1. Introduction
Doripenem is a carbapenem antibiotic that has a broad spectrum, antibacterial activity similar to imipenem against gram-positive pathogens and an antimicrobial spectrum similar to meropenem against gram-negative organisms [
1,
2,
3]. Doripenem activity against
Pseudomonas aeruginosa has been reported to be comparable to that of meropenem [
4,
5]. In the study by Kollef et al., a fixed seven-day course of doripenem was compared with a fixed ten-day course of imipenem/cilastatin for the treatment of ventilator-associated pneumonia (VAP) [
6]. The study demonstrated a lower clinical cure rate and a higher mortality rate among doripenem-treated subjects than among imipenem/cilastatin-treated subjects. The clinical trial of doripenem for VAP treatment was terminated due to safety concerns. In March 2014, the US Food and Drug Administration (FDA) approved doripenem label changes to highlight the increased risk of death in VAP patients. The US FDA approved doripenem only for the treatment of complicated intra-abdominal infection, complicated urinary tract infection, and pyelonephritis [
7,
8]. However, doripenem has been approved in Europe for the treatment of complicated intra-abdominal infection, complicated urinary tract infection, and nosocomial pneumonia, including VAP [
9]. Several studies have reported the clinical effectiveness of doripenem therapy for nosocomial pneumonia [
10,
11]. Therefore, we performed a comprehensive and an updated meta-analysis of the clinical outcomes associated with doripenem therapy for nosocomial pneumonia patients, including VAP and hospital-acquired pneumonia (HAP) patients. We hypothesize that doripenem has clinical efficacy in the treatment of nosocomial pneumonia. The purpose of this meta-analysis was to explore the efficacy and the safety of doripenem in patients with VAP and HAP in comparison with other antimicrobial agents.
2. Method
2.1. Data Search Strategy
The literature search was performed using PubMed, Web of Science, and Cochrane Library databases to identify all included clinical studies and meta-analyses or systematic reviews on the topic from 1 January 2000. In the databases, we used the following search string: ‘(doripenem OR imipenem OR meropenem OR piperacillin-tazobactam) AND (pneumonia OR nosocomial pneumonia OR ventilator-associated pneumonia OR hospital-acquired pneumonia) AND [in PubMed] (random OR prospective OR retrospective OR cohort OR observational OR clinical trial)’, AND [In Web of Science] (random OR prospective OR retrospective OR cohort OR observational), AND [in Cochrane Library] (random OR prospective OR retrospective OR observational). We examined treatment options, including doripenem, imipenem, meropenem, or piperacillin-tazobactam, for patients with pneumonia and searched relevant articles published from inception to 30 April 2022. We included all clinical studies, including retrospective observational studies, prospective observational studies, and randomized controlled trials. Conference abstracts were also searched. Previously published systematic reviews and meta-analyses were reviewed to identify any additional studies that may have been missed in the primary literature search. No language, publication date, or publication status restrictions were imposed.
2.2. Study Selection and Data Extraction
To determine the eligibility of identified trial reports, each study was independently screened and reviewed for eligibility by two authors. After excluding duplicates, two investigators screened the titles and abstracts of all studies retrieved to identify eligible records. After excluding irrelevant studies, all of the relevant articles were reviewed by reading the full texts to determine eligibility. Data regarding author, year of publication, country, study design, pneumonia type, total number of patients receiving doripenem, total number of patients receiving other antimicrobial agents, and administered antibiotics were extracted. Data on the microbiological cure rate, the clinical cure rate, all-cause mortality, and adverse events for patients with nosocomial pneumonia were manually extracted from the eligible full text articles. Any disagreement was subsequently resolved with the consensus of the review team and discussion with a third author.
2.3. Inclusion and Exclusion Criteria
Observational studies with inadequate levels of evidence are not as meaningful as RCTs. Because a very small number of RCTs were available, we included all clinical studies, including retrospective observational studies, prospective observational studies, conference abstracts, and randomized controlled trials. The studies were considered eligible for inclusion only if they directly compared the clinical effectiveness of doripenem with those of other antimicrobial agents in the treatment of adult pneumonia. Doripenem was administered at dosages ranging from 250 mg every 12 h to 1.0 gm every 8 h. Meropenem was administered at a dosage of 1.0 g every 8 h. Imipenem was administered at a dosage of 500 mg every 6 h or 1.0 g every 8 h. Piperacillin-tazobactam was administered at a dosage of 4.5 g every 6 h. All studies were included if they reported one or more of the following outcomes: clinical cure rate, microbiological cure rate, all-cause mortality, adverse events, Pseudomonas aeruginosa (PA) pneumonia clinical cure rate, PA pneumonia microbiological cure rate, and PA pneumonia all-cause mortality. Studies with a population of participants who were younger than 18 years were excluded.
2.4. Definitions and Outcomes
The primary outcome was all-cause mortality. All-cause mortality was the death rate from all causes of death for a population in a given time period. Secondary outcomes were the clinical cure rate, the microbiological cure rate, and adverse events. Clinical cure was defined as resolution of clinical signs and symptoms of pneumonia at the end of treatment. Microbiological cure was defined as the absence of the baseline pathogen after therapy. The adverse event data recorded were the risk of discontinuing due to adverse events, the incidence of serious adverse events, and some common events, such as diarrhea, nausea, headache, constipation, and seizure.
2.5. Quality Assessment
Two investigators assessed the risk of bias in each study using the Cochrane Risk-of-Bias Tool 2.0 for RCTs. The Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was used to evaluate observational studies [
12]. The quality of the evidence was ranked based on the risk of bias according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach at the outcome level [
13,
14].
2.6. Statistical Analysis
We used Cochrane Review Manager software RevMan 5 to perform statistical analysis. The degree of heterogeneity was evaluated with the Q statistic test and the I2 measure was used to assess the degree of statistical heterogeneity. Heterogeneity was defined as significant when the p-value was less than 0.10 or I2 more than 50%. The results that are documented include the between-study pooled risk ratio (RR) and 95% confidence intervals (CIs) that were calculated for dichotomous outcomes. The significance of the pooled ratios was determined by the Z test, and results with a p value of less than 0.05 were considered statistically significant. Funnel and network plots were also generated by Cochrane Review Manager software RevMan 5. A funnel plot associated with therapeutic regimens was used to examine potential publication bias.
