Predicting the Severity of Neurological Impairment Caused by Ischemic Stroke Using Deep Learning Based on Diffusion-Weighted Images
Abstract
:1. Introduction
2. Materials and Methods
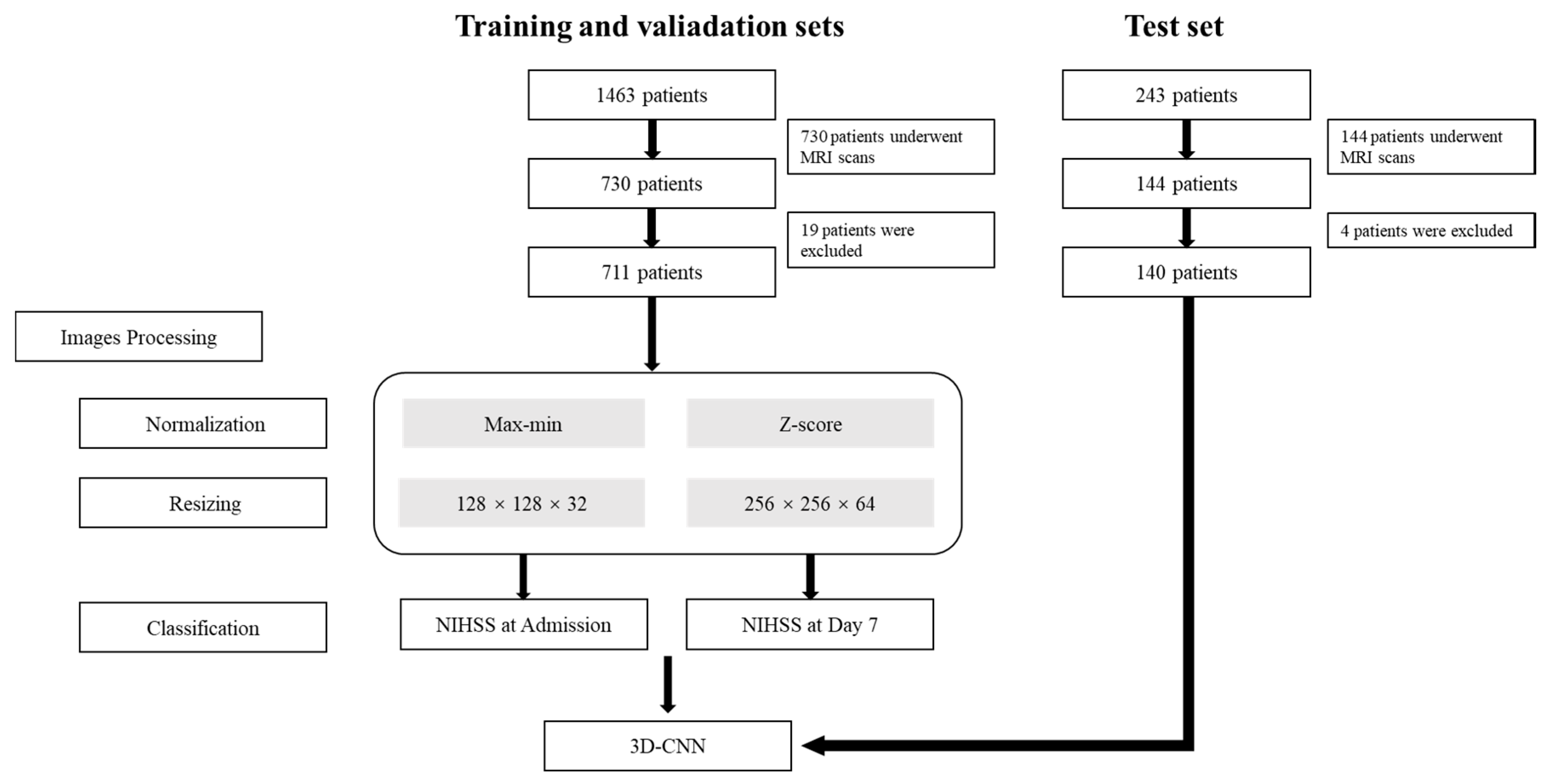
2.1. Patients
2.2. MRI Scanning Protocols
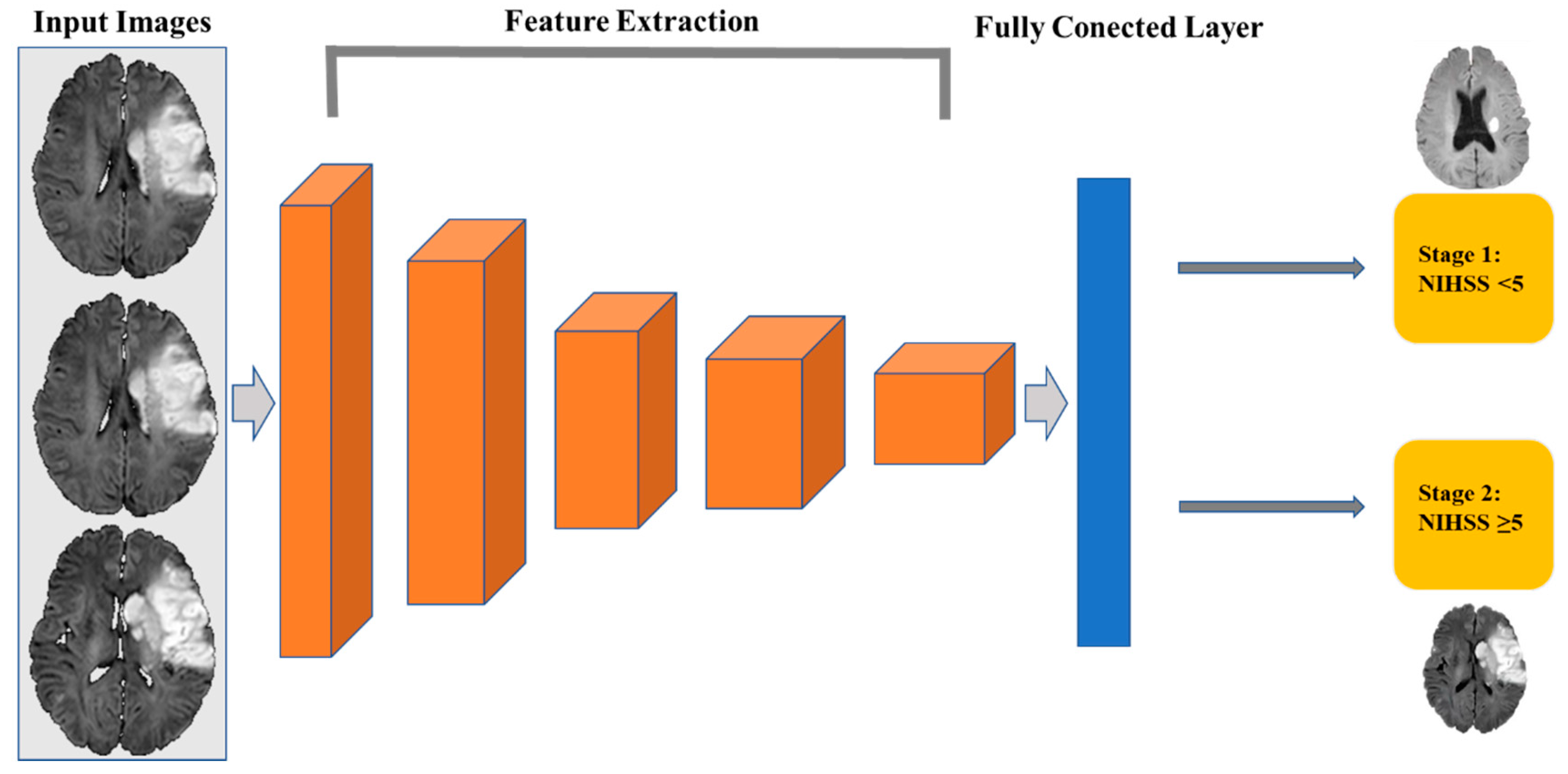
2.3. Classification
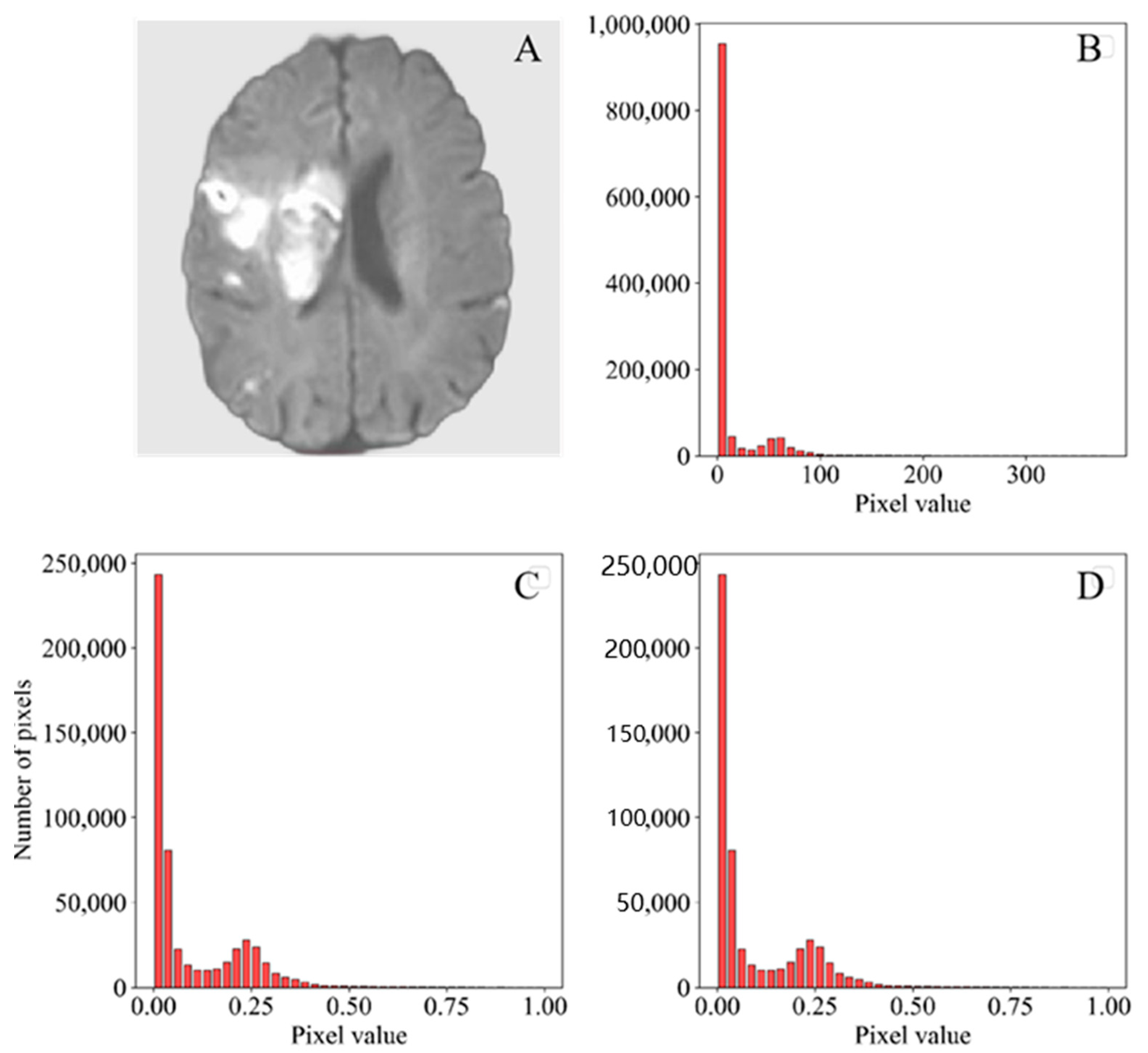
2.4. Image Preprocessing
2.5. Convolutional Neural Network Construction
2.6. Statistical Analysis
3. Results
3.1. Subjects’ Clinical Information
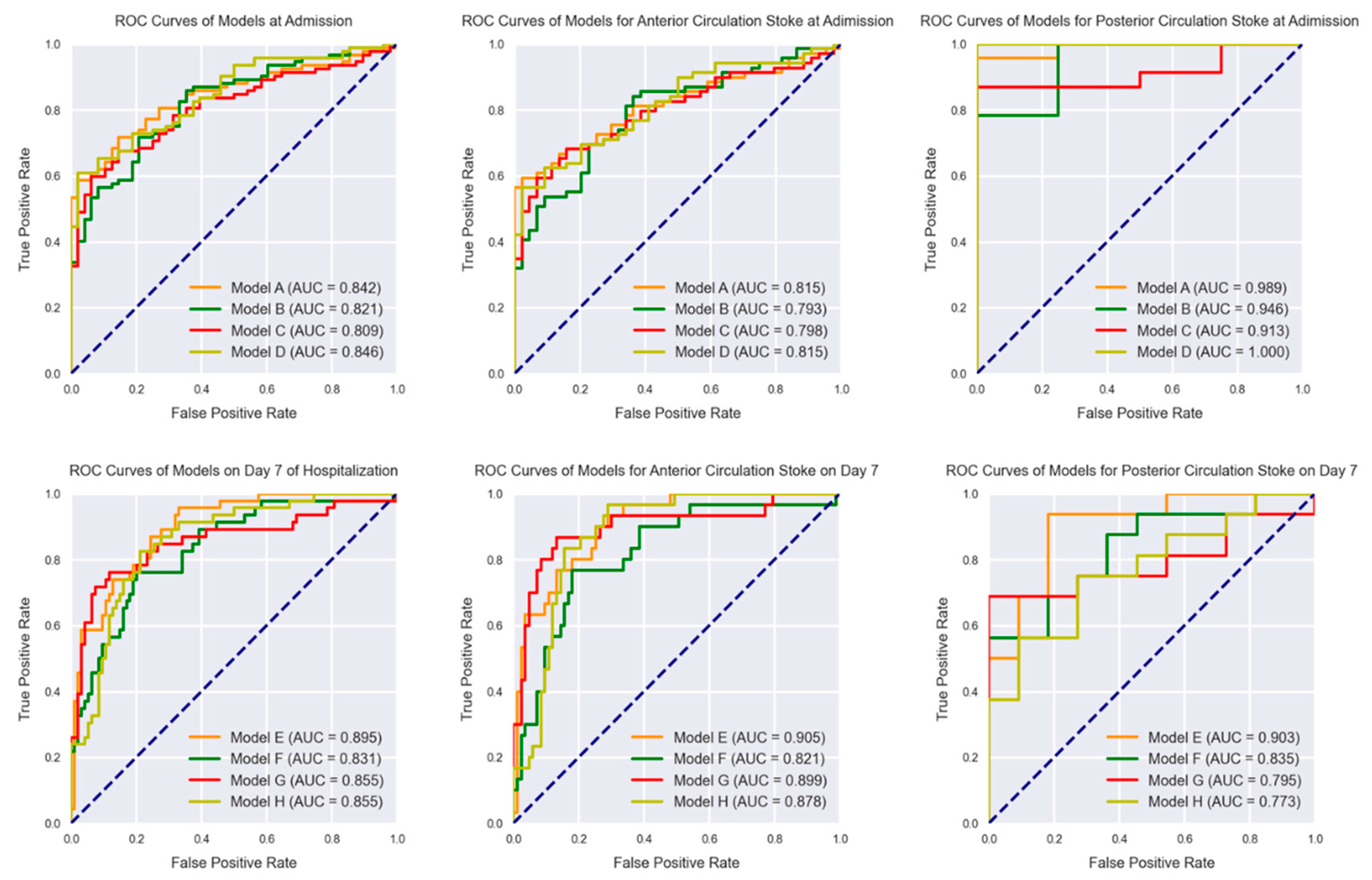
3.2. Classification at Admission
3.3. Classification on Day 7 of Hospitalization
3.4. Classification of IS Based on Different Circulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhamoon, M.S.; Moon, Y.P.; Paik, M.C.; Boden-Albala, B.; Rundek, T.; Sacco, R.L.; Elkind, M.S. Long-term functional recovery after first ischemic stroke: The Northern Manhattan Study. Stroke 2009, 40, 2805–2811. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Wong, K.S.; Bae, H.J.; Pandey, D.K. Large artery intracranial occlusive disease: A large worldwide burden but a relatively neglected frontier. Stroke 2008, 39, 2396–2399. [Google Scholar] [CrossRef]
- Furie, K.L.; Jayaraman, M.V. 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke 2018, 49, 509–510. [Google Scholar] [CrossRef]
- Lyden, P.; Lu, M.; Jackson, C.; Marler, J.; Kothari, R.; Brott, T.; Zivin, J. Underlying structure of the National Institutes of Health Stroke Scale: Results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke 1999, 30, 2347–2354. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.J.