.S.; Ko, S.H. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism 2021, 123, 154838. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Alfredo, T.; Oliveira, S.; Amaro, A.; Rosendo-Silva, D.; Antunes, K.; Pires, A.S.; Teixo, R.; Abrantes, A.M.; Botelho, M.F.; Castelo-Branco, M.; et al. Hypoglycaemic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd Ex Mart. Extract Are Associated with Better Vascular Function of Type 2 Diabetic Rats. Nutrients 2021, 13, 2856. [Google Scholar] [CrossRef]
- Choi, J.H.; Cho, Y.J.; Kim, H.J.; Ko, S.H.; Chon, S.; Kang, J.H.; Kim, K.K.; Kim, E.M.; Kim, H.J.; Song, K.H.; et al. Effect of carbohydrate-restricted diets and intermittent fasting on obesity, type 2 diabetes mellitus, and hypertension management: Consensus statement of the Korean Society for the Study of obesity, Korean Diabetes Association, and Korean Society of Hypertension. Clin. Hypertens. 2022, 28, 26. [Google Scholar] [CrossRef]
- Salzberg, L. Risk Factors and Lifestyle Interventions. Prim. Care. 2022, 49, 201–212. [Google Scholar] [CrossRef]
- Delevatti, R.S.; Bracht, C.G.; Lisboa, S.D.C.; Costa, R.R.; Marson, E.C.; Netto, N.; Kruel, L.F.M. The Role of Aerobic Training Variables Progression on Glycemic Control of Patients with Type 2 Diabetes: A Systematic Review with Meta-analysis. Sports Med. Open 2019, 5, 22. [Google Scholar] [CrossRef]
- Way, K.L.; Keating, S.E.; Baker, M.K.; Chuter, V.H.; Johnson, N.A. The Effect of Exercise on Vascular Function and Stiffness in Type 2 Diabetes: A Systematic Review and Meta-analysis. Curr. Diabetes Rev. 2016, 12, 369–383. [Google Scholar] [CrossRef]
- Sinacore, D.R.; Gulve, E.A. The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: Implications for physical therapy. Phys. Ther. 1993, 73, 878–891. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Combined aerobic and resistance training and vascular function: Effect of aerobic exercise before and after resistance training. J. Appl. Physiol. 2007, 103, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hanssen, H.; Cordes, M.; Rossmeissl, A.; Endes, S.; Schmidt-Trucksass, A. Aerobic, resistance and combined exercise training on arterial stiffness in normotensive and hypertensive adults: A review. Eur. J. Sport Sci. 2015, 15, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Dos Santos Araujo, J.E.; Nunes Macedo, F.; Sales Barreto, A.; Viana Dos Santos, M.R.; Antoniolli, A.R.; Quintans-Junior, L.J. Effects of Resistance and Combined training on Vascular Function in Type 2 Diabetes: A Systematic Review of Randomized Controlled Trials. Rev. Diabet. Stud. 2019, 15, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Y.; Wu, W.; Cai, Z.; Chen, Z.; Yan, X.; Wu, S. Total cholesterol, arterial stiffness, and systolic blood pressure: A mediation analysis. Sci. Rep. 2021, 11, 1330. [Google Scholar] [CrossRef]
- Kwon, T.H.; Kim, K.D. Machine-Learning-Based Noninvasive In Vivo Estimation of HbA1c Using Photoplethysmography Signals. Sensors 2022, 22, 2963. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, J.; Li, H.; Jiang, J.; Chen, S. Steroid Injection and Nonsteroidal Anti-inflammatory Agents for Shoulder Pain: A PRISMA Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2015, 94, e2216. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Song, F.; Eastwood, A.J.; Gilbody, S.; Duley, L.; Sutton, A.J. Publication and related biases. Health Technol. Assess. 2000, 4, 1–115. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Rind, D.; Devereaux, P.J.; Montori, V.M.; Freyschuss, B.; Vist, G.; et al. GRADE guidelines 6. Rating the quality of evidence—Imprecision. J. Clin. Epidemiol. 2011, 64, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Falck-Ytter, Y.; Jaeschke, R.; Vist, G.; et al. GRADE guidelines: 8. Rating the quality of evidence—Indirectness. J. Clin. Epidemiol. 2011, 64, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Montori, V.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Djulbegovic, B.; Atkins, D.; Falck-Ytter, Y.; et al. GRADE guidelines: 5. Rating the quality of evidence—Publication bias. J. Clin. Epidemiol. 2011, 64, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Montori, V.; Akl, E.A.; Djulbegovic, B.; Falck-Ytter, Y.; et al. GRADE guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias). J. Clin. Epidemiol. 2011, 64, 407–415. [Google Scholar] [CrossRef]
