The Spring Distraction System for Growth-Friendly Surgical Treatment of Early Onset Scoliosis: A Preliminary Report on Clinical Results and Safety after Design Iterations in a Prospective Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Period
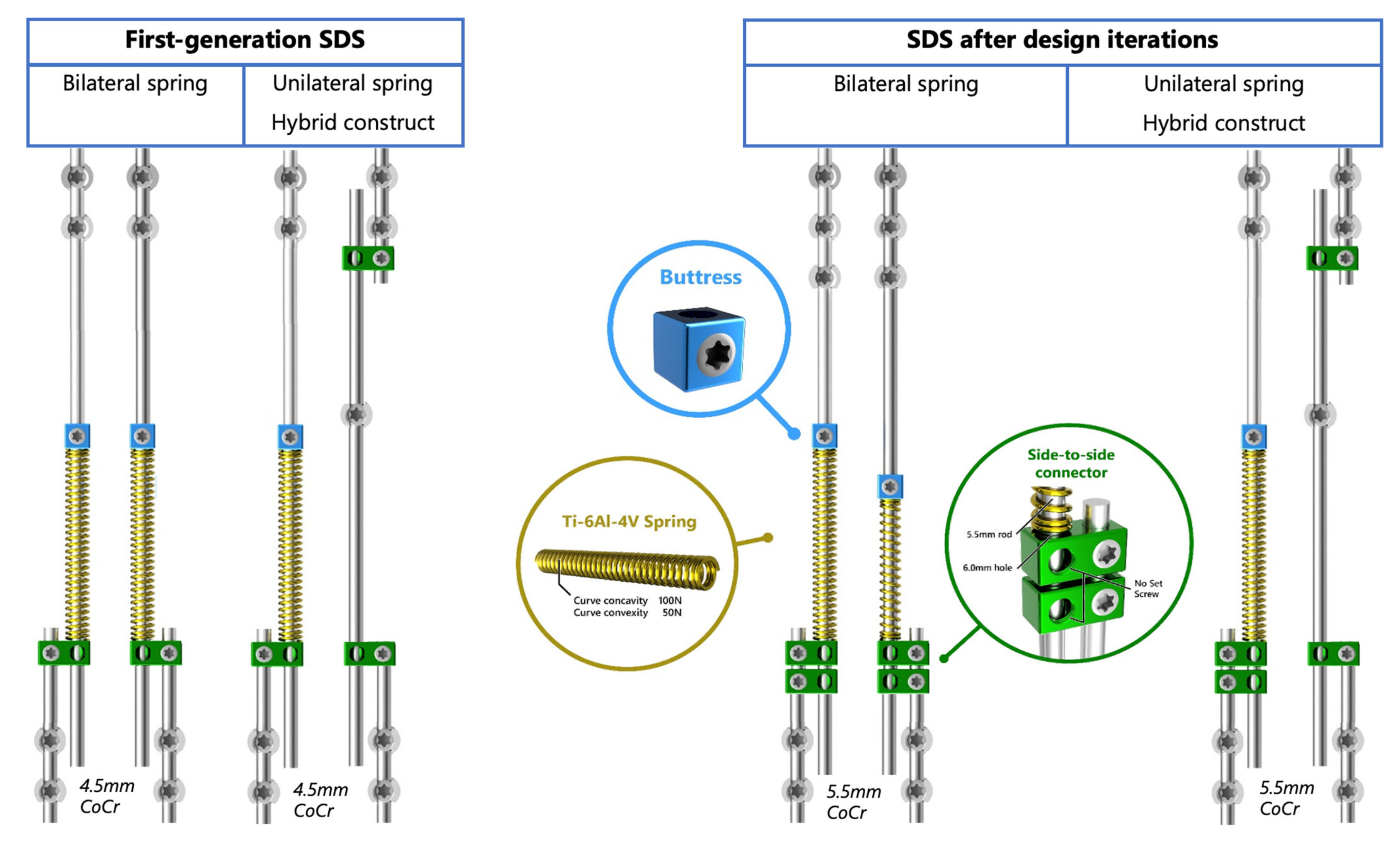
2.2. Surgical Techniques and Implant Configurations
2.3. Outcomes
2.4. Radiographic Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
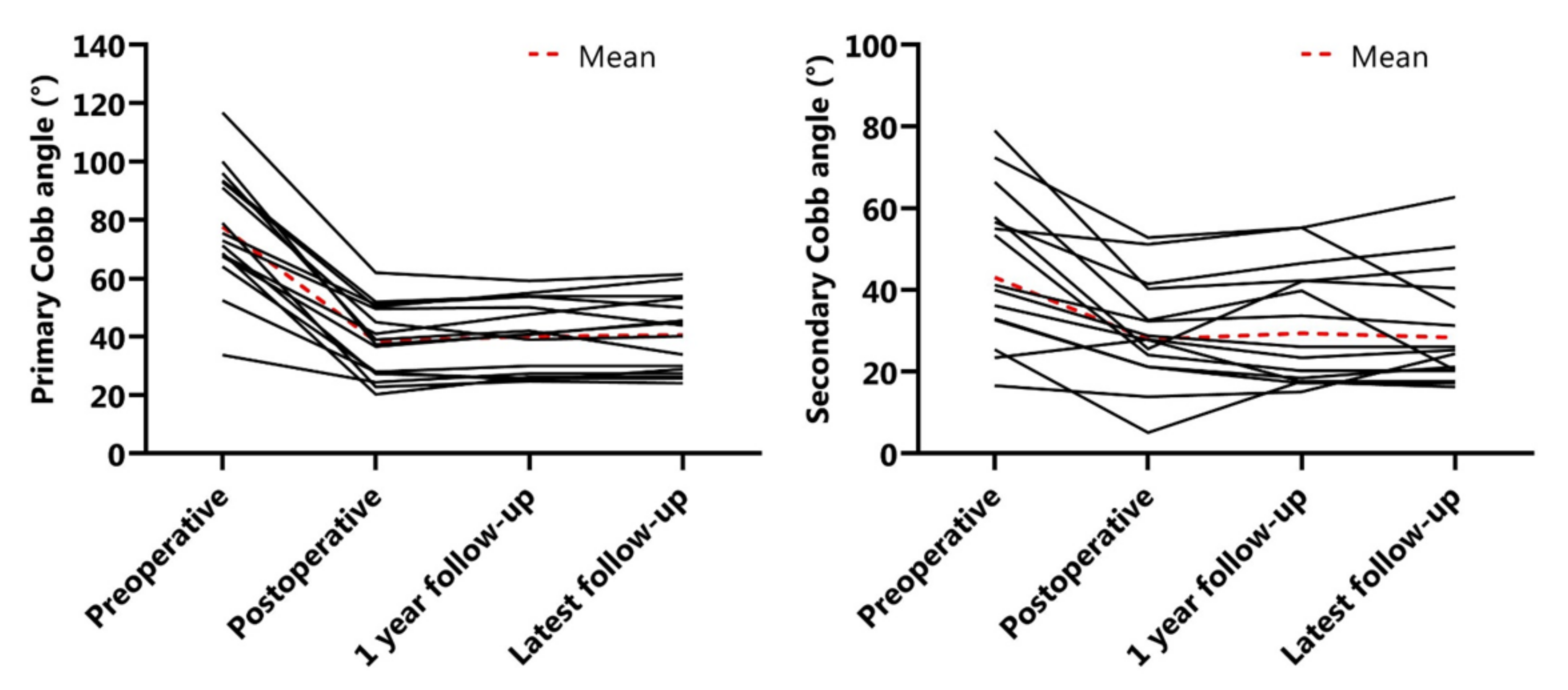
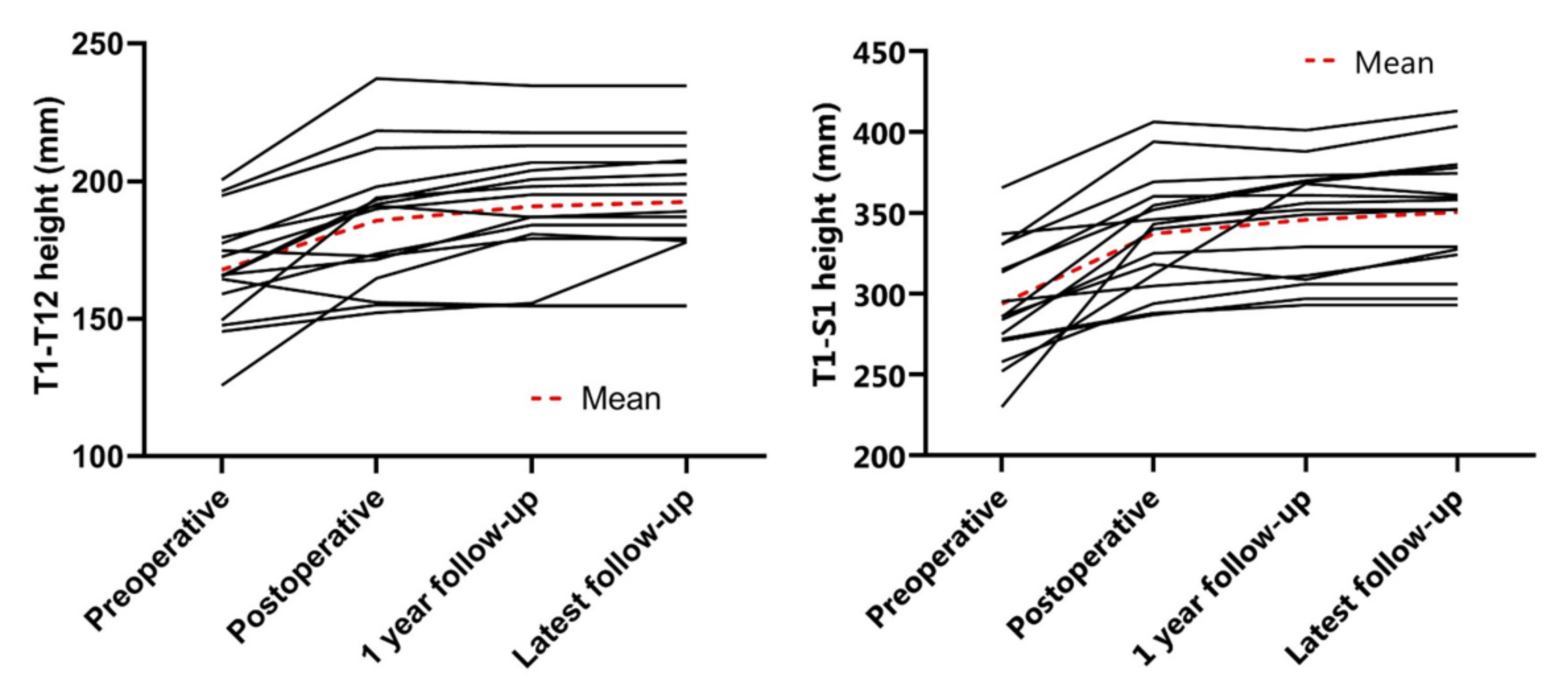
3.2. Radiographic Outcomes
3.3. Severe Adverse Events and Unplanned Returns to the Operating Room
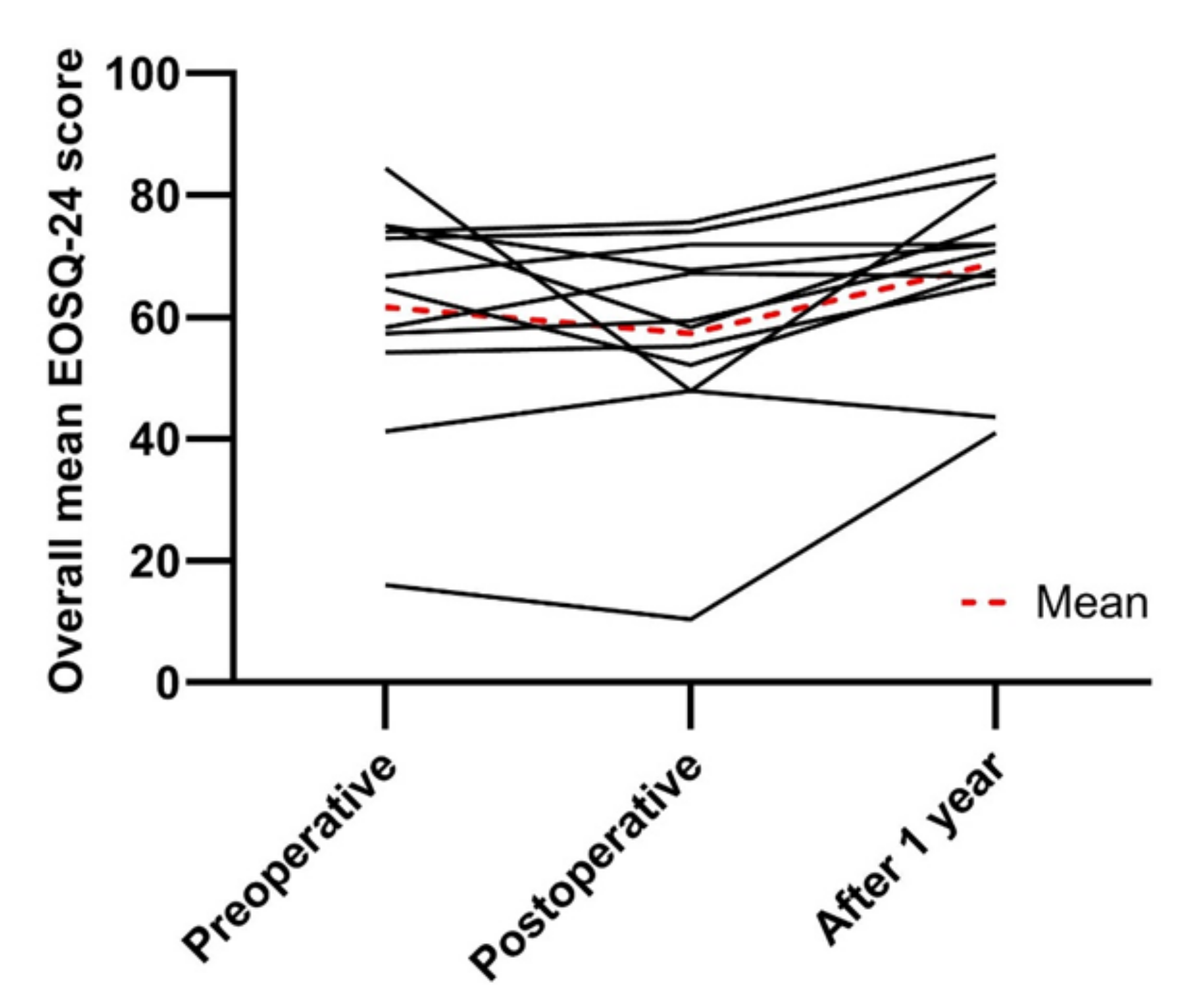
3.4. Health-Related Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.; Matsumoto, H.; McCalla, D.J.; Akbarnia, B.A.; Blakemore, L.C.; Betz, R.R.; Flynn, J.M.; Johnston, C.E.; McCarthy, R.E.; Roye, D.P.; et al. Development and Initial Validation of the Classification of Early-Onset Scoliosis (C-EOS). J. Bone Jt. Surg. 2014, 96, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Pehrsson, K.; Larsson, S.; Oden, A.; Nachemson, A. Long-term follow-up of patients with untreated scoliosis: Study of mortality, causes of death, and symptoms. Spine 1992, 17, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Pehrsson, K.; Danielsson, A.; Nachemson, A. Pulmonary function in adolescent idiopathic scoliosis: A 25 year follow up after surgery or start of brace treatment. Thorax 2001, 56, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, P.; Weinstein, S.L. Natural history of early onset scoliosis. J. Bone Jt. Surg. 2007, 89, 21–33. [Google Scholar]
- Fletcher, N.D.; McClung, A.; Rathjen, K.E.; Denning, J.R.; Browne, R.; Johnston, C.E. Serial Casting as a Delay Tactic in the Treatment of Moderate-to-Severe Early-Onset Scoliosis. J. Pediatr. Orthop. 2012, 32, 664–671. [Google Scholar] [CrossRef]