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V.; et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Fischer, U.; Baumgartner, A.; Arnold, M.; Nedeltchev, K.; Gralla, J.; De Marchis, G.M.; Kappeler, L.; Mono, M.L.; Brekenfeld, C.; Schroth, G.; et al. What is a minor stroke? Stroke 2010, 41, 661–666. [Google Scholar] [CrossRef]
- Barber, P.A.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef]
- Burke, J.F.; Kerber, K.A.; Iwashyna, T.J.; Morgenstern, L.B. Wide variation and rising utilization of stroke magnetic resonance imaging: Data from 11 states. Ann. Neurol. 2012, 71, 179–185. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Armitage, P.; Dennis, M.S.; Lewis, S.; Marshall, I.; Sellar, R. The use of diffusion-weighted magnetic resonance imaging to identify infarctions in patients with minor strokes. J. Stroke Cerebrovasc. Dis. 2000, 9, 70–75. [Google Scholar] [CrossRef]
- Tong, D.C.; Yenari, M.A.; Albers, G.W.; O’Brien, M.; Marks, M.P.; Moseley, M.E. Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (<6.5 h) ischemic stroke. Neurology 1998, 50, 864–870. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.; Kwon, B.; Chang, J.Y.; Song, Y.S.; Lee, D.H.; Kwon, S.U.; Kim, J.S.; Kang, D.W. Clinical-Diffusion mismatch is associated with early neurological improvement after Late-Window endovascular treatment. Cerebrovasc. Dis. 2022, 51, 331–337. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Mao, J.; Huang, Z.; Huang, C.; Xu, W. CNN-RNN: A unified framework for multi-label image classification, 2016. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 2285–2294. [Google Scholar]
