The Use of Thromboelastography in Percutaneous Coronary Intervention and Acute Coronary Syndrome in East Asia: A Systematic Literature Review
Abstract
:1. Introduction
2. Materials and Methods
Literature Search
3. Results
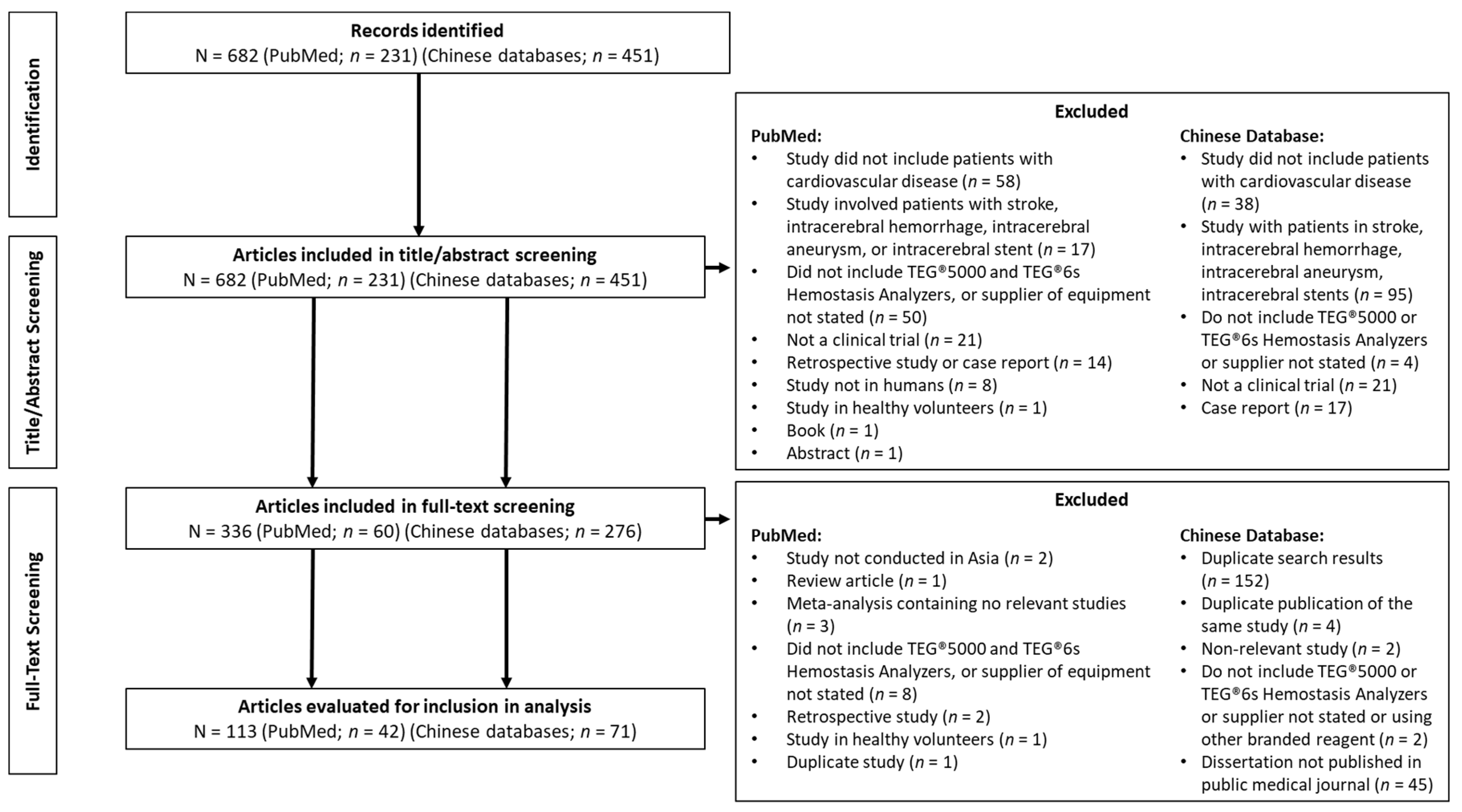
3.1. Literature Search Results
3.2. Clinical Validation of Thromboelastography with PlateletMapping® Assay
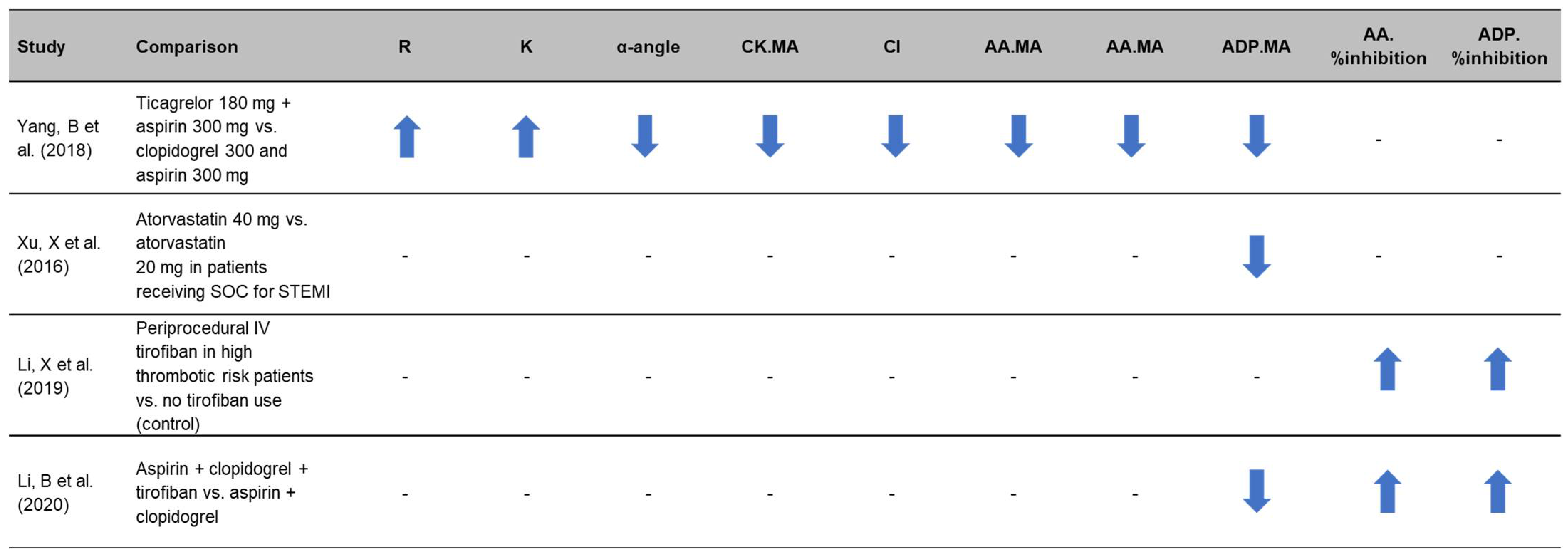