- Loughenbury, P.R.; Tsirikos, A.I. Current concepts in the treatment of neuromuscular scoliosis: Clinical assessment, treatment options, and surgical outcomes. Bone Jt. Open 2022, 3, 85–92. [Google Scholar] [CrossRef]
- Weinstein, S.L.; Dolan, L.A.; Wright, J.G.; Dobbs, M.B. Effects of Bracing in Adolescents with Idiopathic Scoliosis. N. Engl. J. Med. 2013, 369, 1512–1521. [Google Scholar] [CrossRef] [Green Version]
- LaValva, S.; Adams, A.; MacAlpine, E.; Gupta, P.; Hammerberg, K.; Thompson, G.H.; Sturm, P.; Garg, S.; Anari, J.; Sponseller, P.; et al. Serial Casting in Neuromuscular and Syndromic Early-Onset Scoliosis (EOS) Can Delay Surgery over 2 Years. J. Pediatr. Orthop. 2020, 40, e772–e779. [Google Scholar] [CrossRef]
- Nakamura, N.; Uesugi, M.; Inaba, Y.; Machida, J.; Okuzumi, S.; Saito, T. Use of dynamic spinal brace in the management of neuromuscular scoliosis: A preliminary report. J. Pediatr. Orthop. Part B 2014, 23, 291–298. [Google Scholar] [CrossRef]
- Dimeglio, A.; Canavese, F. The growing spine: How spinal deformities influence normal spine and thoracic cage growth. Eur. Spine J. 2012, 21, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Karol, L.A. The Natural History of Early-Onset Scoliosis. J. Pediatr. Orthop. 2019, 39, S38–S43. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Review Results in New Warnings about Using General Anesthetics and Sedation Drug in Young Children and Pregnant Women; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2016.
