Scrambler Therapy for Chronic Pain after Burns and Its Effect on the Cerebral Pain Network: A Prospective, Double-Blinded, Randomized Controlled Trial
Abstract
:1. Introduction
2. Methods
2.1. Clinical Subjects
2.2. Clinical Assessments
2.3. MRI Acquisition and CBV Mapping
2.4. Statistical Analysis
3. Results
3.1. Clinical Features
3.2. CBV Mapping before and after Scrambler Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dauber, A.; Osgood, P.F.; Breslau, A.J.; Vernon, H.L.; Carr, D.B. Chronic persistent pain after severe burns: A survey of 358 burn survivors. Pain Med. 2002, 3, 6–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klifto, K.M.; Dellon, A.L.; Hultman, C.S. Prevalence and associated predictors for patients developing chronic neuropathic pain following burns. Burn. Trauma 2020, 8, tkaa011. [Google Scholar] [CrossRef] [PubMed]
- Hamed, K.; Giles, N.; Anderson, J.; Phillips, J.; Dawson, L.F.; Drummond, P.; Wallace, H.; Wood, F.M.; Rea, S.M.; Fear, M. Changes in cutaneous innervation in patients with chronic pain after burns. Burns 2011, 37, 631–637. [Google Scholar] [CrossRef]
- Klifto, K.M.; Yesantharao, P.S.; Dellon, A.L.; Hultman, C.S.; Lifchez, S.D. Chronic neuropathic pain following hand burns: Etiology, treatment, and long-term outcomes. J. Hand Surg. 2021, 46, 67.e1–67.e9. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruvalcaba, I.; Sánchez-Hernández, V.; Mercado-Sesma, A.R. Effect of a combined continuous and intermittent transcutaneous electrical nerve stimulation on pain perception of burn patients evaluated by visual analog scale: A pilot study. Local Reg. Anesth. 2015, 8, 119–122. [Google Scholar]
- Udina-Cortés, C.; Fernández-Carnero, J.; Romano, A.A.; Cuenca-Zaldívar, J.N.; Villafañe, J.H.; Castro-Marrero, J.; Alguacil-Diego, I.M. Effects of neuro-adaptive electrostimulation therapy on pain and disability in fibromyalgia: A prospective, randomized, double-blind study. Medicine 2020, 99, e23785. [Google Scholar] [CrossRef]
- Bridger, C.; Prabhala, T.; Dawson, R.; Khazen, O.; MacDonell, J.; DiMarzio, M.; Staudt, M.D.; De, E.J.B.; Argoff, C.; Pilitsis, J.G. Neuromodulation for chronic pelvic pain: A single-institution experience with a collaborative team. Neurosurgery 2020, 88, 819–827. [Google Scholar] [CrossRef]