3. Results
3.1. Characteristics of the Included Trials
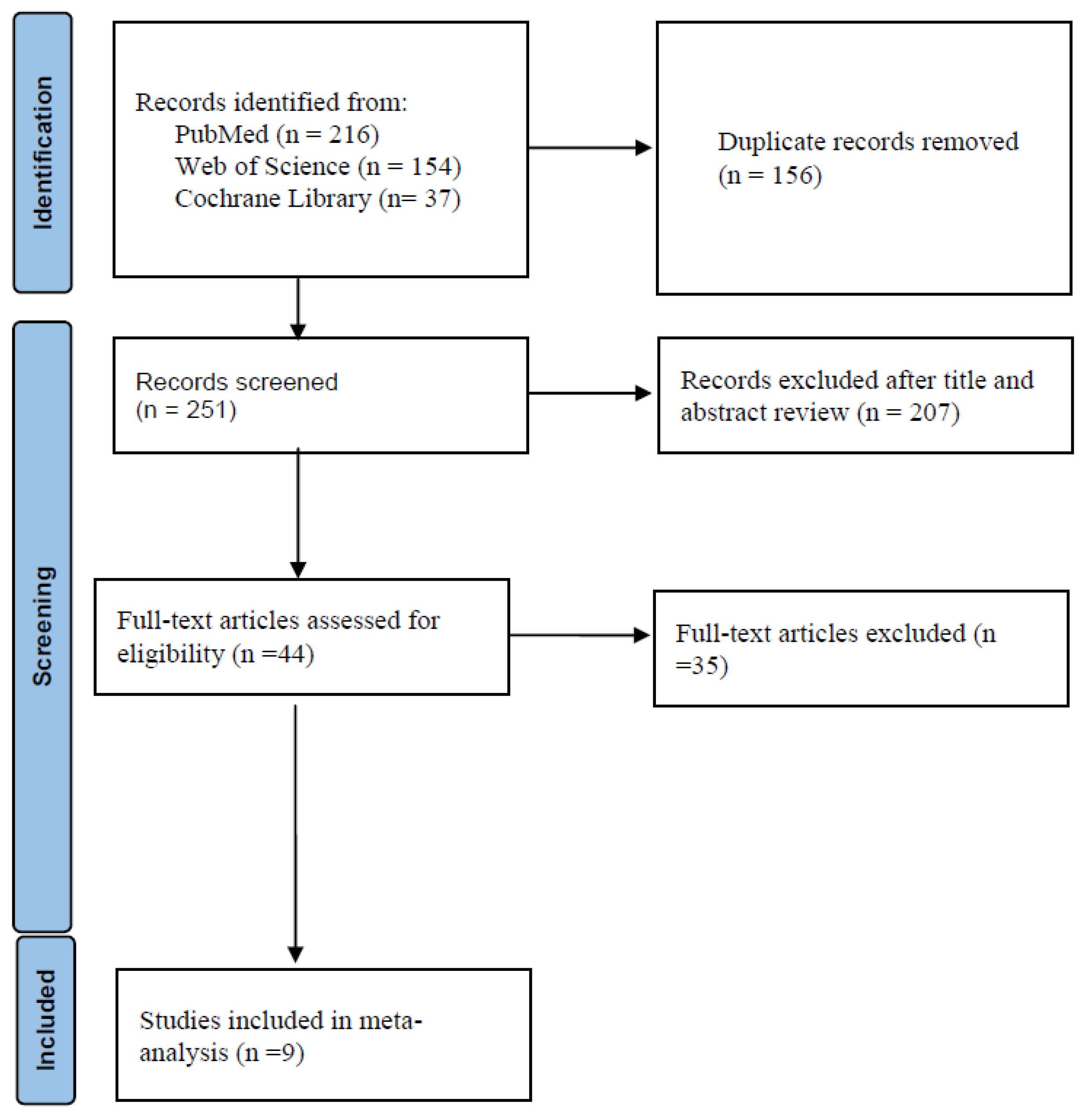
The flow diagram in
Figure 1 shows the details of the study selection process. There were 156 duplicate articles. After excluding duplicates and irrelevant studies, 44 potentially relevant articles remained. After full-text article review, 35 articles were excluded because they lacked results comparing doripenem to other antimicrobial agents in adult pneumonia patients. Finally, nine studies were included in the meta-analysis [
6,
15,
16,
17,
18,
19,
20,
21,
22]. The main characteristics of the nine included studies are shown in
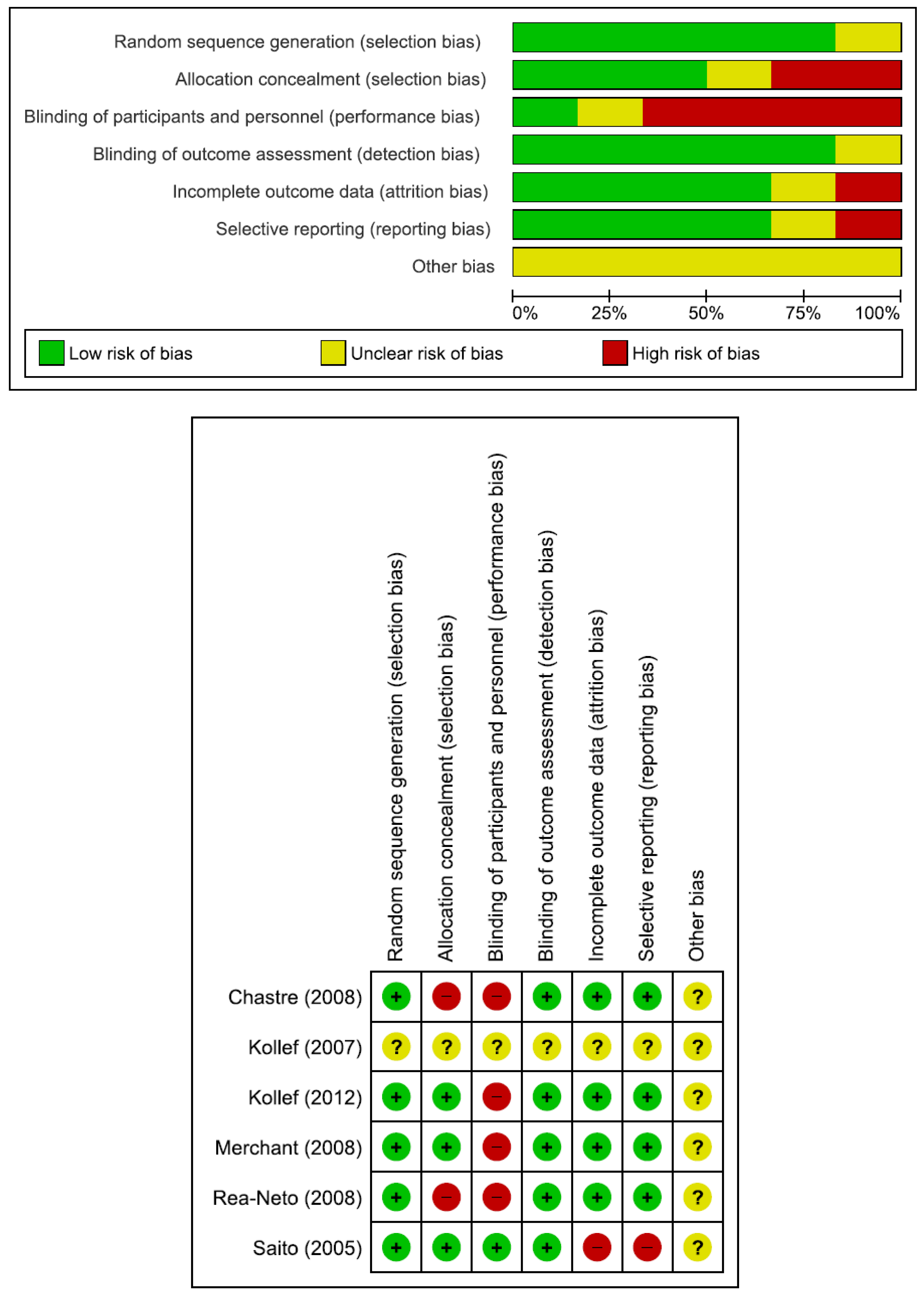
Table 1. There were 952 patients in the doripenem group and 1183 patients in the comparator group. Six were RCTs, one was prospective observational study, and two were