3.3. Use of Thromboelastography to Quantify Antiplatelet Efficacy in Therapy Comparison Studies
3.4. Utility of Thromboelastography to Monitor Response to Therapy and for Individualized Treatment
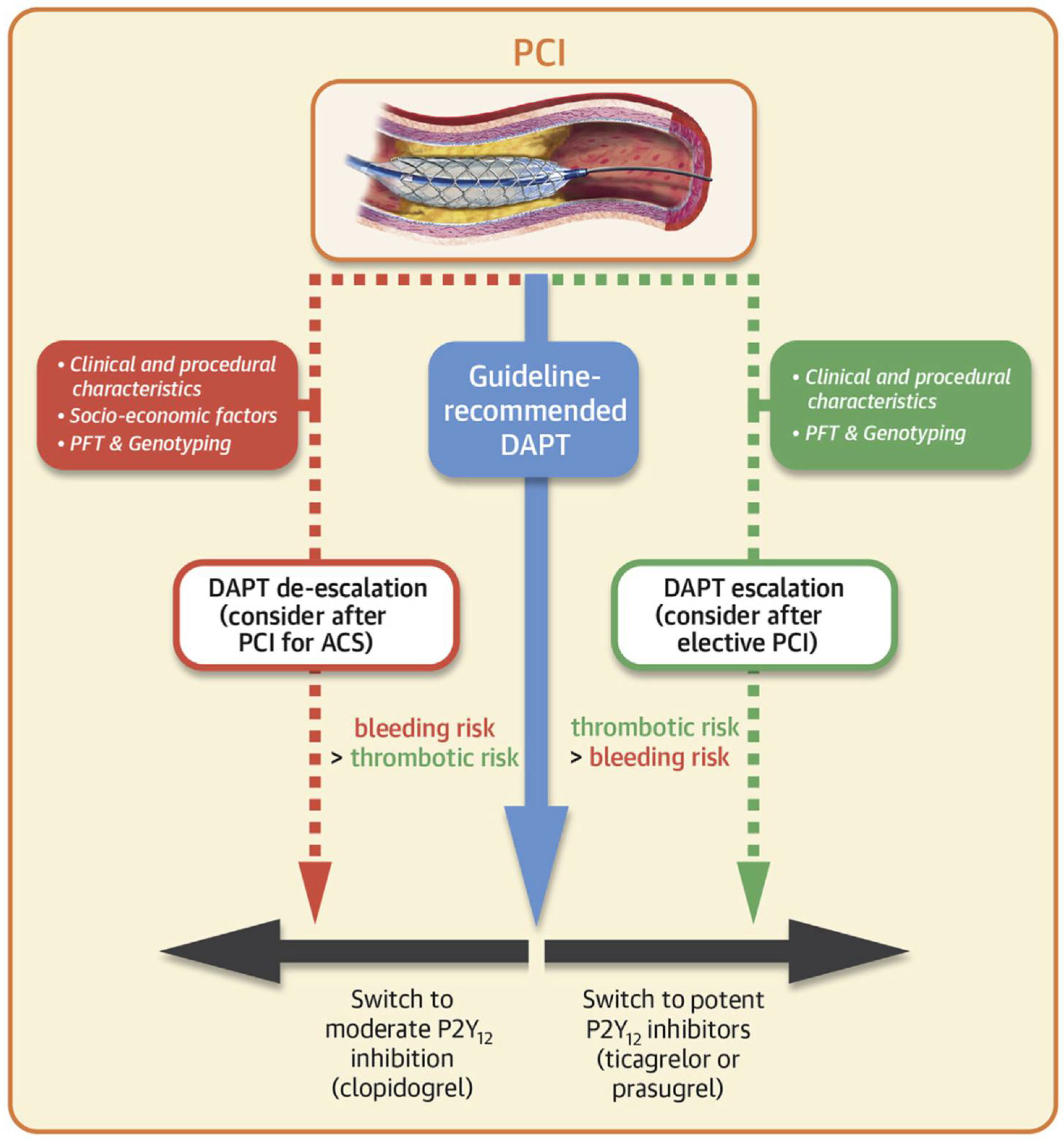
3.5. Utility of Thromboelastography for Guiding Escalation of Antiplatelet Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Museedi, A.S.; Grossman, S.A. Acute Coronary Syndrome. In StatPearls; Treasure Island: Las Vegas, NV, USA, 2021. [Google Scholar]
- Braun, M.; Kassop, D. Acute Coronary Syndrome: Management. FP Essent 2020, 490, 20–28. [Google Scholar] [PubMed]
- Beavers, C.J.; Bagai, J. Anticoagulation Monitoring during Cardiac Procedures: Considerations for Anticoagulation Safety. Society for Cardiovascular Angiography and Interventions (SCAI). 2020. Available online: https://scai.org/anticoagulation-monitoring-during-cardiac-procedures-considerations-anticoagulation-safety (accessed on 7 March 2022).
