Risk Factors for Postsurgical Gout Flares after Thoracolumbar Spine Surgeries
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuo, C.-F.; Grainge, M.J.; Mallen, C.; Zhang, W.; Doherty, M. Rising burden of gout in the UK but continuing suboptimal management: A nationwide population study. Ann. Rheum. Dis. 2014, 74, 661–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Care Res. 2011, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-F.; Grainge, M.J.; See, L.-C.; Yu, K.-H.; Luo, S.-F.; Zhang, W.; Doherty, M. Epidemiology and management of gout in Taiwan: A nationwide population study. Arthritis Res. Ther. 2015, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.C.; Pillinger, M.H.; Keenan, R.T. Inpatient Gout: A Review. Curr. Rheumatol. Rep. 2014, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Sarkin, A.; Shieh, M.; Khanna, D.; Terkeltaub, R.; Lee, S.; Kavanaugh, A.; Hirsch, J. Health Care Utilization in Patients with Gout. Semin. Arthritis Rheum. 2011, 40, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Roberts, L. Healthcare burden of in-hospital gout. Intern. Med. J. 2012, 42, 1261–1263. [Google Scholar] [CrossRef]
- Friedman, J.E.; Dallal, R.M.; Lord, J.L. Gouty attacks occur frequently in postoperative gastric bypass patients. Surg. Obes. Relat. Dis. 2008, 4, 11–13. [Google Scholar] [CrossRef]
- Zhuo, Y.; Cai, X.; Hou, Z.; Zhu, Z.; Cai, D. Postoperative Recurrent Gout Flares: A Cross-sectional Study from China. JCR J. Clin. Rheumatol. 2020, 26, 197–203. [Google Scholar] [CrossRef]
- Jeong, H.; Jeon, C.H. Clinical characteristics and risk factors for gout flare during the postsurgical period. Adv. Rheumatol. 2019, 59, 31. [Google Scholar] [CrossRef] [Green Version]
- Craig, M.H.; Poole, G.V.; Hauser, C.J. Postsurgical gout. Am. Surg. 1995, 61, 56–59. [Google Scholar]
- Kang, E.H.; Lee, E.Y.; Lee, Y.J.; Song, Y.W. Clinical features and risk factors of postsurgical gout. Ann. Rheum. Dis. 2007, 67, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, E.I.; Quion-Verde, H.; Kaptein, E.M.; Massry, S.G. Severe Hyperuricemia in Patients with Volume Depletion. Am. J. Nephrol. 1984, 4, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.; Schneiderhan, J.; Zick, S.M. Diets for Health: Goals and Guidelines. Am. Fam. Physician 2018, 97, 721–728. [Google Scholar]