- Caldas, J.C.S.; Pais-Ribeiro, J.L.; Carneiro, S.R. General anesthesia, surgery and hospitalization in children and their effects upon cognitive, academic, emotional and sociobehavioral development—A review. Paediatr. Anaesth. 2004, 14, 910–915. [Google Scholar] [CrossRef]
- Peiro-Garcia, A.; Bourget-Murray, J.; Suarez-Lorenzo, I.; Ferri-De-Barros, F.; Parsons, D. Early Complications in Vertical Expandable Prosthetic Titanium Rib and Magnetically Controlled Growing Rods to Manage Early Onset Scoliosis. Int. J. Spine Surg. 2021, 15, 368–375. [Google Scholar] [CrossRef]
- Akbarnia, B.A.; Cheung, K.; Noordeen, H.; Elsebaie, H.; Yazici, M.; Dannawi, Z.; Kabirian, N. Next Generation of Growth-Sparing Techniques: Preliminary Clinical Results of a Magnetically Controlled Growing Rod in 14 Patients with Early-Onset Scoliosis. Spine 2013, 38, 665–670. [Google Scholar] [CrossRef]
- Cheung, K.M.-C.; Cheung, J.P.-Y.; Samartzis, D.; Mak, K.-C.; Wong, Y.-W.; Cheung, W.-Y.; Akbarnia, A.B.; Luk, K.D.-K. Magnetically controlled growing rods for severe spinal curvature in young children: A prospective case series. Lancet 2012, 379, 1967–1974. [Google Scholar] [CrossRef] [Green Version]
- Kwan, K.Y.H.; Alanay, A.; Yazici, M.; Demirkiran, G.; Helenius, I.; Nnadi, C.; Ferguson, J.; Akbarnia, B.A.; Cheung, J.P.Y.; Cheung, K. Unplanned Reoperations in Magnetically Controlled Growing Rod Surgery for Early Onset Scoliosis with a Minimum of Two-Year Follow-Up. Spine 2017, 42, E1410–E1414. [Google Scholar] [CrossRef]
- Teoh, K.H.; Winson, D.M.; James, S.H.; Jones, A.; Howes, J.; Davies, P.R.; Ahuja, S. Do magnetic growing rods have lower complication rates compared with conventional growing rods? Spine J. 2016, 16, S40–S44. [Google Scholar] [CrossRef]