retrospective observational studies. Nine studies compared doripenem with other antimicrobial agents, including imipenem/cilastatin in five studies, meropenem in four studies, and piperacillin/tazobactam in two studies. Seven studies had high risk of bias (
Figure 2 and
Table 2).
3.2. Efficacy and Safety Outcomes
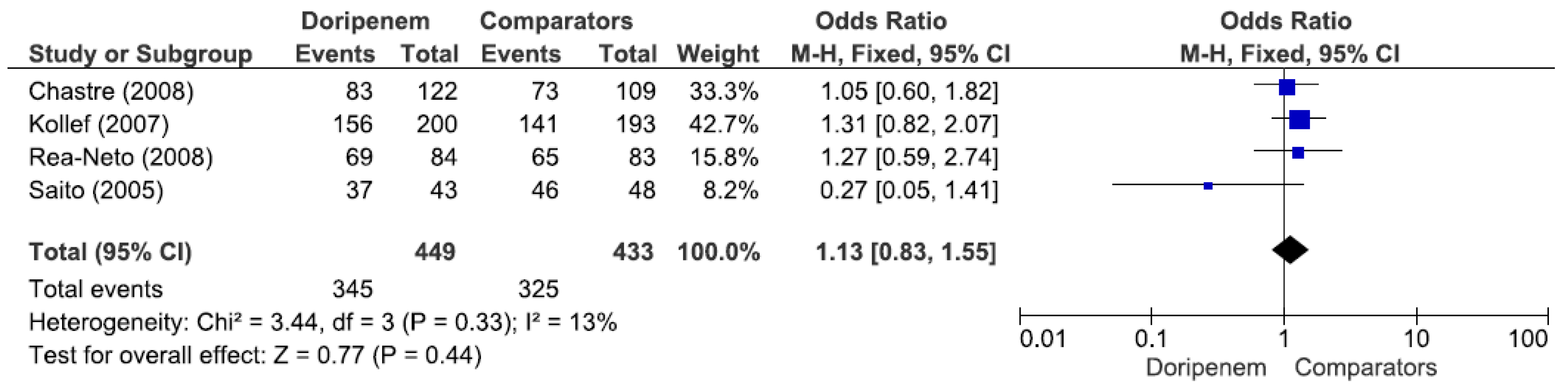
Four studies involving 882 patients (449 receiving doripenem therapy, 433 receiving other antimicrobial agent therapies) reported microbiological cure rates. There was no statistically significant difference in the microbiological cure rate between patients treated with doripenem and those treated with other antimicrobial agents (OR = 1.13, 95% CI = 0.83–1.55,
p = 0.44, I
2 = 13%) (
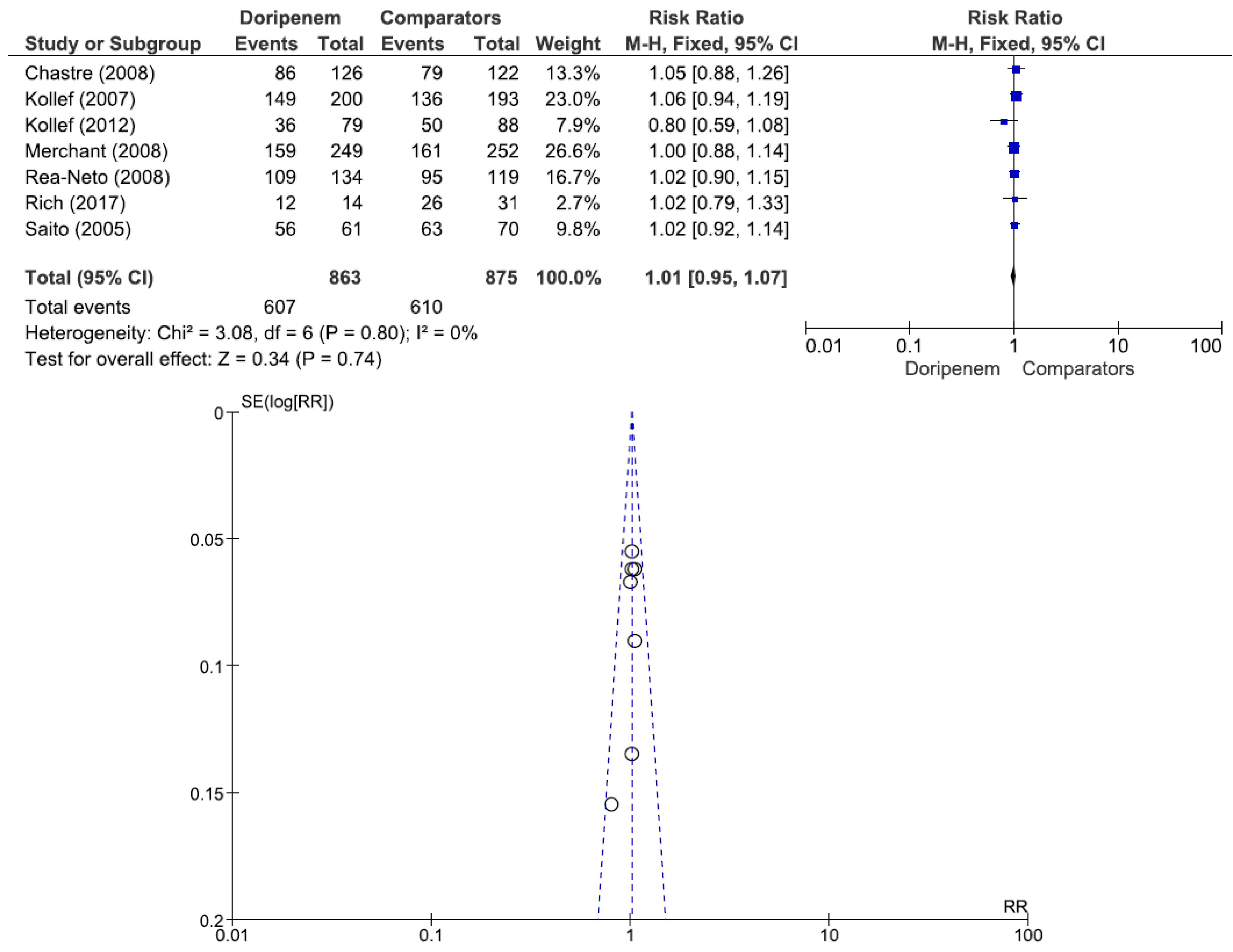