- Rihn, T.L.; Díez, J. Unfractionated heparin in cardiology: Redefining the standard of practice. Pharmacotherapy 2004, 24, 132s–141s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa-Uva, M.; Neumann, F.J.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardio-Thorac. Surg. 2019, 55, 4–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, J.D.; Pottgiesser, T.; Hartmann, J.; Duerschmied, D.; Bode, C.; Achneck, H.E. Comparison of three common whole blood platelet function tests for in vitro P2Y12 induced platelet inhibition. J. Thromb. Thrombolysis 2020, 50, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Frontroth, J.P. Light transmission aggregometry. Methods Mol. Biol. 2013, 992, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhao, S.; Hao, Y. Net platelet clot strength of thromboelastography platelet mapping assay for the identification of high on-treatment platelet reactivity in post-PCI patients. Biosci. Rep. 2020, 40, BSR20201346. [Google Scholar] [CrossRef]
- Rali, A.S.; Salem, A.M.; Gebre, M.; Garies, T.M.; Taduru, S.; Bracey, A.W., Jr. Viscoelastic Haemostatic Assays in Cardiovascular Critical Care. Card. Fail. Rev. 2020, 7, e01. [Google Scholar] [CrossRef]
- Li, Y.; Chang, H.; Ni, L.; Xue, P.; Li, C.; Yuan, L.; Cui, H.; Yu, C. Analysis of thrombelastogram-guided medication in patients with coronary heart disease after percutaneous coronary intervention. Exp. Ther. Med. 2019, 17, 3047–3052. [Google Scholar] [CrossRef] [Green Version]
- Ranucci, M.; Baryshnikova, E. Sensitivity of Viscoelastic Tests to Platelet Function. J. Clin. Med. 2020, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y(12) Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef]
- Cimmino, G.; Gallinoro, E.; Di Serafino, L.; De Luca, N.; Cirillo, P. Antiplatelet Therapy in Acute Coronary Syndromes. Lights and Shadows of Platelet Function Tests to Guide the Best Therapeutic Approach. Curr. Vasc. Pharmacol. 2020, 18, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Warlo, E.M.K.; Arnesen, H.; Seljeflot, I. A brief review on resistance to P2Y(12) receptor antagonism in coronary artery disease. Thromb. J. 2019, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Shi, X.; Xu, X.; Lin, Y. Both CYP2C19 and PON1 Q192R Genotypes Influence Platelet Response to Clopidogrel by Thrombelastography in Patients with Acute Coronary Syndrome. Cardiovasc. Ther. 2019, 2019, 3470145. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Arabi, A.; El-Menyar, A.; Abdulkarim, S.; AlJundi, A.; Alqahtani, A.; Arafa, S.; Al Suwaidi, J. Impact of Polypharmacy on Adherence to Evidence-Based Medication in Patients who Underwent Percutaneous Coronary Intervention. Curr. Vasc. Pharmacol. 2016, 14, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Aradi, D.; Kirtane, A.; Bonello, L.; Gurbel, P.A.; Tantry, U.S.; Huber, K.; Freynhofer, M.K.; ten Berg, J.; Janssen, P.; Angiolillo, D.J.; et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 1762–1771. [Google Scholar] [CrossRef] [Green Version]
- Nooney, V.B.; Hurst, N.L.; De Caterina, R.; Chirkov, Y.Y.; Horowitz, J.D. Does high on-treatment platelet aggregability reflect poor individual response to clopidogrel? Thromb. Res. 2020, 196, 510–515. [Google Scholar] [CrossRef]
- Sienkiewicz-Oleszkiewicz, B.; Wiela-Hojeńska, A. CYP2C19 polymorphism in relation to the pharmacotherapy optimization of commonly used drugs. Pharmazie 2018, 73, 619–624. [Google Scholar] [CrossRef]