- Khanna, D.; Khanna, P.P.; Fitzgerald, J.D.; Singh, M.K.; Bae, S.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S.; et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: Therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012, 64, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020, 72, 744–760. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Rao, P.J.; Kam, A.C.; Mobbs, R.J. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: Systematic review and meta-analysis. Eur. Spine J. 2015, 24, 1017–1030. [Google Scholar] [CrossRef]
- Khan, N.R.; Clark, A.J.; Lee, S.L.; Venable, G.T.; Rossi, N.B.; Foley, K.T. Surgical Outcomes for Minimally Invasive vs Open Transforaminal Lumbar Interbody Fusion. Neurosurgery 2015, 77, 847–874. [Google Scholar] [CrossRef] [Green Version]
- McAdams-DeMarco, M.A.; Maynard, J.W.; Coresh, J.; Baer, A.N. Anemia and the onset of gout in a population-based cohort of adults: Atherosclerosis Risk in Communities study. Arthritis Res. Ther. 2012, 14, R193. [Google Scholar] [CrossRef] [Green Version]
- Eun, Y.; Han, K.-D.; Kim, D.H.; Kim, I.Y.; Park, E.-J.; Lee, S.; Cha, H.-S.; Koh, E.-M.; Lee, J.; Kim, H. Association between anemia and hyperuricemia: Results from the Korean National Health and Nutrition Examination Survey. Sci. Rep. 2019, 9, 19067. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D. Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin. Nephrol. 2004, 24, 469–473. [Google Scholar] [CrossRef]
- Choi, H.K.; Mount, D.B.; Reginato, A.M. Pathogenesis of Gout. Ann. Intern. Med. 2005, 143, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Jatuworapruk, K.; Grainger, R.; Dalbeth, N.; Taylor, W.J. Development of a prediction model for inpatient gout flares in people with comorbid gout. Ann. Rheum. Dis. 2019, 79, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Asada, A.; Kasahara, E.; Sato, E.F.; Shindo, M.; Inoue, M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation 2002, 105, 1155–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Alcohol intake and risk of incident gout in men: A prospective study. Lancet 2004, 363, 1277–1281. [Google Scholar] [CrossRef]
- Neogi, T.; Chen, C.; Niu, J.; Chaisson, C.; Hunter, D.J.; Zhang, Y. Alcohol Quantity and Type on Risk of Recurrent Gout Attacks: An Internet-based Case-crossover Study. Am. J. Med. 2014, 127, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.-H.; Chen, D.-Y.; Chen, J.-H.; Chen, S.-Y.; Chen, S.-M.; Cheng, T.-T.; Hsieh, S.-C.; Hsieh, T.-Y.; Hsu, P.-F.; Kuo, C.-F.; et al. Management of gout and hyperuricemia: Multidisciplinary consensus in Taiwan. Int. J. Rheum. Dis. 2018, 21, 772–787. [Google Scholar] [CrossRef]