- Pachman, D.R.; Weisbrod, B.L.; Seisler, D.K.; Barton, D.L.; Fee-Schroeder, K.C.; Smith, T.; Lachance, D.H.; Liu, H.; Shelerud, R.A.; Cheville, A.L.; et al. Pilot evaluation of scrambler therapy for the treatment of chemotherapy-induced peripheral neuropathy. Support Care Cancer 2015, 23, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Yarchoan, M.; Naidoo, J.; Smith, T.J. Successful treatment of scar pain with scrambler therapy. Cureus 2019, 11, e5903. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Cheville, A.L.; Loprinzi, C.L.; Longo-Schoberlein, D. Scrambler therapy for the treatment of chronic post-mastectomy pain (cPMP). Cureus 2017, 9, e1378. [Google Scholar] [CrossRef] [Green Version]
- Marineo, G.; Iorno, V.; Gandini, C.; Moschini, V.; Smith, T.J. Scrambler therapy may relieve chronic neuropathic pain more effectively than guideline-based drug management: Results of a pilot, randomized, controlled trial. J. Pain Symptom Manag. 2012, 43, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Reichling, D.B.; Levine, J.D. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009, 32, 611–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, R.S.; Tuckett, R.P.; English, K.B.; Johansson, O.; Saffle, J.R. Substance P axons and sensory threshold increase in burn-graft human skin. J. Surg. Res. 2004, 118, 154–160. [Google Scholar] [CrossRef]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, A.R.; Farmer, M.A.; Baliki, M.N.; Apkarian, A.V. Chronic pain: The role of learning and brain plasticity. Restor. Neurol. Neurosci. 2014, 32, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Apkarian, V.A.; Hashmi, J.A.; Baliki, M.N. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 2011, 152, S49–S64. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.K.; Nabekura, J. Functional and structural plasticity in the primary somatosensory cortex associated with chronic pain. J. Neurochem. 2017, 141, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Joo, S.Y.; Park, C.H.; Cho, Y.S.; Seo, C.H.; Ohn, S.H. Plastic Changes in pain and motor network induced by chronic burn pain. J. Clin. Med. 2021, 10, 2592. [Google Scholar] [CrossRef]