- Dean, L. Clopidogrel Therapy and CYP2C19 Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kane, M.S., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Gurbel, P.A.; Bliden, K.P.; Navickas, I.A.; Mahla, E.; Dichiara, J.; Suarez, T.A.; Antonino, M.J.; Tantry, U.S.; Cohen, E. Adenosine diphosphate-induced platelet-fibrin clot strength: A new thrombelastographic indicator of long-term poststenting ischemic events. Am. Heart J. 2010, 160, 346–354. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.D.; Lopez-Espina, C.G.; Bliden, K.; Gurbel, P.; Hartmann, J.; Achneck, H.E. TEG(R)6s system measures the contributions of both platelet count and platelet function to clot formation at the site-of-care. Platelets 2020, 31, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.F.; Han, Y.L.; Zhang, J.H.; Wang, J.; Zhang, Y.; Xu, B.; Gao, Z.; Qiao, S.B.; Chen, J.; Wu, Y.; et al. Comparing of light transmittance aggregometry and modified thrombelastograph in predicting clinical outcomes in Chinese patients undergoing coronary stenting with clopidogrel. Chin. Med. J. 2015, 128, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.S.; Park, Y.; Tantry, U.S.; Ahn, J.H.; Kang, M.G.; Kim, K.; Jang, J.Y.; Park, H.W.; Park, J.R.; Hwang, S.J.; et al. Pharmacodynamic effects of a new fixed-dose clopidogrel-aspirin combination compared with separate administration of clopidogrel and aspirin in patients treated with coronary stents: The ACCEL-COMBO trial. Platelets 2017, 28, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Yin, S.; Sun, Z.; Xu, X.; Qin, J. The relationship between on-clopidogrel platelet reactivity, genotype, and post-percutaneous coronary intervention outcomes in Chinese patients. Scand. J. Clin. Lab. Investig. 2015, 75, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.P.; Guan, J.; Cai, L.L.; Li, Y.R.; Deng, X.L.; Liu, Q.Y.; Zheng, B.X.; Cong, Y.L. Evaluation of PFA P2Y assay in monitoring platelet function in elderly patients with cardiovascular disease receiving clopidogrel treatment. Nan Fang Yi Ke Da Xue Bao 2016, 37, 533–536. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Fan, X.; Li, Z.; Liu, C.; Gu, J. A comparative study of the efficacy of clopidogrel after PCI was monitored by thromboelastography and optical turbidity. J. Clin. Blood Transfus. Test. 2017, 19, 59–63. [Google Scholar]
- World Chinese Association of laboratory and pathologists; Laboratory physicians branch of Chinese Medical Association; Professional Committee of cardiovascular Laboratory Medicine. There is a consensus among experts on the application of platelet function detection in patients with acute coronary syndrome in antiplatelet therapy. J. Clin. Med. Res. Pract. 2018, 3, 201. [Google Scholar]
- Miao, L.; Lu, Y.; Qu, C.; Yanyan, B.; Guan, J.; Yanjun, Y. Patients with coronary heart disease were monitored by antiplatelet drug therapy and average platelet volume changes after coronary artery intervention. Chin. J. Clin. Lab. Sci. 2017, 35, 439–443. [Google Scholar]
- Yao, Y.; Zhang, J.H.; Tang, X.F.; He, C.; Ma, Y.L.; Xu, J.J.; Song, Y.; Liu, R.; Meng, X.M.; Song, L.; et al. Head to Head Comparison of Two Point-of-care Platelet Function Tests Used for Assessment of On-clopidogrel Platelet Reactivity in Chinese Acute Myocardial Infarction Patients Undergoing Percutaneous Coronary Intervention. Chin. Med. J. 2016, 129, 2269–2274. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Lu, G. TEG testing had an effect on the incidence of MACE in the process of double-linked antiplatelet drug therapy after PCI in the artery of the elderly coronary heart disease. Clin. Res. J. 2019, 27, 19–21. [Google Scholar]
- Yang, B.; Zheng, C.; Yu, H.; Zhang, R.; Li, S.; Tan, L.; Leng, M.; Cai, S. Comparison of Ticagrelor and Clopidogrel for Patients Undergoing Emergency Percutaneous Coronary Intervention. Iran. J. Public Health 2018, 47, 952–957. [Google Scholar] [PubMed]