Figure 3). A total of 7 studies involving 1738 patients (863 receiving doripenem therapy, 875 receiving other antimicrobial agent therapies) reported clinical cure rates. There was no statistically significant difference in the clinical cure rate between patients treated with doripenem and those treated with other antimicrobial agents (OR = 1.01, 95% CI = 0.95–1.07,
p = 0.74, I
2 = 0%) (
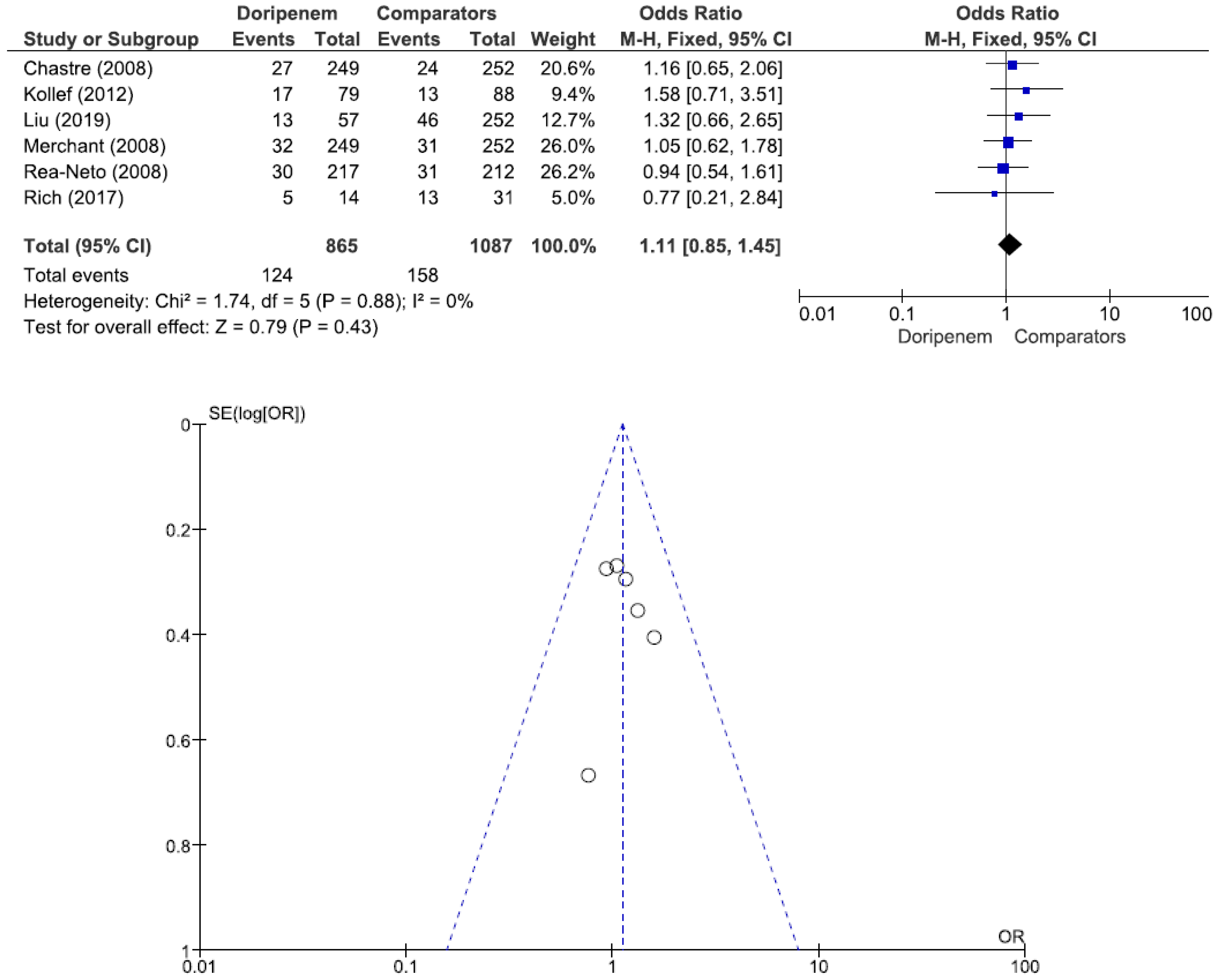
Figure 4). Six studies involving 1952 patients (865 receiving doripenem therapy, 1087 receiving other antimicrobial agent therapies) reported all-cause mortality. There was no statistically significant difference in all-cause mortality between patients treated with doripenem and those treated with other antimicrobial agents (OR = 1.11, 95% CI = 0.85–1.45,
p = 0.43, I
2 = 0%) (
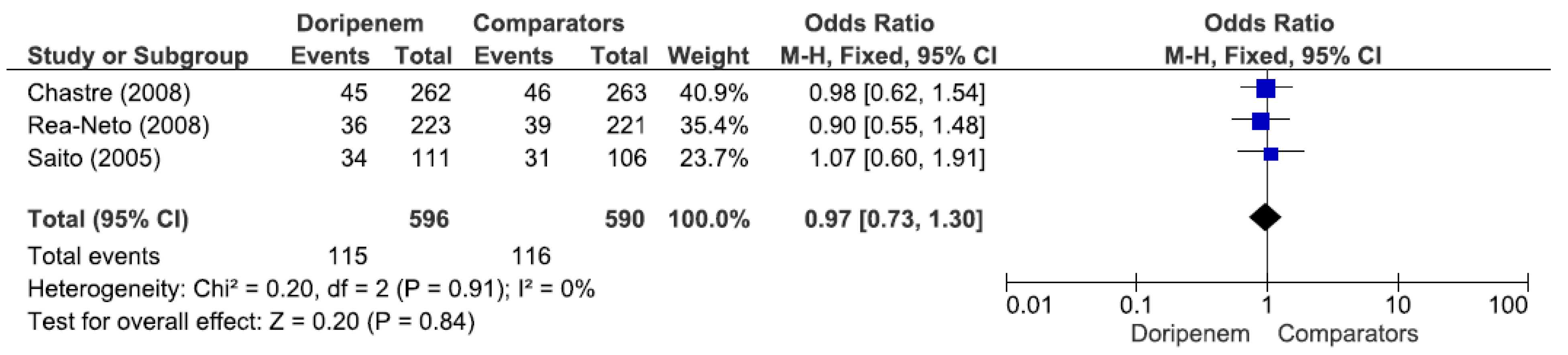