- Bess, S.; Akbarnia, B.A.; Thompson, G.H.; Sponseller, P.D.; Shah, S.A.; El Sebaie, H.; Hazem, F.R.C.S.; Karlin, L.I.; Connie, R.N.; Skaggs, D.L.; et al. Complications of growing-rod treatment for early-onset scoliosis: Analysis of one hundred and forty patients. J. Bone Jt. Surg. Am. 2010, 92, 2533–2543. [Google Scholar] [CrossRef]
- Akbarnia, B.A.; Pawelek, J.B.; Cheung, K.; Demirkiran, G.; Elsebaie, H.B.; Emans, J.B.; Johnston, C.E.; Mundis, G.M.; Noordeen, H.; Skaggs, D.L.; et al. Traditional Growing Rods versus Magnetically Controlled Growing Rods for the Surgical Treatment of Early-Onset Scoliosis: A Case-Matched 2-Year Study. Spine Deform. 2014, 2, 493–497. [Google Scholar] [CrossRef]
- Thakar, C.; Kieser, D.C.; Mardare, M.; Haleem, S.; Fairbank, J.; Nnadi, C. Systematic review of the complications associated with magnetically controlled growing rods for the treatment of early onset scoliosis. Eur. Spine J. 2018, 27, 2062–2071. [Google Scholar] [CrossRef]
- Joyce, T.; Smith, S.L.; Rushton, P.; Bowey, A.J.; Gibson, M.J. Analysis of Explanted Magnetically Controlled Growing Rods From Seven UK Spinal Centers. Spine 2018, 43, E16–E22. [Google Scholar] [CrossRef] [PubMed]
- Hothi, H. The impact and surgeon perceptions of the suspension of the CE certification of MAGEC devices on clinical practice. Bone Jt. Open 2022, 3, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.C.; Nafo, W.; Lu, W.W.; Cheung, K.M.C. A biomechanical study on the effect of lengthening magnitude on spine off-loading in magnetically controlled growing rod surgery: Implications on lengthening frequency. J. Orthop. Surg. 2021, 29, 23094990211042237. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Subramanian, T.; Panteliadis, P.; Wilson-Macdonald, J.; Rothenfluh, D.A.; Nnadi, C. Quantifying the “law of diminishing returns” in magnetically controlled growing rods. Bone Jt. J. 2017, 99, 1658–1664. [Google Scholar] [CrossRef]