- Seo, C.H.; Park, C.-H.; Jung, M.H.; Jang, S.; Joo, S.Y.; Kang, Y.; Ohn, S.H. Preliminary investigation of pain-related changes in cerebral blood volume in patients with phantom limb pain. Arch. Phys. Med. Rehab. 2017, 98, 2206–2212. [Google Scholar] [CrossRef]
- Tanwar, S.; Mattoo, B.; Kumar, U.; Bhatia, R. Repetitive transcranial magnetic stimulation of the prefrontal cortex for fibromyalgia syndrome: A randomised controlled trial with 6-months follow up. Adv. Rheumatol. 2020, 60, 34. [Google Scholar] [CrossRef]
- Park, C.-H.; Seo, C.H.; Jung, M.H.; Joo, S.Y.; Jang, S.; Lee, H.Y.; Ohn, S.H. Investigation of cognitive circuits using steady-state cerebral blood volume and diffusion tensor imaging in patients with mild cognitive impairment following electrical injury. Neuroradiology 2017, 59, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Dydyk, A.M.; Grandhe, S. Pain Assessment; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Jensen, M.P.; Chen, C.; Brugger, A.M. Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. J. Pain 2003, 4, 407–414. [Google Scholar] [CrossRef]
- Kim, H.W.; Shin, C.; Lee, S.H.; Han, C. Standardization of the Korean version of the patient health questionnaire-4 (PHQ-4). Clin. Psychopharmacol. Neurosci. 2021, 19, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tao, W.; Hou, Y.Y.; Wang, W.; Lu, Y.G.; Pan, Z.Z. Persistent pain facilitates response to morphine reward by downregulation of central amygdala GABAergic function. Neuropsychopharmacology 2014, 39, 2263–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayback-Beebe, A.; Panula, T.; Arzola, S.; Goff, B. Scrambler therapy treatment: The importance of examining clinically meaningful improvements in chronic pain and quality of life. Mil. Med. 2020, 185, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starkweather, A.R.; Coyne, P.; Lyon, D.E.; Elswick, R.K., Jr.; An, K.; Sturgill, J. Decreased low back pain intensity and differential gene expression following Calmare®: Results from a double-blinded randomized sham-controlled study. Res. Nurs. Health 2015, 38, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Ohrtman, E.A.; Morales-Quezada, L.; Simko, L.C.; Ryan, C.M.; Zafonte, R.; Schneider, J.C.; Fregni, F. Distinct behavioral response of primary motor cortex stimulation in itch and pain after burn injury. Neurosci. Lett. 2019, 690, 89–94. [Google Scholar] [CrossRef]