- Wong, K.K.; Cummock, J.S.; Li, G.; Ghosh, R.; Xu, P.; Volpi, J.J.; Wong, S. Automatic segmentation in acute ischemic stroke: Prognostic significance of topological stroke volumes on stroke outcome. Stroke 2022. online ahead of print. [Google Scholar] [CrossRef]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, J.; Sun, Y.; Li, C.; Wu, W.; Jin, L.; Yang, Z.; Ni, B.; Gao, P.; Wang, P.; et al. 3D deep learning from CT scans predicts tumor invasiveness of subcentimeter pulmonary adenocarcinomas. Cancer Res. 2018, 78, 6881–6889. [Google Scholar] [CrossRef]
- Cetinoglu, Y.K.; Koska, I.O.; Uluc, M.E.; Gelal, M.F. Detection and vascular territorial classification of stroke on diffusion-weighted MRI by deep learning. Eur. J. Radiol. 2021, 145, 110050. [Google Scholar] [CrossRef]
- Liu, L.; Kurgan, L.; Wu, F.X.; Wang, J. Attention convolutional neural network for accurate segmentation and quantification of lesions in ischemic stroke disease. Med. Image Anal. 2020, 65, 101791. [Google Scholar] [CrossRef]
- Chen, L.; Bentley, P.; Rueckert, D. Fully automatic acute ischemic lesion segmentation in DWI using convolutional neural networks. Neuroimage Clin. 2017, 15, 633–643. [Google Scholar] [CrossRef]
- Kim, Y.C.; Lee, J.E.; Yu, I.; Song, H.N.; Baek, I.Y.; Seong, J.K.; Jeong, H.G.; Kim, B.J.; Nam, H.S.; Chung, J.W.; et al. Evaluation of diffusion lesion volume measurements in acute ischemic stroke using Encoder-Decoder convolutional network. Stroke 2019, 50, 1444–1451. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Z.; Mane, R.; Wang, S.; Jing, J.; Fu, H.; Wu, Z.; Li, H.; Jiang, Y.; Meng, X.; et al. Predicting functional outcome in patients with acute brainstem infarction using deep neuroimaging features. Eur. J. Neurol. 2021, 29, 744–752. [Google Scholar] [CrossRef]