- Sankar, W.N.; Skaggs, D.L.; Yazici, M.; Johnston, C.E.; Shah, S.A.; Javidan, P.; Kadakia, R.V.; Day, T.F.; Akbarnia, B.A. Lengthening of dual growing rods and the law of diminishing returns. Spine 2011, 36, 806–809. [Google Scholar] [CrossRef]
- Wijdicks, S.P.J.; Lemans, J.V.C.; Verkerke, G.J.; Noordmans, H.J.; Castelein, R.M.; Kruyt, M.C. The potential of spring distraction to dynamically correct complex spinal deformities in the growing child. Eur. Spine J. 2021, 30, 714–723. [Google Scholar] [CrossRef]
- Lemans, J.V.; Wijdicks, S.P.; Castelein, R.M.; Kruyt, M.C. Spring distraction system for dynamic growth guidance of early onset scoliosis: Two-year prospective follow-up of 24 patients. Spine J. 2020, 21, 671–681. [Google Scholar] [CrossRef]
- Lemans, J.V.C.; Tabeling, C.S.; Castelein, R.M.; Kruyt, M.C. Identifying complications and failure modes of innovative growing rod configurations using the (hybrid) magnetically controlled growing rod (MCGR) and the spring distraction system (SDS). Spine Deform. 2021, 9, 1679–1689. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Skov, S.T.; Wijdicks, S.P.; Bünger, C.; Castelein, R.M.; Li, H.; Kruyt, M.C. Treatment of early-onset scoliosis with a hybrid of a concave magnetic driver (magnetic controlled growth rod) and a contralateral passive sliding rod construct with apical control: Preliminary report on 17 cases. Spine J. 2018, 18, 122–129. [Google Scholar] [CrossRef]
- Matsumoto, H.; Williams, B.; Park, H.Y.; Yoshimachi, J.Y.; Roye, B.D.; Roye, D.P.; Akbarnia, B.A.; Emans, J.; Skaggs, D.; Smith, J.T.; et al. The Final 24-Item Early Onset Scoliosis Questionnaires (EOSQ-24): Validity, Reliability and Responsiveness. J. Pediatr. Orthop. 2018, 38, 144–151. [Google Scholar] [CrossRef]