- Xu, X.R.; Li, K.B.; Wang, P.; Xu, L.; Liu, Y.; Yang, Z.S.; Yang, X.C. The impact of different doses of atorvastatin on plasma endothelin and platelet function in acute ST-segment elevation myocardial infarction after emergency percutaneous coronary intervention. Zhonghua Nei Ke Za Zhi 2016, 55, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Wang, Z.; Ji, Q.; Wang, Q.; Li, X.; Lv, Q. Platelet Function and Risk of Bleeding in Patients With Acute Coronary Syndrome Following Tirofiban Infusion. Front. Pharmacol. 2019, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Li, B. Application effect of triple antiplatelet scheme after PCI in STEMI patients. Lin Chuang Yi Xue 2020, 57–59. [Google Scholar]
- Zhong, T.; Xu, W.; Hu, H.L.; Liu, S.J.; Wen, H.Q.; Xia, K.; Bian, M.H. Monitoring of Coagulation Status and Evaluation of Antiplatelet Aggregation in Patients with CHD by Using TEG. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018, 26, 1484–1491. [Google Scholar] [CrossRef]
- Wu, H.Y.; Zhang, C.; Zhao, X.; Qian, J.Y.; Wang, Q.B.; Ge, J.B. Residual platelet reactivity is preferred over platelet inhibition rate in monitoring antiplatelet efficacy: Insights using thrombelastography. Acta Pharmacol. Sin. 2020, 41, 192–197. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, L.; Ma, Z. Thromboelastography attempts to apply value analysis in antiplatelet therapy in PCI patients. Lin Chuang Xue Ye Xue Za Zhi 2016, 29, 120–122. [Google Scholar]
- Tái, Q.W.; Chén, Y.N. The application value of thromboelastography in antiplatelet therapy in coronary artery intervention patients is discussed. China Med. Guide 2016, 14, 165–166. [Google Scholar]
- Hou, X.; Han, W.; Gan, Q.; Liu, Y.; Fang, W. CYP2C19 and ABCB1 genetic polymorphisms correlate with the recurrence of ischemic cardiovascular adverse events after clopidogrel treatment. J. Clin. Lab. Anal. 2018, 32, e22369. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Miao, L.; Li, J.; Wang, L.; Yan, P.; Zhang, X. The relationship between CYP2C19 gene polymorphism and platelet inhibition rate was discussed in patients with coronary heart disease intervention. J. Integr. Cardiovasc. Cerebrovasc. Dis. 2018, 16, 461–463. [Google Scholar]
- Tang, Y.D.; Wang, W.; Yang, M.; Zhang, K.; Chen, J.; Qiao, S.; Yan, H.; Wu, Y.; Huang, X.; Xu, B.; et al. Randomized Comparisons of Double-Dose Clopidogrel or Adjunctive Cilostazol Versus Standard Dual Antiplatelet in Patients with High Posttreatment Platelet Reactivity: Results of the CREATIVE Trial. Circulation 2018, 137, 2231–2245. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Miao, L.; Li, J.; Wang, L.; Zhang, X. CYP2C19 gene polymorphism combined thromboelastography attempts to guide clinical research on postoperative antiplatelet therapy for coronary heart disease intervention. Zhong Hua Xin Xue Guan Bing Yan Jiu 2018, 16, 34–38. [Google Scholar]
- Deppe, A.C.; Weber, C.; Zimmermann, J.; Kuhn, E.W.; Slottosch, I.; Liakopoulos, O.J.; Choi, Y.H.; Wahlers, T. Point-of-care thromboelastography/thromboelastometry-based coagulation management in cardiac surgery: A meta-analysis of 8332 patients. J. Surg. Res. 2016, 203, 424–433. [Google Scholar] [CrossRef]
- Serraino, G.F.; Murphy, G.J. Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: Updated systematic review and meta-analysis. Br. J. Anaesth. 2017, 118, 823–833. [Google Scholar] [CrossRef] [Green Version]
- Sambu, N.; Radhakrishnan, A.; Dent, H.; Calver, A.L.; Corbett, S.; Gray, H.; Simpson, I.A.; Curzen, N. Personalised antiplatelet therapy in stent thrombosis: Observations from the Clopidogrel Resistance in Stent Thrombosis (CREST) registry. Heart 2012, 98, 706–711. [Google Scholar] [CrossRef]