Figure 5). Three studies involving 1186 patients (596 receiving doripenem therapy, 590 receiving other antimicrobial agent therapies) reported adverse events. There was no statistically significant difference in adverse events between patients treated with doripenem and those treated with other antimicrobial agents (OR = 0.97, 95% CI = 0.73–1.30,
p = 0.84, I
2 = 0%) (
Figure 6).
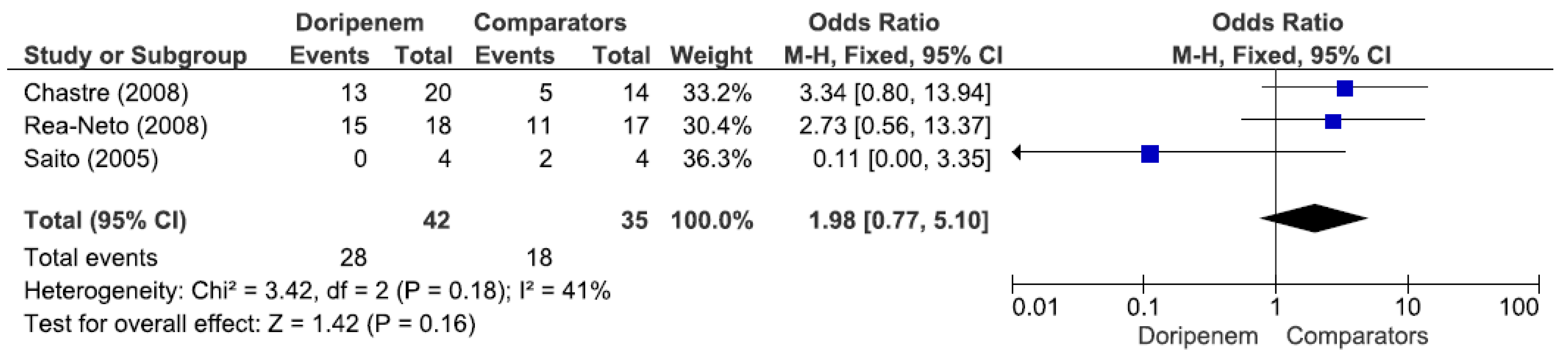
Three studies involving 77 patients (42 receiving doripenem therapy, 35 receiving other antimicrobial agent therapies) reported PA pneumonia microbiological cure rates. There was no statistically significant difference in the PA pneumonia microbiological cure rate between patients treated with doripenem and those treated with other antimicrobial agents (OR = 1.98, 95% CI = 0.77–5.10,
p = 0.16, I
2 = 41%) (
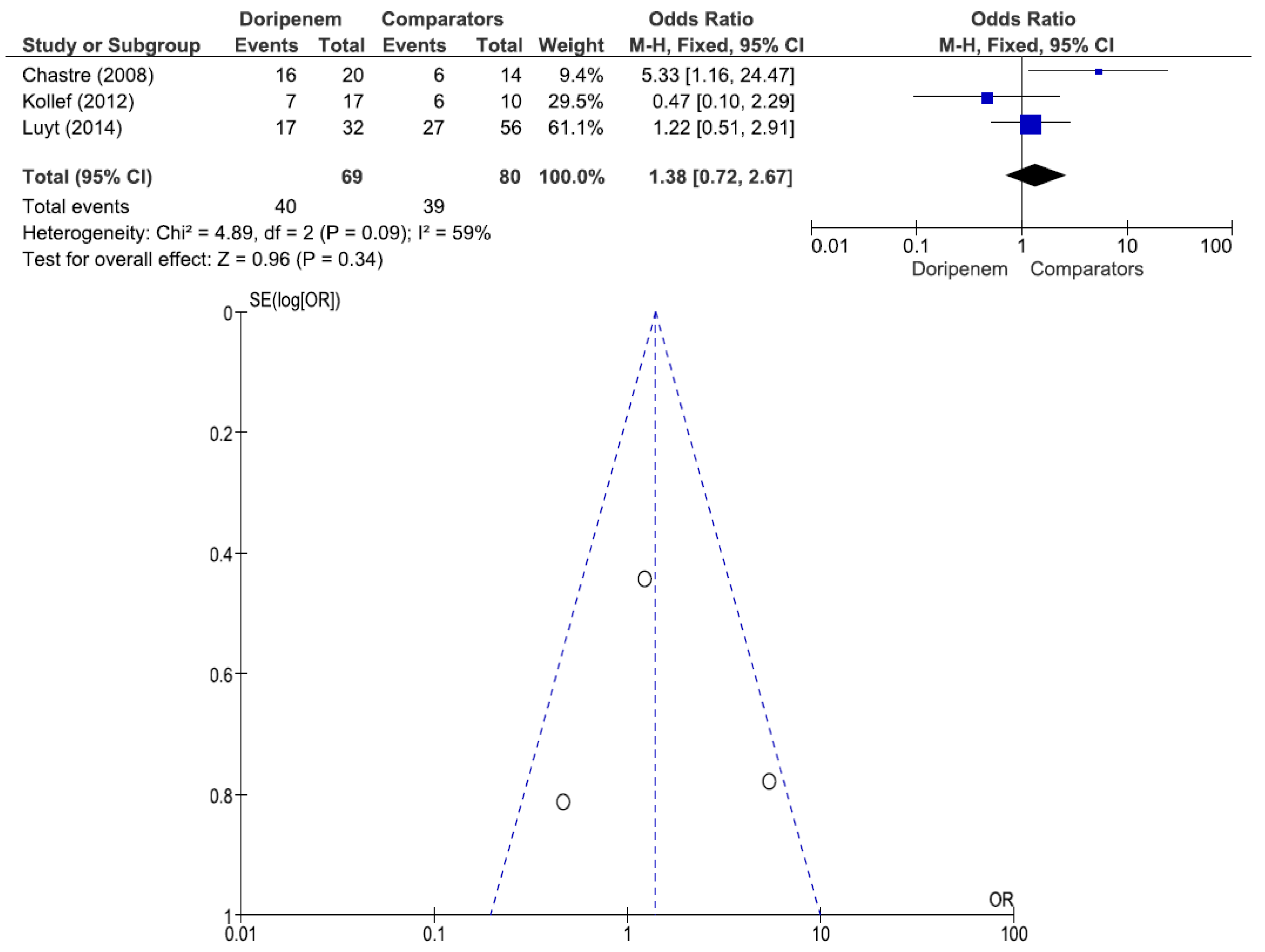