| All (n = 128) | Flare-Up (n = 56) | No Flare (n = 72) | p Value | |
|---|---|---|---|---|
| Age (years) | 64.8 ± 11.7 | 66.2 ± 9.8 | 63.9 ± 13.0 | 0.27 |
| Sex (men:women) | 102:26 | 48:8 | 54:18 | 0.10 |
| BMI (kg/m2) | 27.8 ± 4.3 | 28.4 ± 4.4 | 27.1 ± 4.0 | 0.11 |
| Comorbidities (number of patients) | ||||
| Hypertension | 86 (67%) | 37 (66%) | 49 (68%) | 0.81 |
| Diabetes mellitus | 37 (29%) | 16 (29%) | 21 (29%) | 0.94 |
| Hyperlipidemia | 22 (17%) | 5 (9%) | 17 (24%) | 0.03 * |
| Cardiovascular disease | 22 (17%) | 9 (16%) | 13 (18%) | 0.77 |
| Chronic kidney disease | 7 (5%) | 2 (4%) | 5 (7%) | 0.41 |
| COPD | 5 (4%) | 3 (5%) | 2 (3%) | 0.45 |
| Malignancy | 6 (5%) | 2 (4%) | 4 (6%) | 0.70 |
| Gout medication use (number of patients) | 66 (52%) | 20 (36%) | 46 (64%) | 0.002 * |
| Benzbromarone | 28 (22%) | 9 (16%) | 19 (26%) | |
| Allopurinol | 15 (12%) | 4 (7%) | 11 (15%) | |
| Febuxostat | 13 (10%) | 3 (5%) | 10 (14%) | |
| Colchicine | 12 (9%) | 4 (7%) | 8 (11%) | |
| Sulfinpyrazone | 1 (1%) | 1 (2%) | - | |
| Probenecid | 1 (1%) | - | 1 (1%) | |
| Diuretic use (number of patients) | 23 (18%) | 8 (14%) | 15 (21%) | 0.34 |
| Smoking (number of patients) | 52 (41%) | 30 (54%) | 22 (31%) | 0.01 * |
| Alcohol use (number of patients) | 4 (3%) | 1 (2%) | 3 (4%) | 0.63 |
| All (n = 128) | Flare-Up (n = 56) | No Flare (n = 72) | p Value | |
|---|---|---|---|---|
| Operative time (min) | 235 ± 98 | 237 ± 94 | 235 ± 106 | 0.90 |
| Type of surgery | 0.80 | |||
| Decompression or discectomy | 13 (10%) | 6 (11%) | 7 (10%) | |
| Instrumentation without fusion | 20 (16%) | 8 (14%) | 12 (17%) | |
| Instrumentation & MISS fusion | 10 (8%) | 3 (5%) | 7 (10%) | |
| Instrumentation & open fusion | 85 (66%) | 39 (70%) | 46 (64%) | |
| Number of operated levels | 3.3 ± 1.1 | 3.3 ± 1.0 | 3.3 ± 1.2 | 0.90 |
| Number of operations with instrumentation | 115 (90%) | 50 (89%) | 65 (90%) | 0.83 |
| Number of operations with fusion | 92 (72%) | 40 (71%) | 52 (72%) | 0.98 |
| MISS procedure | 15 (12%) | 4 (7%) | 11 (15%) | 0.25 |
| Revision surgery | 20 (16%) | 9 (16%) | 12 (17%) | 0.93 |
| Intraoperative blood loss (mL) | 620 ± 430 | 626 ± 389 | 616 ± 461 | 0.90 |
| Intraoperative fluid intake (mL) | 2316 ± 1235 | 2369 ± 1232 | 2279 ± 1258 | 0.73 |
| Intraoperative transfusion (number of patients) | 55 (43%) | 25 (45%) | 31 (43%) | 0.86 |
| Postoperative transfusion (number of patients) | 16 (13%) | 10 (18%) | 6 (8%) | 0.12 |