- Kamnitsas, K.; Ledig, C.; Newcombe, V.; Simpson, J.P.; Kane, A.D.; Menon, D.K.; Rueckert, D.; Glocker, B. Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Med. Image Anal. 2017, 36, 61–78. [Google Scholar] [CrossRef]
- Nagi, J.; Ducatelle, F.; Caro, G.; Dan, C.; Gambardella, L.M. Max-pooling convolutional neural networks for vision-based hand gesture recognition. In Proceedings of the 2011 IEEE International Conference on Signal and Image Processing Applications (ICSIPA), Kuala Lumpur, Malaysia, 16–18 November 2011; pp. 342–347. [Google Scholar]
- Yin, C.; Feng, Z.; Jiang, W.; Xiao, L.; Belongie, S. Kernel pooling for convolutional neural networks. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 3049–3058. [Google Scholar]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef]
- Sacco, R.L. Risk factors and outcomes for ischemic stroke. Neurology 1995, 45, S10–S14. [Google Scholar]
- Sun, T.K.; Chiu, S.C.; Yeh, S.H.; Chang, K.C. Assessing reliability and validity of the Chinese version of the stroke scale: Scale development. Int. J. Nurs. Stud. 2006, 43, 457–463. [Google Scholar] [CrossRef]
- Cai, Y.F.; Jia, Z.; Zhang, X.C.; Zhao, Y.Q.; Wang, L.X.; Guo, J.W.; Ai-Hua, O.U.; Liang, W.X.; Pei, J. Study on multi-center investigation of the Chinese version of National Institutes of Health Stroke Scale. J. Beijing Univ. Tradit. Chin. Med. 2008, 31, 494–498. [Google Scholar]
- Davalos, A.; Blanco, M.; Pedraza, S.; Leira, R.; Castellanos, M.; Pumar, J.M.; Silva, Y.; Serena, J.; Castillo, J. The clinical-DWI mismatch: A new diagnostic approach to the brain tissue at risk of infarction. Neurology 2004, 62, 2187–2192. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Murray, V.; Berge, E.; Del, Z.G.; Sandercock, P.; Lindley, R.L.; Cohen, G. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. Lancet 2012, 379, 2364–2372. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, F.; Li, C.X.; Yan, Y.; Kempf, D.; Nair, G.; Wang, S.; Muly, E.C.; Zola, S.; Howell, L. Temporal evolution of ischemic lesions in nonhuman primates: A diffusion and perfusion MRI study. PLoS ONE 2015, 10, e117290. [Google Scholar] [CrossRef]
- Pereira, S.; Pinto, A.; Alves, V.; Silva, C.A. Brain tumor segmentation using convolutional neural networks in MRI images. IEEE Trans. Med. Imaging 2016, 35, 1240–1251. [Google Scholar] [CrossRef]
- Martin-Schild, S.; Albright, K.C.; Tanksley, J.; Pandav, V.; Jones, E.B.; Grotta, J.C.; Savitz, S.I. Zero on the NIHSS does not equal the absence of stroke. Ann. Emerg. Med. 2011, 57, 42–45. [Google Scholar] [CrossRef]
- Aoki, J.; Kimura, K.; Shibazaki, K.; Sakamoto, Y. DWI-ASPECTS as a predictor of dramatic recovery after intravenous recombinant tissue plasminogen activator administration in patients with middle cerebral artery occlusion. Stroke 2013, 44, 534–537. [Google Scholar] [CrossRef]
- Jiang, L.; Peng, M.; Geng, W.; Chen, H.; Su, H.; Zhao, B.; Chen, Y.C.; Yin, X. FLAIR hyperintensities-DWI mismatch in acute stroke: Associations with DWI volume and functional outcome. Brain Imaging Behav. 2020, 14, 1230–1237. [Google Scholar] [CrossRef]
- de Margerie-Mellon, C.; Turc, G.; Tisserand, M.; Naggara, O.; Calvet, D.; Legrand, L.; Meder, J.F.; Mas, J.L.; Baron, J.C.; Oppenheim, C. Can DWI-ASPECTS substitute for lesion volume in acute stroke? Stroke 2013, 44, 3565–3567. [Google Scholar] [CrossRef]