- Brok, J.; Thorlund, K.; Gluud, C.; Wetterslev, J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J. Clin. Epidemiol. 2008, 61, 763–769. [Google Scholar] [CrossRef]
- Thorlund, K.; Devereaux, P.J.; Wetterslev, J.; Guyatt, G.; Ioannidis, J.P.; Thabane, L.; Gluud, L.L.; Als-Nielsen, B.; Gluud, C. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int. J. Epidemiol. 2009, 38, 276–286. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Liu, S.; Li, H.; Jiang, J.; Chen, S.; Chen, J. Intra-articular Steroid Injection for Frozen Shoulder: A Systematic Review and Meta-analysis of Randomized Controlled Trials With Trial Sequential Analysis. Am. J. Sports Med. 2017, 45, 2171–2179. [Google Scholar] [CrossRef]
- Guo, S.; Guo, X.; Zhang, H.; Zhang, X.; Li, Z. The Effect of Diacerein on Type 2 Diabetic Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis. J. Diabetes Res. 2020, 2020, 2593792. [Google Scholar] [CrossRef]
- Wetterslev, J.; Thorlund, K.; Brok, J.; Gluud, C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med. Res. Methodol. 2009, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Hiuge, A.; Makino, H.; Nagumo, A.; Takaki, H.; Goto, H.K.; Yoshimasa, Y.; Miyamoto, Y. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J. Atheroscler. Thromb. 2010, 17, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Loimaala, A.; Groundstroem, K.; Rinne, M.; Nenonen, A.; Huhtala, H.; Parkkari, J.; Vuori, I. Effect of long-term endurance and strength training on metabolic control and arterial elasticity in patients with type 2 diabetes mellitus. Am. J. Cardiol. 2009, 103, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Loimaala, A.; Huikuri, H.V.; Kööbi, T.; Rinne, M.; Nenonen, A.; Vuori, I. Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes 2003, 52, 1837–1842. [Google Scholar] [CrossRef]
- Barone, G.B.; Dobrosielski, D.; Bonekamp, S.; Stewart, K.; Clark, J. A randomized trial of exercise for blood pressure reduction in type 2 diabetes: Effect on flow-mediated dilation and circulating biomarkers of endothelial function. Atherosclerosis 2012, 224, 446–453. [Google Scholar] [CrossRef]
- Dobrosielski, D.; Gibbs, B.; Ouyang, P.; Bonekamp, S.; Clark, J.; Wang, N.; Silber, H.; Shapiro, E.; Stewart, K. Effect of exercise on blood pressure in type 2 diabetes: A randomized controlled trial. J. Gen. Intern. Med. 2012, 27, 1453–1459. [Google Scholar] [CrossRef]
- Magalhães, J.; Melo, X.; Correia, I.; Ribeiro, R.; Raposo, J.; Dores, H.; Bicho, M.; Sardinha, L. Effects of combined training with different intensities on vascular health in patients with type 2 diabetes: A 1-year randomized controlled trial. Cardiovasc. Diabetol. 2019, 18, 34. [Google Scholar] [CrossRef]
- Maiorana, A.; O’Driscoll, G.; Cheetham, C.; Dembo, L.; Stanton, K.; Goodman, C.; Taylor, R.; Green, D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J. Am. Coll. Cardiol. 2001, 38, 860–866. [Google Scholar] [CrossRef]
- Jayedi, A.; Emadi, A.; Shab-Bidar, S. Dose-Dependent Effect of Supervised Aerobic Exercise on HbA(1c) in Patients with Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. Sports Med. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Jiahao, L.; Jiajin, L.; Yifan, L. Effects of resistance training on insulin sensitivity in the elderly: A meta-analysis of randomized controlled trials. J. Exerc. Sci. Fit. 2021, 19, 241–251. [Google Scholar] [CrossRef]
- Mannucci, E.; Bonifazi, A.; Monami, M. Comparison between different types of exercise training in patients with type 2 diabetes mellitus: A systematic review and network metanalysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Miche, E.; Herrmann, G.; Nowak, M.; Wirtz, U.; Tietz, M.; Hürst, M.; Zoller, B.; Radzewitz, A. Effect of an exercise training program on endothelial dysfunction in diabetic and non-diabetic patients with severe chronic heart failure. Clin. Res. Cardiol. 2006, 95 (Suppl. S1), i117–i124. [Google Scholar] [CrossRef] [PubMed]