Figure 7). A total of 3 studies involving 149 patients (69 receiving doripenem therapy, 80 receiving other antimicrobial agent therapies) reported PA pneumonia clinical cure rates. There was no statistically significant difference in the PA pneumonia clinical cure rate between patients treated with doripenem and those treated with other antimicrobial agents (OR = 1.38, 95% CI = 0.72–2.67,
p = 0.34, I
2 = 59%) (
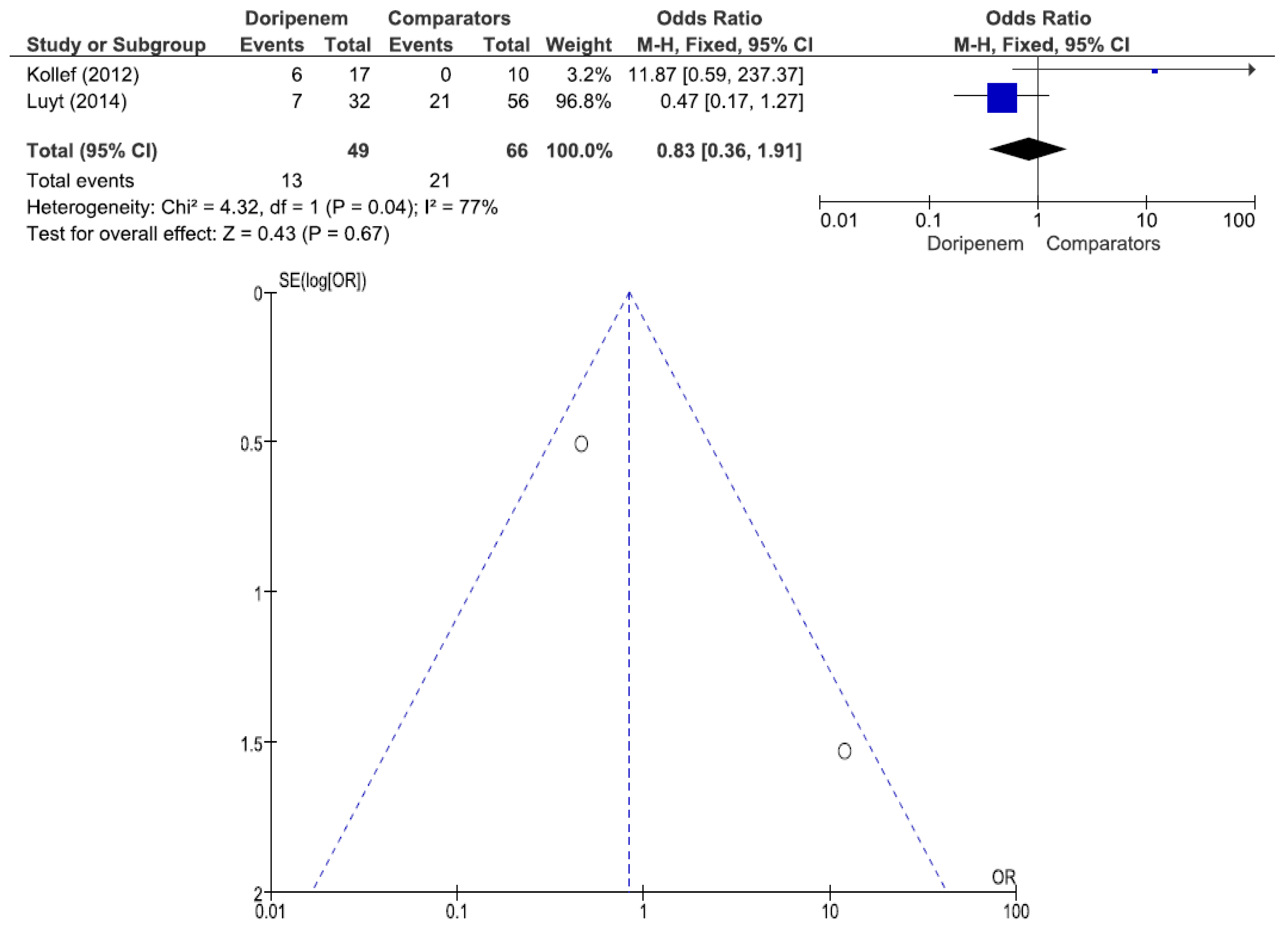
Figure 8). A total of 2 studies involving 115 patients (49 receiving doripenem therapy, 66 receiving other antimicrobial agent therapies) reported PA pneumonia all-cause mortality. There was no statistically significant difference in PA pneumonia all-cause mortality between patients treated with doripenem and those treated with other antimicrobial agents (OR = 0.83, 95% CI = 0.36–1.91,
p = 0.67, I
2 = 77%) (
Figure 9).