- Ramos, L.A.; van Os, H.; Hilbert, A.; Olabarriaga, S.D.; van der Lugt, A.; Roos, Y.; van Zwam, W.H.; van Walderveen, M.; Ernst, M.; Zwinderman, A.H.; et al. Combination of radiological and clinical baseline data for outcome prediction of patients with an acute ischemic stroke. Front. Neurol. 2022, 13, 809343. [Google Scholar] [CrossRef]
| Model | Type | Filter Size | Number of Filters | Stride |
|---|---|---|---|---|
| Layer 1 | Conv1 + Maximum Pooling | 3 × 3 × 3 | 16 | (1, 1, 1) |
| Layer 2 | Conv2 + Maximum Pooling | 3 × 3 × 3 | 32 | (2, 2, 2) |
| Layer 3 | Conv3 | 3 × 3 × 3 | 64 | (1, 1, 1) |
| Layer 4 | Conv4 + Maximum Pooling | 3 × 3 × 3 | 64 | (2, 2, 2) |
| Layer 5 | Conv6 | 3 × 3 × 3 | 96 | (1, 1, 1) |
| Layer 6 | Conv6 + Maximum Pooling | 3 × 3 × 3 | 96 | (2, 2, 2) |
| Layer 7 | Conv7 | 3 × 3 × 3 | 128 | (1, 1, 1) |
| Layer 8 | Conv8 + Maximum Pooling | 3 × 3 × 3 | 128 | (2, 2, 2) |
| Layer 9 | FC1 | - | - | - |
| Layer 10 | FC2 | - | - | - |
| Layer 11 | FC3 (SoftMax) | - | - | - |
| Predicted NIHSS Stage | Normalization | Voxels | |
|---|---|---|---|
| Model A | Admission | Maximum–minimum | 128 × 128 × 32 |
| Model B | Admission | Maximum–minimum | 256 × 256 × 64 |
| Model C | Admission | Z-score | 128 × 128 × 32 |
| Model D | Admission | Z-score | 256 × 256 × 64 |
| Model E | Hospital Day 7 | Maximum–minimum | 128 × 128 × 32 |
| Model F | Hospital Day 7 | Maximum–minimum | 256 × 256 × 64 |
| Model G | Hospital Day 7 | Z-score | 128 × 128 × 32 |
| Model H | Hospital Day 7 | Z-score | 256 × 256 × 64 |
| Characteristics | Training and Validation Sets | Test Set | p-Value |
|---|---|---|---|
| Sample capacity | 711 | 140 | |
| Age (years) a | 66.02 ± 11.22 | 65.00 ± 10.26 | 0.31 |
| Women (%) b | 33.1 (237) | 35.7 (50) | 0.65 |
| Anterior circulation (%) | 80.9 (538) | 82.9 (113) | 0.08 |