- Wijdicks, S.P.; Dompeling, S.D.; de Reuver, S.; Kempen, D.H.; Castelein, R.M.; Kruyt, M.C. Reliability and Validity of the Adapted Dutch Version of the Early-Onset Scoliosis-24-Item Questionnaire (EOSQ-24). Spine 2019, 44, E965–E973. [Google Scholar] [CrossRef]
- Wijdicks, S.P.; Tromp, I.N.; Yazici, M.; Kempen, D.H.; Castelein, R.M.; Kruyt, M.C. A comparison of growth among growth-friendly systems for scoliosis: A systematic review. Spine J. 2019, 19, 789–799. [Google Scholar] [CrossRef]
- Choi, E.; Yaszay, B.; Mundis, G.; Hosseini, P.; Pawelek, J.; Alanay, A.; Berk, H.; Cheung, K.; Demirkiran, G.; Ferguson, J.; et al. Implant Complications after Magnetically Controlled Growing Rods for Early Onset Scoliosis: A Multicenter Retrospective Review. J. Pediatr. Orthop. 2017, 37, e588–e592. [Google Scholar] [CrossRef]


| Patient Characteristics | |
|---|---|
| Patients | 17 (7 female) |
| Age at surgery (years) | 9.5 ± 2.5 |
| EOS etiology | |
| Congenital | 3 |
| Idiopathic | 4 |
| Neuromuscular | 9 |
| Syndromic | 1 |
| Surgery time skin-to-skin (minutes) | 169 (range: 100–240) * (N = 17) |
| Blood loss (milliliter) | 395 (range: 100–700) † (N = 17) |
| Time to discharge (days) | 5 (range: 4–7) |
| Mean follow-up (years) | 1.9 ± 0.5 |
| Implant configuration (N) | |
| Concave + convex springs | 9 |
| Concave spring + convex apical screw | 6 |
| Concave spring + convex epiphysiodesis | 1 |
| Unilateral concave spring distraction only | 1 |
| Preoperative | Postoperative | After 1 Year | Latest Follow-Up | |
|---|---|---|---|---|
| Primary Cobb angle (°) | 78 ± 20 | 38 ± 12 | 40 ± 12 | 41 ± 13 |