- Alencar de Castro, R.J.; Leal, P.C.; Sakata, R.K. Pain management in burn patients. Braz. J. Anesthesiol. 2013, 63, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Seo, C.H.; Park, C.-H.; Jung, M.H.; Baek, S.; Song, J.; Cha, E.; Ohn, A.S.H. Increased white matter diffusivity associated with phantom limb pain. Korean J. Pain 2019, 32, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Winkler, A.M.; Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation inference for the general linear model. NeuroImage 2014, 92, 381–397. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Nichols, T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 2009, 44, 83–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Matielo, H.; Gonçalves, E.S.; Campos, M.; Oliveira, V.R.; Toniolo, E.F.; Alves, A.S.; Lebrun, I.; de Andrade, D.C.; Teixeira, M.J.; Britto, L.R.; et al. Electrical stimulation of the posterior insula induces mechanical analgesia in a rodent model of neuropathic pain by modulating GABAergic signaling and activity in the pain circuitry. Brain Res. 2021, 1754, 147237. [Google Scholar] [CrossRef] [PubMed]
- Kalous, A.; Osborne, P.B.; Keast, J.R. Spinal cord compression injury in adult rats initiates changes in dorsal horn remodeling that may correlate with development of neuropathic pain. J. Comp. Neurol. 2009, 513, 668–684. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [Green Version]
- Tomasello, C.; Pinto, R.M.; Mennini, C.; Conicella, E.; Stoppa, F.; Raucci, U. Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: A preliminary study. Pediatr. Blood Cancer 2018, 65, e27064. [Google Scholar] [CrossRef]
- Portilla, A.S.; Bravo, G.L.; Miraval, F.K.; Villamar, M.F.; Schneider, J.C.; Ryan, C.M.; Fregni, F. A feasibility study assessing cortical plasticity in chronic neuropathic pain following burn injury. J. Burn Care Res. 2013, 34, e48–e52. [Google Scholar] [CrossRef]