| Posterior circulation (%) | 25.2 (173) | 25.0 (27) | 0.83 |
| NIHSS (0 days) <5 (%) | 55.3 (393) | 34.2 (48) | <0.01 |
| NIHSS (0 days) ≥5 (%) | 44.7 (318) | 65.7 (92) | <0.01 |
| NIHSS (7 days) <5 (%) | 62.7 (445) | 67.1 (94) | 0.35 |
| NIHSS (7 days) ≥5 (%) | 37.3 (268) | 32.9 (46) | 0.32 |
| Model | AUC | Sensitivity | Specificity |
|---|---|---|---|
| Model A | 0.842 (0.771–0.898) | 71.7% (64.1–80.6%) | 77.1% (62.7–88.0%) |
| Model B | 0.821 (0.747–0.881) | 71.7% (61.4–80.6%) | 79.2% (65.0–98.5%) |
| Model C | 0.809 (0.734–0.871) | 59.8% (49.0–64.8%) | 93.7% (82.8–98.7%) |
| Model D | 0.846 (0.776–0.902) | 60.9% (50.1–70.9%) | 97.9% (88.9–99.9%) |
| Model E | 0.895 (0.832–0.940) | 95.7% (88.5–99.9%) | 67.0% (56.6–76.4%) |
| Model F | 0.831 (0.759–0.889) | 76.1% (61.2–87.4%) | 79.8% (70.2–87.4%) |
| Model G | 0.855 (0.785–0.908) | 76.1% (61.2–87.4%) | 88.3% (80.0–94.0%) |
| Model H | 0.855 (0.758–0.909) | 82.6% (68.6–92.2%) | 78.7% (69.1–86.5%) |
| Model | AUC | Sensitivity | Specificity |
|---|---|---|---|
| Model A | 0.815 (0.731–0.881) | 59.4% (46.9–71.1%) | 97.7% (88.0–99.9%) |
| Model B | 0.793 (0.707–0.886) | 84.1% (77.3–91.8%) | 63.6% (47.8–77.6%) |
| Model C | 0.798 (0.712–0.867) | 59.4% (46.9–71.1%) | 93.2% (81.3–98.6%) |
| Model D | 0.815 (0.731–0.881) | 56.5% (44.0–68.4%) | 97.7% (88.0–99.9%) |
| Model E | 0.905 (0.836–0.952) | 90.0% (73.5–97.9%) | 74.7% (64.0–83.6%) |
| Model F | 0.821 (0.738–0.887) | 76.7% (57.7–90.1%) | 81.9% (72.0–89.5%) |
| Model G | 0.899 (0.828–0.948) | 86.7% (69.3–96.2%) | 86.7% (77.5–93.2%) |
| Model H | 0.878 (0.803–0.932) | 96.7% (82.8–99.9%) | 72.7% (39.0–94.0%) |
| Model | AUC | Sensitivity | Specificity |
|---|---|---|---|