| Secondary Cobb angle (°) | 43 ± 21 | 28 ± 15 | 29 ± 16 | 28 ± 15 |
| T5–T12 kyphosis (°) | 33 ± 19 | 22 ± 12 | 23 ± 14 | 22 ± 17 |
| L1–S1 lordosis (°) | 54 ± 16 | 47 ± 15 | 44 ± 20 | 51 ± 17 |
| T1–T12 height (mm) | 167.8 ± 20.0 | 185.7 ± 24.1 | 190.8 ± 22.9 | 192.5 ± 21.5 |
| T1–S1 height (mm) | 293.8 ± 35.8 | 337.2 ± 35.8 | 345.8 ± 33.9 | 350.5 ± 35.6 |
| Patient | Sex | Age at SAE | Underlying Disease | Initial Surgery | SAEs | UPRORs and Treatment |
|---|---|---|---|---|---|---|
| P03 | F | 9.1 years | VACTERL association | SDS T2-L1 Bilateral hemivertebra resection and unilateral hemiepiphysiodesis T7-L1 | Adding on above proximal anchor | Extension to C4 |
| P06 | F | 11.2 years | Microcephalus | SDS T2-L4 | Adding on below distal anchor | Extension to L5 |
| P12 | M | 10.2 years | Myelomeningocele; Chiari II malformation | SDS T2-Ilium | SSI after initial surgery | Spring retension |
| Preoperative | Postoperative | After 1 Year | |
|---|---|---|---|
| General health | 67.7 ± 27.4 | 66.7 ± 17.9 | 70.8 ± 18.7 |
| Pain/discomfort | 60.4 ± 21.2 | 53.6 ± 19.7 | 61.5 ± 19.6 |
| Pulmonary function | 80.2 ± 25.8 | 78.1 ± 28.8 | 89.6 ± 13.9 |
| Transfer | 66.7 ± 30.8 | 57.3 ± 25.3 | 79.2 ± 23.4 |
| Physical function | 55.6 ± 31.8 | 46.5 ± 29.0 | 57.6 ± 31.5 |
| Daily living | 30.2 ± 24.1 | 30.2 ± 27.9 | 36.5 ± 25.3 |
| Fatigue/energy level | 67.7 ± 30.4 | 51.0 ± 27.4 | 66.7 ± 24.6 |
| Emotion | 62.5 ± 18.5 | 56.3 ± 18.8 | 74.0 ± 24.1 |
| Parental burden | 60.8 ± 26.2 | 60.0 ± 20.8 | 73.3 ± 19.0 |
| Financial burden | 83.3 ± 24.6 | 83.3 ± 30.8 | 93.8 ± 15.5 |
| Overall satisfaction | 62.5 ± 26.7 | 61.5 ± 17.2 | 70.8 ± 15.4 |