- Brozic, A.P.; Marzolini, S.; Goodman, J.M. Effects of an adapted cardiac rehabilitation programme on arterial stiffness in patients with type 2 diabetes without cardiac disease diagnosis. Diab. Vasc. Dis. Res. 2017, 14, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.R.; Min, K.W.; Ahn, H.J.; Seok, H.G.; Lee, J.H.; Park, G.S.; Han, K.A. Effects of Aerobic Exercise vs. Resistance Training on Endothelial Function in Women with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2011, 35, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Byrkjeland, R.; Stensæth, K.H.; Anderssen, S.; Njerve, I.U.; Arnesen, H.; Seljeflot, I.; Solheim, S. Effects of exercise training on carotid intima-media thickness in patients with type 2 diabetes and coronary artery disease. Influence of carotid plaques. Cardiovasc. Diabetol. 2016, 15, 13. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kotani, K.; Okada, K.; Ando, A.; Hasegawa, H.; Kanai, H.; Ishibashi, S.; Yamada, T.; Taniguchi, N. Arterial wall elasticity measured using the phased tracking method and atherosclerotic risk factors in patients with type 2 diabetes. J. Atheroscler. Thromb. 2013, 20, 678–687. [Google Scholar] [CrossRef][Green Version]
- Kotb, N.A.; Gaber, R.; Salama, M.; Nagy, H.M.; Elhendy, A. Clinical and biochemical predictors of increased carotid intima-media thickness in overweight and obese adolescents with type 2 diabetes. Diab. Vasc. Dis. Res. 2012, 9, 35–41. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and Atherosclerosis: Epidemiology, Pathophysiology, and Management. JAMA J. Am. Med. Assoc. 2002, 287, 2570. [Google Scholar] [CrossRef]
- Pulakazhi Venu, V.K.; El-Daly, M.; Saifeddine, M.; Hirota, S.A.; Ding, H.; Triggle, C.R.; Hollenberg, M.D. Minimizing Hyperglycemia-Induced Vascular Endothelial Dysfunction by Inhibiting Endothelial Sodium-Glucose Cotransporter 2 and Attenuating Oxidative Stress: Implications for Treating Individuals With Type 2 Diabetes. Can. J. Diabetes 2019, 43, 510–514. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hatley, M.E.; Bolick, D.T.; Palmer, L.A.; Edelstein, D.; Brownlee, M.; Hedrick, C.C. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia 2004, 47, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.C.; Chen, Y.C.; Cheng, H.H.; Lee, T.L.; Tsai, J.S.; Lee, I.T.; Peng, K.T.; Lee, C.W.; Hsu, L.F.; Chen, Y.L. Combined exposure to fine particulate matter and high glucose aggravates endothelial damage by increasing inflammation and mitophagy: The involvement of vitamin D. Part. Fibre Toxicol. 2022, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Kohnert, K.D.; Heinke, P.; Zander, E.; Vogt, L.; Salzsieder, E. Glycemic Key Metrics and the Risk of Diabetes-Associated Complications. Rom. J. Diabetes Nutr. Metab. Dis. 2016, 23, 403–413. [Google Scholar] [CrossRef]
- D’Souza, J.M.; D’Souza, R.P.; Vijin, V.F.; Shetty, A.; Arunachalam, C.; Pai, V.R.; Shetty, R.; Faarisa, A. High predictive ability of glycated hemoglobin on comparison with oxidative stress markers in assessment of chronic vascular complications in type 2 diabetes mellitus. Scand. J. Clin. Lab. Investig. 2016, 76, 51–57. [Google Scholar] [CrossRef]
- Kostov, K.; Blazhev, A. Use of Glycated Hemoglobin (A1c) as a Biomarker for Vascular Risk in Type 2 Diabetes: Its Relationship with Matrix Metalloproteinases-2, -9 and the Metabolism of Collagen IV and Elastin. Medicina 2020, 56, 231. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, R.; Hwang, M.H.; Hamilton, M.T.; Park, Y. The effects of exercise on vascular endothelial function in type 2 diabetes: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 15. [Google Scholar] [CrossRef]
- Dhawan, P.; Vasishta, S.; Balakrishnan, A.; Joshi, M.B. Mechanistic insights into glucose induced vascular epigenetic reprogramming in type 2 diabetes. Life Sci. 2022, 298, 120490. [Google Scholar] [CrossRef]
- Martinez, M.; Santamarina, J.; Pavesi, A.; Musso, C.; Umpierrez, G.E. Glycemic variability and cardiovascular disease in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2021, 9, e002032. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).