4. Discussion
The current meta-analysis of nine studies provides evidence that doripenem has a microbiological cure rate, a clinical cure rate, an all-cause mortality, and adverse events similar to those of comparator antimicrobial agents for the treatment of VAP and HAP. In terms of PA pneumonia, doripenem has a microbiological cure rate, a clinical cure rate, and an all-cause mortality similar to those of comparator antimicrobial agents for the treatment of PA pneumonia. There were only two meta-analyses reported in the literature that explored doripenem therapy for bacterial infections [
23,
24]. The two meta-analyses also reported that doripenem had a microbiological cure rate, a clinical cure rate, and an all-cause mortality similar to those of comparator antimicrobial agent therapies for bacterial infections. Only three references assessing nosocomial pneumonia were cited in the two meta-analyses [
6,
17,
18]. Our current meta-analysis focused on nosocomial pneumonia and cited nine references. Our study provides more reliable evidence of doripenem efficacy in patients with nosocomial pneumonia.
Regarding the microbiological cure rate, four of the nine studies reported no statistically significant difference in the microbiological cure rate of nosocomial pneumonia between the doripenem and the comparator therapy groups. The five other studies did not compare microbiological cure rates between the two groups. Regarding the clinical cure rate, six of the nine studies reported no statistically significant difference in the nosocomial pneumonia clinical cure rate between the doripenem and the comparator therapy groups. One study did not compare the clinical cure rate between the two groups. One study compared the PA pneumonia clinical cure rate between the two groups. One study reported that the clinical cure rate was lower in patients in the doripenem arm than in those in the imipenem arm (36/79 = 45.6% versus 50/88 = 56.8%; 95% CI, −26.3% to 3.8%). There was no statistically significant difference between the two groups (p = 0.165 by Fisher’s exact test). Regarding all-cause mortality, five of the nine studies reported no statistically significant difference in the all-cause mortality of nosocomial pneumonia between the doripenem and the comparator therapy groups. Two studies did not compare all-cause mortality between the two groups. One study compared PA pneumonia all-cause mortality between the two groups. One study reported that all-cause mortality was higher in patients in the doripenem arm than in those in the imipenem arm (17/79 = 21.5% versus 13/88 = 14.8%; 95% CI, −5.0 to 18.5). There was no statistically significant difference between the two groups (p = 0.314 by Fisher’s exact test). Regarding adverse events, three of the nine studies reported no statistically significant difference in adverse events between the doripenem and the comparator therapy groups. Six other studies did not compare adverse events between the two groups.
Regarding the PA pneumonia microbiological cure rate, three of the nine studies reported no statistically significant difference in the microbiological cure rate of nosocomial pneumonia between the doripenem and the comparator therapy groups. Six other studies did not compare the microbiological cure rate between the two groups. Regarding the PA pneumonia clinical cure rate, two of the nine studies reported no statistically significant difference in the clinical cure rate of nosocomial pneumonia between the doripenem and the comparator therapy groups. Six studies did not compare the clinical cure rate between the two groups. One study reported that the PA pneumonia clinical cure rate was lower in patients with Pseudomonas aeruginosa VAP in the doripenem arm than in the imipenem arm (7/17 = 41.2% versus 6/10 = 60.0%; 95% CI, −57.2 to 19.5). There was no statistically significant difference between the two groups (p = 0.440 by Fisher’s exact test). Regarding PA pneumonia all-cause mortality, one of the nine studies reported no statistically significant difference in all-cause mortality in nosocomial pneumonia patients between the doripenem and the comparator therapy groups. Seven studies did not compare all-cause mortality between the two groups. One study reported that PA pneumonia all-cause mortality was higher in patients in the doripenem arm than in those in the imipenem arm (6/17 = 35.3% versus 0/10 = 0.0%; 95% CI, 12.6 to 58.0). There was no statistically significant difference between the two groups (p = 0.057 by Fisher’s exact test). The analysis of PA pneumonia was limited by a small sample size and heterogeneity among the studies. Therefore, the evidence related to PA pneumonia was considered low quality in the current meta-analysis.
Kollef et al.’s study showed that there was no statistically significant difference in the clinical cure rate and all-cause mortality between the doripenem and the imipenem subgroups in the VAP patient group. There was also no statistically significant difference in the clinical cure rate and all-cause mortality between the doripenem therapy and imipenem therapy subgroups in the
Pseudomonas aeruginosa VAP group [
6]. The Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend the following: VAP patients should receive a seven-day course of antimicrobial therapy rather than a longer duration. There may be situations in which a shorter or a longer duration of antibiotics is indicated, depending upon the rates of improvements in clinical, radiologic, and laboratory parameters [
25]. A fixed seven-day course of doripenem therapy for VAP does not depend upon the rates of improvements in clinical, radiologic, and laboratory parameters; thus, the antibiotic course does not adhere to clinical treatment norms. Kollef noted one important limitation of their study. There were larger numbers of cases of VAP attributed to
Pseudomonas aeruginosa,
Acinetobacter baumannii, and Methicillin-resistant
Staphylococcus aureus (MRSA) infection in the doripenem arm than in the imipenem/cilastatin arm. The clinical outcome of VAP treated with a seven-day course of doripenem should be worse than that of VAP treated with a ten-day course of imipenem/cilastatin. Medical experts should re-evaluate the clinical validity of Kollef et al.’s study. However, the outcomes of the current meta-analysis of nosocomial pneumonia treatments showed that doripenem was as effective as other antimicrobial agents.
5. Limitations
Few prospective RCTs have explored this issue. We included the findings of observational studies in the current meta-analysis. Selection bias and confounding were impossible to eliminate. In addition, the numbers of included studies with certain comparisons were small. There is insufficient data for analysis on the treatment of Pseudomonas aeruginosa pneumonia, which was a limitation of this meta-analysis. A pharmacokinetic/pharmacodynamic evaluation would help to verify the effectiveness of doripenem therapy for nosocomial pneumonia. We did not explore this issue in the current meta-analysis, which was another limitation. There was low quality evidence in the current meta-analysis. However, the conclusions of this meta-analysis are similar to the conclusions of most studies in the literature.
6. Conclusions
The current meta-analysis displayed that doripenem treatment for nosocomial pneumonia was associated with a similar microbiological cure rate, clinical cure rate, all-cause mortality, and similar adverse events to comparator antimicrobial agents. However, the evidence in the current meta-analysis was of low quality, and RCTs are urgently needed to confirm the role of doripenem therapy for nosocomial pneumonia.