| Overall mean score | 61.6 ± 18.5 | 57.3 ± 17.7 | 68.9 ± 14.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabeling, C.S.; Lemans, J.V.C.; Top, A.; Scholten, E.P.; Stempels, H.W.; Schlösser, T.P.C.; Ito, K.; Castelein, R.M.; Kruyt, M.C. The Spring Distraction System for Growth-Friendly Surgical Treatment of Early Onset Scoliosis: A Preliminary Report on Clinical Results and Safety after Design Iterations in a Prospective Clinical Trial. J. Clin. Med. 2022, 11, 3747. https://doi.org/10.3390/jcm11133747
Tabeling CS, Lemans JVC, Top A, Scholten EP, Stempels HW, Schlösser TPC, Ito K, Castelein RM, Kruyt MC. The Spring Distraction System for Growth-Friendly Surgical Treatment of Early Onset Scoliosis: A Preliminary Report on Clinical Results and Safety after Design Iterations in a Prospective Clinical Trial. Journal of Clinical Medicine. 2022; 11(13):3747. https://doi.org/10.3390/jcm11133747
Chicago/Turabian StyleTabeling, Casper S., Justin V. C. Lemans, Anouk Top, E. Pauline Scholten, Hilde W. Stempels, Tom P. C. Schlösser, Keita Ito, René M. Castelein, and Moyo C. Kruyt. 2022. "The Spring Distraction System for Growth-Friendly Surgical Treatment of Early Onset Scoliosis: A Preliminary Report on Clinical Results and Safety after Design Iterations in a Prospective Clinical Trial" Journal of Clinical Medicine 11, no. 13: 3747. https://doi.org/10.3390/jcm11133747
APA StyleTabeling, C. S., Lemans, J. V. C., Top, A., Scholten, E. P., Stempels, H. W., Schlösser, T. P. C., Ito, K., Castelein, R. M., & Kruyt, M. C. (2022). The Spring Distraction System for Growth-Friendly Surgical Treatment of Early Onset Scoliosis: A Preliminary Report on Clinical Results and Safety after Design Iterations in a Prospective Clinical Trial. Journal of Clinical Medicine, 11(13), 3747. https://doi.org/10.3390/jcm11133747