| Model A | 0.989 (0.853–1.000) | 78.3% (56.3–92.5%) | 100.0% (39.8–100.0%) |
| Model B | 0.989 (0.853–1.000) | 95.6% (77.8–99.9%) | 100.0% (39.8–100.0%) |
| Model C | 0.946 (0.785–0.996) | 78.3% (56.3–92.5%) | 100.0% (39.8–100.0%) |
| Model D | 1.000 (0.872–1.000) | 100.0% (85.2–100.0%) | 100.0% (39.8–100.0%) |
| Model E | 0.903 (0.727–0.983) | 93.7% (69.8–99.8%) | 81.8% (48.2–97.7%) |
| Model F | 0.835 (0.643–0.949) | 56.2% (29.2–80.2%) | 100.0% (71.5–100.0%) |
| Model G | 0.899 (0.828–0.948) | 86.7% (69.3–96.2%) | 72.7% (39.0–94.0%) |
| Model H | 0.773 (0.572–0.910) | 75.0% (47.6–92.7%) | 72.7% (39.0–94.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Long, C.; Zhao, W.; Liu, J. Predicting the Severity of Neurological Impairment Caused by Ischemic Stroke Using Deep Learning Based on Diffusion-Weighted Images. J. Clin. Med. 2022, 11, 4008. https://doi.org/10.3390/jcm11144008
Zeng Y, Long C, Zhao W, Liu J. Predicting the Severity of Neurological Impairment Caused by Ischemic Stroke Using Deep Learning Based on Diffusion-Weighted Images. Journal of Clinical Medicine. 2022; 11(14):4008. https://doi.org/10.3390/jcm11144008
Chicago/Turabian StyleZeng, Ying, Chen Long, Wei Zhao, and Jun Liu. 2022. "Predicting the Severity of Neurological Impairment Caused by Ischemic Stroke Using Deep Learning Based on Diffusion-Weighted Images" Journal of Clinical Medicine 11, no. 14: 4008. https://doi.org/10.3390/jcm11144008
APA StyleZeng, Y., Long, C., Zhao, W., & Liu, J. (2022). Predicting the Severity of Neurological Impairment Caused by Ischemic Stroke Using Deep Learning Based on Diffusion-Weighted Images. Journal of Clinical Medicine, 11(14), 4008. https://doi.org/10.3390/jcm11144008