Author Contributions
C.H., I.C. and Y.Y. made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The meta-analysis was registered at the Prospero international prospective register of systematic reviews (registration No. CRD42022337480).
Informed Consent Statement
All of the meta-analyses in the literature are no informed consent statement is needed.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ge, Y.; Wikler, M.A.; Sahm, D.F.; Blosser-Middleton, R.S.; Karlowsky, J.A. In vitro antimicrobial activity of doripenem, a new carbapenem. Antimicrob. Agents Chemother. 2004, 48, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Huynh, H.K.; Biedenbach, D.J.; Fritsche, T.R.; Sader, H.S. Doripenem (s-4661), a novel carbapenem: Comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluations. J. Antimicrob. Chemother. 2004, 54, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Huynh, H.K.; Biedenbach, D.J. Activities of doripenem (s-4661) against drug-resistant clinical pathogens. Antimicrob. Agents Chemother. 2004, 48, 3136–3140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fritsche, T.R.; Stilwell, M.G.; Jones, R.N. Antimicrobial activity of doripenem (s-4661): A global surveillance report (2003). Clin. Microbiol. Infect. 2005, 11, 974–984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watanabe, A.; Takahashi, H.; Kikuchi, T.; Kobayashi, T.; Gomi, K.; Fujimura, S.; Tokue, Y.; Nukiwa, T. Comparative in vitro activity of s-4661, a new parenteral carbapenem, and other antimicrobial agents against respiratory pathogens. Chemotherapy 2000, 46, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Chastre, J.; Clavel, M.; Restrepo, M.I.; Michiels, B.; Kaniga, K.; Cirillo, I.; Kimko, H.; Redman, R. A randomized trial of 7-day doripenem versus 10-day imipenem–cilastatin forventilator-associated pneumonia. Crit Care 2012, 16, R218. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Statement on Recentlyterminated Clinical Trial with Doribax (Doripenem). Available online: http://www.fda.gov/Drugs/DrugSafety/ucm285883.htm (accessed on 5 January 2012).
- US Food and Drug Administration. FDA Drug Safetycommunication: FDA Approves Label Changes for AntibacterialDorbax (Doripenem) Describing Increased Risk of Death for Ventilatorpatients with Pneumonia. Available online: http://www.fda.gov/Drugs/DrugSafety/ucm387971.htm (accessed on 6 March 2014).
- European Medicines Agency Advises Doctors Treating Patients with Nosocomial Pneumonia with Doribax. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2012/06/WC500129087.pdf (accessed on 6 March 2014).
- Chao, C.M.; Chen, C.C.; Huang, H.L.; Chuang, Y.C.; Lai, C.C.; Tang, H.J. Clinical experience of patients receiving doripenem-containing regimens for the treatment of healthcare-associated infections. PLoS ONE 2016, 11, e0167522. [Google Scholar] [CrossRef][Green Version]
- Muscedere, J.G.; Day, A.; Heyland, D.K. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin. Infect. Dis. 2010, 51 (Suppl. S1), S120–S125. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Atkins, D.; Eccles, M.; Flottorp, S.; Guyatt, G.H.; Henry, D.; Hill, S.; Liberati, A.; O’Connell, D.; Oxman, A.D.; Phillips, B.; et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 2004, 4, 38. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Watanabe, A.; Nakata, K.; Odagiri, S.; Aoki, N.; Matsushima, T.; Kohno, S.; Nasu, M.; Nakashima, M.; Yamaguchi, K.; et al. Comparative study of doripenem and meropenem in respiratory infections. Phase III double-blind comparative study Jpn. J. Chemother. 2005, 53 (Suppl. 1), 185–204. (In Japanese) [Google Scholar] [CrossRef]
- Kollef, M.H.; Rello, J.; Prokocimer, P.; Lee, M.; Kaniga, K.; Friedland, I. Efficacy of doripenem in nosocomial pneumonia: Nonventilator-associated and ventilator-associated pneumonia. Chest 2007, 132, 498c–499. [Google Scholar] [CrossRef]
- Réa-Neto, A.; Niederman, M.; Lobo, S.M.; Schroeder, E.; Lee, M.; Kaniga, K.; Ketter, N.; Prokocimer, P.; Friedland, I. Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: A randomized, open-label, multicenter study. Curr. Med. Res. Opin. 2008, 24, 2113–2126. [Google Scholar] [CrossRef]
- Chastre, J.; Wunderink, R.; Prokocimer, P.; Lee, M.; Kaniga, K.; Friedland, I. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: A multicenter, randomized study. Crit. Care Med. 2008, 36, 1089–1096. [Google Scholar] [CrossRef]
- Merchant, S.; Gast, C.; Nathwani, D.; Lee, M.; Quintana, A.; Ketter, N.; Friedland, I.; Ingham, M. Hospital resource utilization with doripenem versus imipenem in the treatment of ventilator-associated pneumonia. Clin. Ther. 2008, 30, 717–733. [Google Scholar] [CrossRef]
- Luyt, C.E.; Aubry, A.; Lu, Q.; Micaelo, M.; Bréchot, N.; Brossier, F.; Brisson, H.; Rouby, J.J.; Trouillet, J.L.; Combes, A.; et al. Imipenem, meropenem, or doripenem to treat patients with Pseudomonas aeruginosa ventilator-associated pneumonia. Antimicrob. Agents Chemother. 2014, 58, 1372–1380. [Google Scholar] [CrossRef]
- Rich, R.; Montero, J. Doripenem versus meropenem for gram-negative pneumonia in critically ill patients. Crit Care Med. 2018, 46, 307. [Google Scholar] [CrossRef]
- Liu, W.D.; Shih, M.C.; Chuang, Y.C.; Wang, J.T.; Sheng, W.H. Comparative efficacy of doripenem versus meropenem for hospital-acquired and ventilator-associated pneumonia. J. Microbiol. Immunol. Infect. 2019, 52, 788–795. [Google Scholar] [CrossRef]
- Qu, X.Y.; Hu, T.T.; Zhou, W. A meta-analysis of efficacy and safety of doripenem for treating bacterial infections. Braz. J. Infect. Dis. 2015, 19, 156–162. [Google Scholar] [CrossRef]
- Lai, C.C.; Cheng, I.L.; Chen, Y.H.; Tang, H.J. The efficacy and safety of doripenem in the treatment of acute bacterial infections—A systemic review and meta-analysis of randomized controlled trials. J. Clin. Med. 2019, 8, 958. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical practice guidelines by the infectious diseases society of america and the american thoracic society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).