| Preoperative Hb (g/dL) | 13.2 ± 1.9 | 12.8 ± 2.1 | 13.6 ± 1.8 | 0.02 * |
| Postoperative Hb (g/dL) | 11.2 ± 1.8 | 10.5 ± 1.8 | 11.8 ± 1.7 | 0.001 * |
| Hb level decrease on the first postoperative day (g/dL) | 2.1 ± 1.2 | 2.2 ± 1.2 | 1.8 ± 1.0 | 0.03 * |
| Variables | Regression Coefficients | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|---|
| Gout medication use | −1.23 | 0.32 | (0.14, 0.75) | 0.009 * |
| Smoking | 1.31 | 3.23 | (1.34, 7.80) | 0.009 * |
| Hyperlipidemia | −0.09 | 0.53 | (0.19, 1.44) | 0.213 |
| Hb, preoperative | 0.66 | 0.68 | (0.53, 0.87) | 0.002 * |
| Hb level decrease | −0.41 | 1.93 | (1.25, 2.96) | 0.003 * |
| Variables | Values |
|---|---|
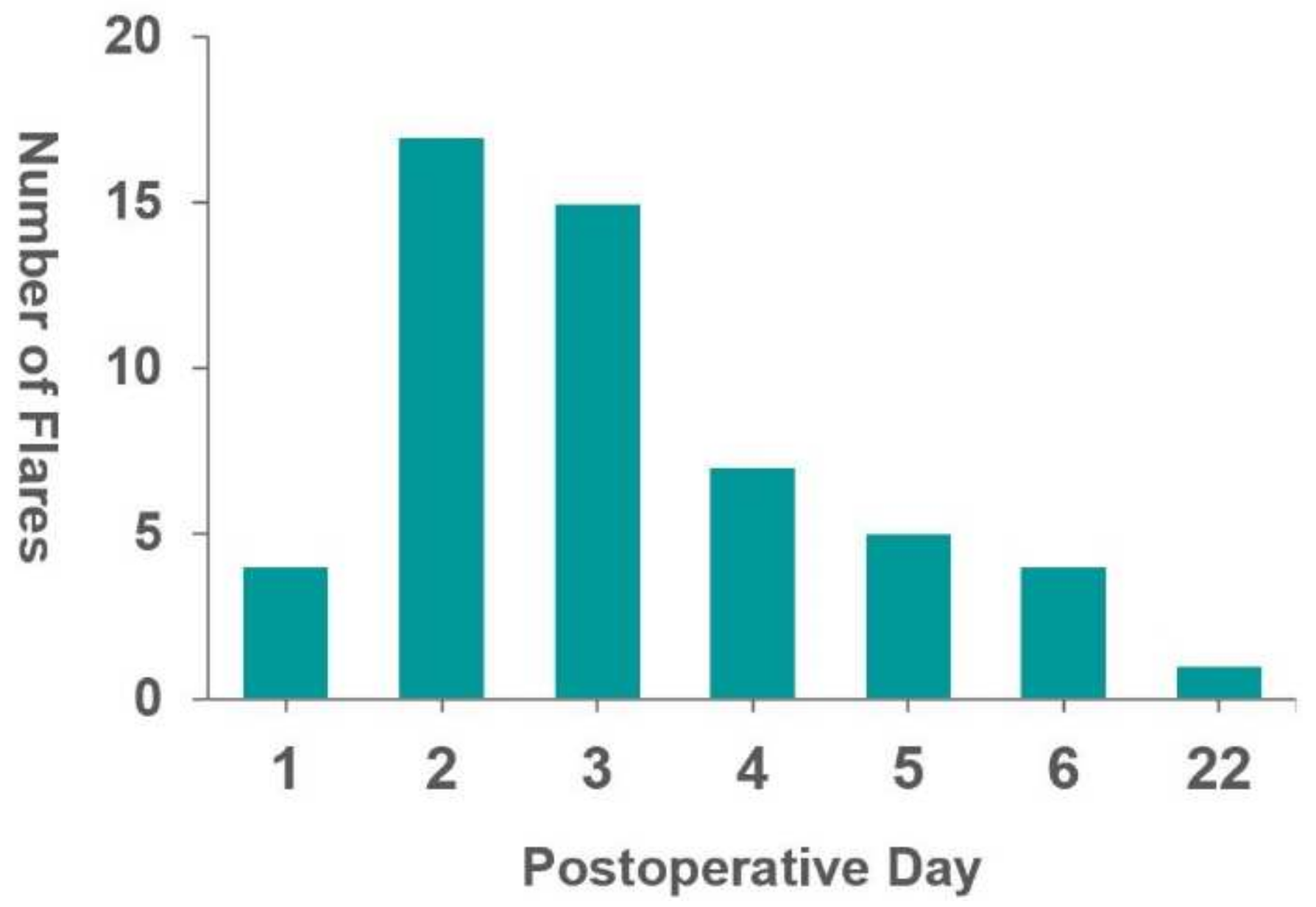
| Postoperative day of onset (days) (mean and range) | 3.5 (1–22) |
| Number of involved joint (number) (%) | |
| Monoarticular | 26 (46) |
| Oligo or polyarticular | 21 (38) |
| Unspecified | 9 (16) |
| Involved joints at flare (number) (%) | |
| Lower extremity | 40 (71) |
| Upper extremity | 3 (5) |
| Both upper & lower extremities | 4 (7) |
| Unspecified | 9 (16) |
| Flare site (number) (%) | |
| Knee | 31 (55) |
| Ankle | 24 (43) |
| 1st MTP joint | 7 (13) |
| Foot except 1st MTP joint | 5 (9) |
| Wrist | 5 (9) |
| Elbow | 3 (5) |
| Hand | 1 (2) |
| Uric acid level on gout flare (mg/dL) | 6.2 ± 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.-J.; Huang, Y.-C.; Yao, Y.-C.; Hsiung, W.; Chou, P.-H.; Wang, S.-T.; Chang, M.-C.; Lin, H.-H. Risk Factors for Postsurgical Gout Flares after Thoracolumbar Spine Surgeries. J. Clin. Med. 2022, 11, 3749. https://doi.org/10.3390/jcm11133749
Chen K-J, Huang Y-C, Yao Y-C, Hsiung W, Chou P-H, Wang S-T, Chang M-C, Lin H-H. Risk Factors for Postsurgical Gout Flares after Thoracolumbar Spine Surgeries. Journal of Clinical Medicine. 2022; 11(13):3749. https://doi.org/10.3390/jcm11133749
Chicago/Turabian StyleChen, Kuan-Jung, Yen-Chun Huang, Yu-Cheng Yao, Wei Hsiung, Po-Hsin Chou, Shih-Tien Wang, Ming-Chau Chang, and Hsi-Hsien Lin. 2022. "Risk Factors for Postsurgical Gout Flares after Thoracolumbar Spine Surgeries" Journal of Clinical Medicine 11, no. 13: 3749. https://doi.org/10.3390/jcm11133749
APA StyleChen, K.-J., Huang, Y.-C., Yao, Y.-C., Hsiung, W., Chou, P.-H., Wang, S.-T., Chang, M.-C., & Lin, H.-H. (2022). Risk Factors for Postsurgical Gout Flares after Thoracolumbar Spine Surgeries. Journal of Clinical Medicine, 11(13), 3749. https://doi.org/10.3390/jcm11133749