- Kang, B.; Ma, J.; Shen, J.; Xu, H.; Wang, H.; Zhao, C.; Xie, J.; Zhong, S.; Gao, C.; Xu, X.; et al. Altered brain activity in end-stage knee osteoarthritis revealed by resting-state functional magnetic resonance imaging. Brain Behav. 2022, 12, e2479. [Google Scholar] [CrossRef]
- Barroso, J.; Vigotsky, A.D.; Branco, P.; Reis, A.M.; Schnitzer, T.J.; Galhardo, V.; Apkarian, A.V. Brain gray matter abnormalities in osteoarthritis pain: A cross-sectional evaluation. Pain 2020, 161, 2167–2178. [Google Scholar] [CrossRef]
- Lewis, G.N.; Parker, R.S.; Sharma, S.; Rice, D.A.; McNair, P.J. Structural brain alterations before and after total knee arthroplasty: A longitudinal assessment. Pain Med. 2018, 19, 2166–2176. [Google Scholar] [CrossRef] [Green Version]
- Hosseini Amiri, M.; Tavousi, S.H.; Mazlom, S.R.; Manzari, Z.S. Effect of transcranial direct current stimulation on pain anxiety during burn wound care. Burns 2016, 42, 872–876. [Google Scholar] [CrossRef]
- Thibaut, A.; Shie, V.L.; Ryan, C.M.; Zafonte, R.; Ohrtman, E.A.; Schneider, J.C.; Fregni, F. A review of burn symptoms and potential novel neural targets for non-invasive brain stimulation for treatment of burn sequelae. Burns 2020, 47, 525–537. [Google Scholar] [CrossRef] [PubMed]
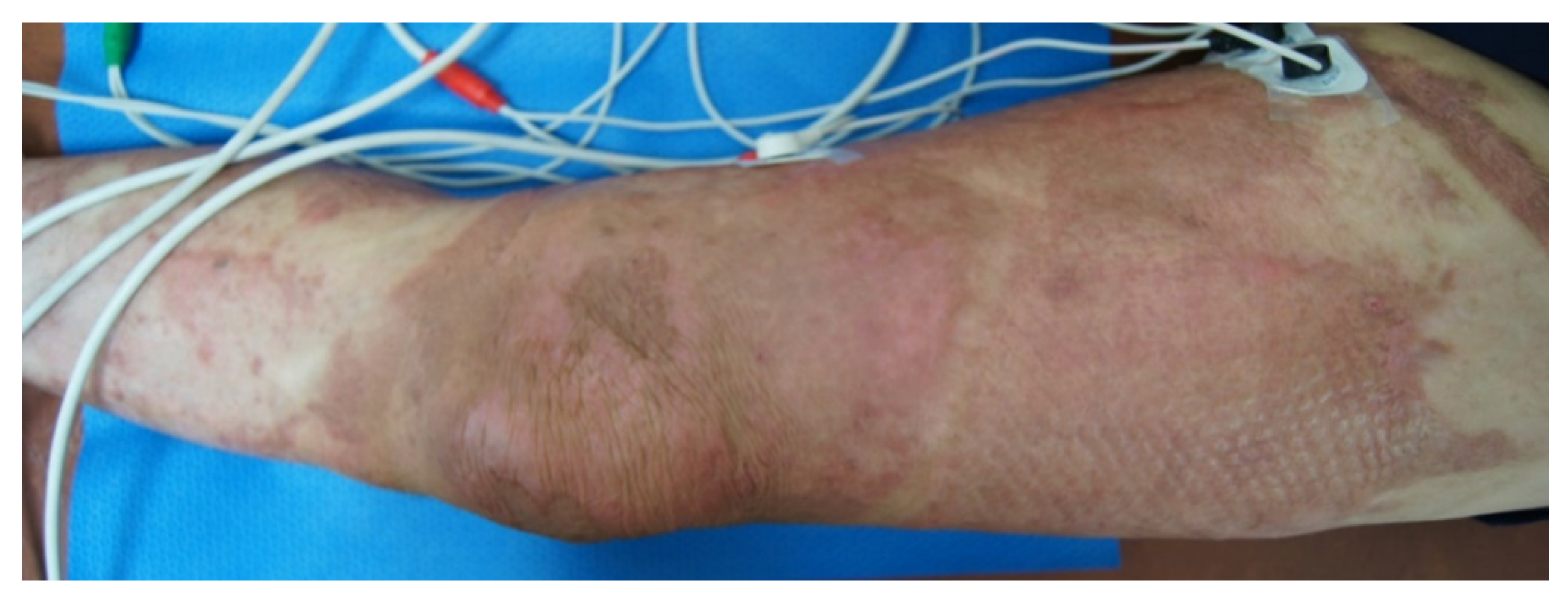
| Experimental Group (n = 14) | Sham Group (n = 23) | p | |
|---|---|---|---|
| Male:Female | 12:2 | 18:5 | 0.69 |
| Age (years) | 47 (39–59) | 49 (30–57) | 0.83 |
| TBSA (%) | 11 (5–23) | 20 (5–23) | 0.65 |
| Days between burn and MRI acquisition | 76 (53–93) | 74 (52–107) | 1.00 |
| The sites of burn injury | |||
| Arm: Forearm/Hand: Thigh: Leg/Foot | 5:5:1:3 | 6:8:1:8 | 0.82 |
| VAS | 6 (5–8) | 7 (6–8) | 0.17 |
| BPI | |||
| Sensory dimension | 23 (13–26) | 26 (18–30) | 0.29 |
| Reactive dimension | 38 (27–47) | 42 (34–50) | 0.41 |
| HDRS | 4 (1–4) | 2 (1–4) | 0.44 |
| Experimental Group | Sham Stimulation Group | |||||
|---|---|---|---|---|---|---|
| Baseline | After 2 Weeks | p | Baseline | After 2 Weeks | p | |
| VAS, median (IQR) | 6 (5–8) | 3 (3–4) | 0.004 | 7 (6–8) | 6 (5–7) | 0.001 |
| Comparison of VAS after 2 weeks between groups | <0.001 | |||||
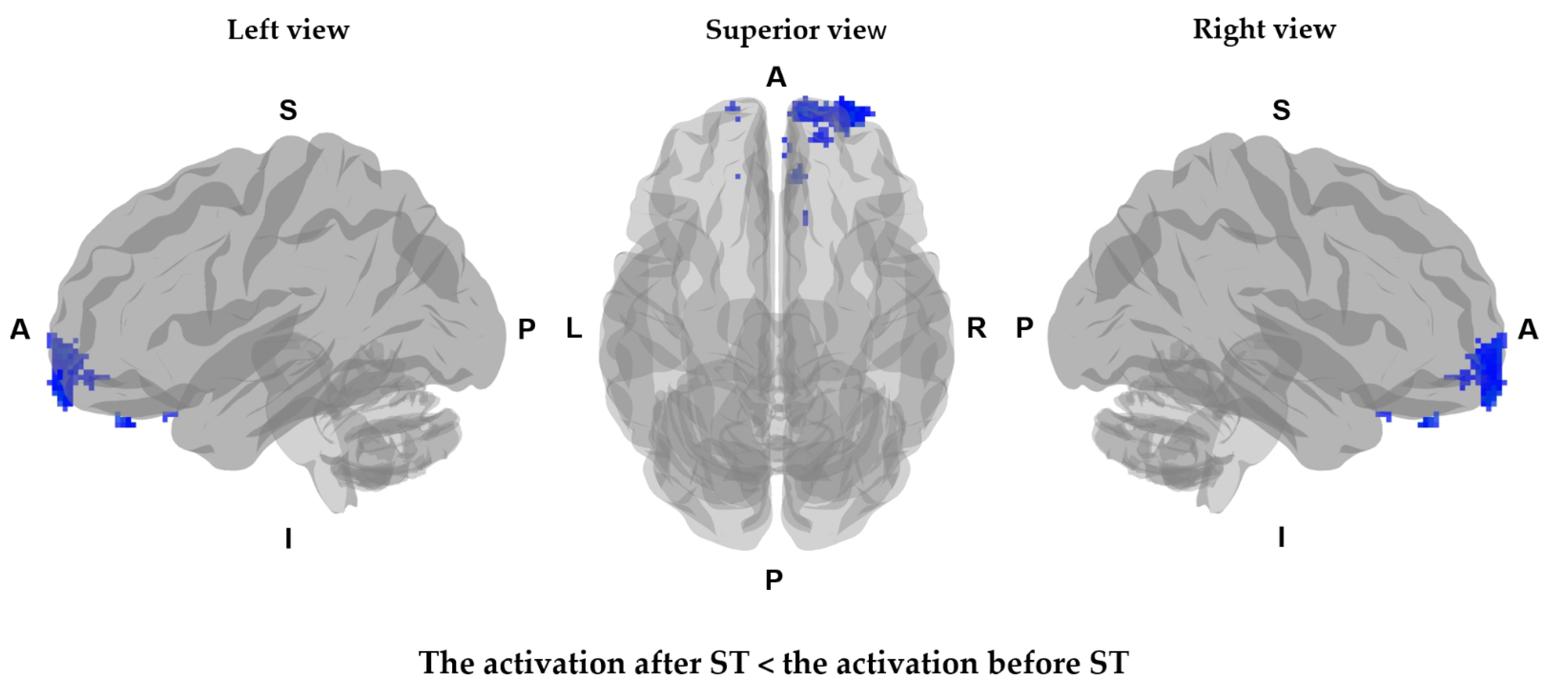
| Comparison | Cluster No | Voxel Count | Grey Matter Label | T Value | p Value | Coordinates (mm) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Decreased CBV | 1 | 232 | Right orbito-frontal gyrus | 450.661 | 0.004 | 30 | 60 | −8 |
| Right middle frontal gyrus | 441.674 | 0.004 | 26 | 58 | −6 | |||
| Right superior frontal gyrus | 337.098 | 0.004 | 26 | 66 | −6 | |||
| 3 | 16 | Right gyrus rectus | 252.559 | 0.004 | 8 | 38 | −30 | |
| 4 | 5 | Left orbito-frontal gyrus | 356.164 | 0.004 | −16 | 64 | −12 | |
| Left superior frontal gyrus | 345.580 | 0.004 | −14 | 64 | −12 | |||
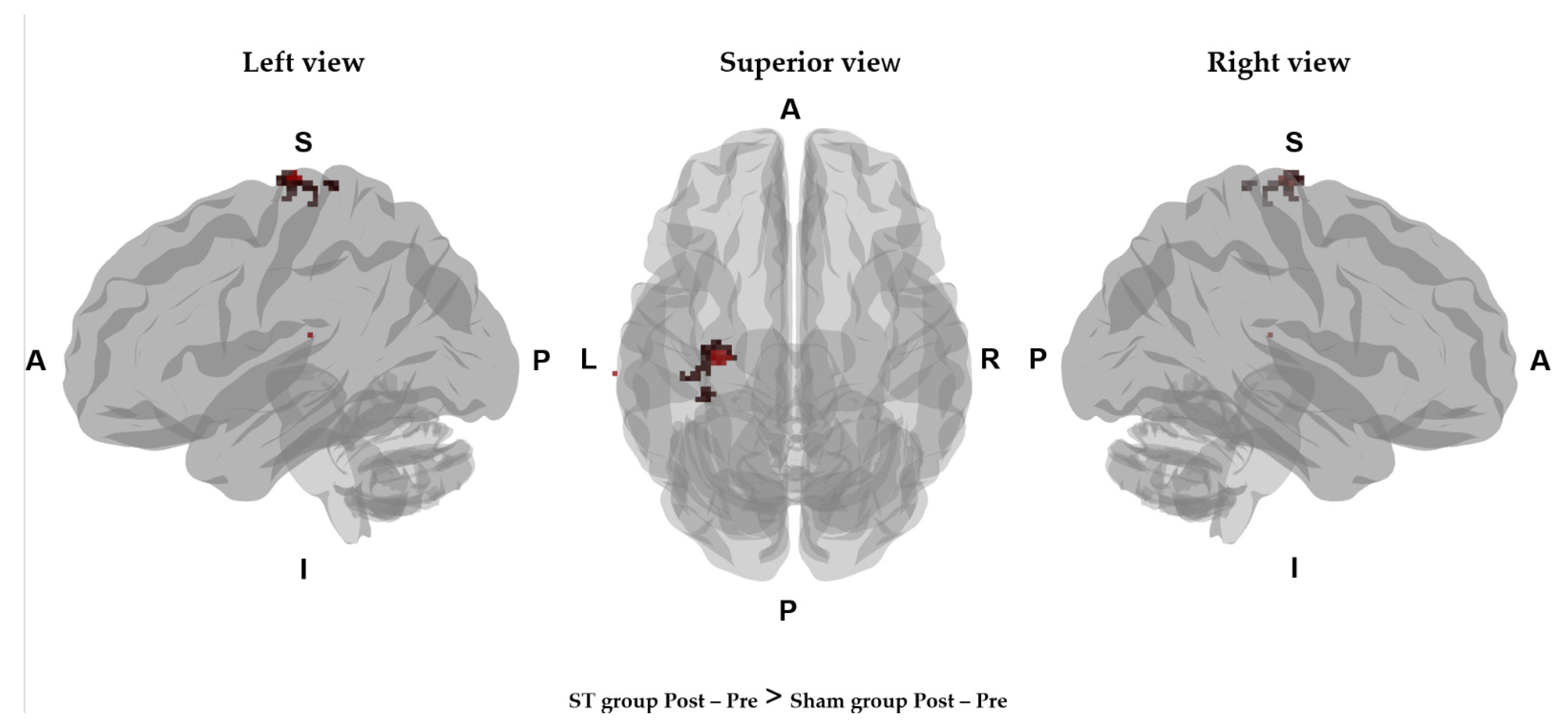
| Comparison | Gray Matter Label | T Value | p Value | Coordinates (mm) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| STpost – pre >Sham post − pre | Left precentral gyrus | 411.954 | 0.002 | −30 | −20 | 74 |
| Left postcentral gyrus | 373.215 | 0.004 | −32 | −26 | 72 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Park, C.-h.; Cho, Y.S.; Kim, L.; Yoo, J.W.; Joo, S.Y.; Seo, C.H. Scrambler Therapy for Chronic Pain after Burns and Its Effect on the Cerebral Pain Network: A Prospective, Double-Blinded, Randomized Controlled Trial. J. Clin. Med. 2022, 11, 4255. https://doi.org/10.3390/jcm11154255
Lee SY, Park C-h, Cho YS, Kim L, Yoo JW, Joo SY, Seo CH. Scrambler Therapy for Chronic Pain after Burns and Its Effect on the Cerebral Pain Network: A Prospective, Double-Blinded, Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(15):4255. https://doi.org/10.3390/jcm11154255
Chicago/Turabian StyleLee, Seung Yeol, Chang-hyun Park, Yoon Soo Cho, Laurie Kim, Ji Won Yoo, So Young Joo, and Cheong Hoon Seo. 2022. "Scrambler Therapy for Chronic Pain after Burns and Its Effect on the Cerebral Pain Network: A Prospective, Double-Blinded, Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 15: 4255. https://doi.org/10.3390/jcm11154255
APA StyleLee, S. Y., Park, C.-h., Cho, Y. S., Kim, L., Yoo, J. W., Joo, S. Y., & Seo, C. H. (2022). Scrambler Therapy for Chronic Pain after Burns and Its Effect on the Cerebral Pain Network: A Prospective, Double-Blinded, Randomized Controlled Trial. Journal of Clinical Medicine, 11(15), 4255. https://doi.org/10.3390/jcm11154255