- Olechowski, B.; Dalton, R.T.; Khanna, V.; Ashby, A.; Vavyla, M.; Mariathas, M.; Harris, S.; Nicholas, Z.; Mahmoudi, M.; Curzen, N. Detection of individual responses to clopidogrel: Validation of a novel, rapid analysis using thrombelastography 6s. Cardiovasc. Ther. 2018, 36, e12433. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Tantry, U.S.; Monroe, A.L.; Muresan, A.A.; Brunner, N.E.; Lopez-Espina, C.G.; Delmenico, P.R.; Cohen, E.; Raviv, G.; et al. First report of the point-of-care TEG: A technical validation study of the TEG-6S system. Platelets 2016, 27, 642–649. [Google Scholar] [CrossRef]
- Neal, M.D.; Moore, E.E.; Walsh, M.; Thomas, S.; Callcut, R.A.; Kornblith, L.Z.; Schreiber, M.; Ekeh, A.P.; Singer, A.J.; Lottenberg, L.; et al. A comparison between the TEG 6s and TEG 5000 analyzers to assess coagulation in trauma patients. J. Trauma Acute Care Surg. 2020, 88, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Craft, R.M.; Chavez, J.J.; Bresee, S.J.; Wortham, D.C.; Cohen, E.; Carroll, R.C. A novel modification of the Thrombelastograph assay, isolating platelet function, correlates with optical platelet aggregation. J. Lab. Clin. Med. 2004, 143, 301–309. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bliden, K.P.; Gurbel, P.A. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J. Am. Coll. Cardiol. 2005, 46, 1705–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Chew, D.P.; Abdul Kader, M.A.S.; Ako, J.; Bahl, V.K.; Chan, M.; Park, K.W.; Chandra, P.; Hsieh, I.C.; Huan, D.Q.; et al. 2020 Asian Pacific Society of Cardiology Consensus Recommendations on the Use of P2Y12 Receptor Antagonists in the Asia-Pacific Region. Eur. Cardiol. 2021, 16, e02. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Wang, Q.; Xu, Q.; Lv, Q. Clopidogrel-associated genetic variants on inhibition of platelet activity and clinical outcome for acute coronary syndrome patients. Basic Clin. Pharmacol. Toxicol. 2019, 124, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.E.; Kim, Y.J.; Park, J.J.; Kim, S.; Park, K.; Cho, M.S.; Nam, G.B.; Park, D.W. Safety and Effectiveness of Contemporary P2Y(12) Inhibitors in an East Asian Population With Acute Coronary Syndrome: A Nationwide Population-Based Cohort Study. J. Am. Heart Assoc. 2019, 8, e012078. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, S. Clopidogrel resistance: Identifying and overcoming a barrier to effective antiplatelet treatment. Cardiovasc. Ther. 2011, 29, e100–e111. [Google Scholar] [CrossRef]
- Tantry, U.S.; Hartmann, J.; Neal, M.D.; Schöechl, H.; Bliden, K.P.; Agarwal, S.; Mason, D.; Dias, J.D.; Mahla, E.; Gurbel, P.A. The role of viscoelastic testing in assessing peri-interventional platelet function and coagulation. Platelets 2021, 33, 520–530. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| English language article OR Chinese language article | Any language other than English or Chinese |
| Studies of humans or human blood samples (adults or pediatrics) | Non-human studies, any studies performed in animals |
| Authors/investigators from any center involved are from an Asian country | Authors/investigators from a country not in Asia |
| Clinical trials and meta-analyses | Reviews, case reports, editorials, responses, comments, congress abstracts |
| Studies with prospective data collection | Studies based on retrospective data collection |
| Reports data relevant to cardiology | Reports data from a setting other than cardiology |
| Utilization of standard TEG (5000 and 6s) in the context of predicting or improving patient outcomes | No Viscoelastic testing data reported Viscoelastic data reported but not directly linked to assessment/treatment of cardiovascular disease Use of other VHA device (e.g., ROTEM, SONOCLOT, Multiplate®, VerifyNow®, Haema system, CFMS LEPU) only Information about the supplier of equipment not provided |
| Assay | Parameter | Description |
|---|---|---|
| Thromboelastography | α-angle | Rate of clot formation |
| CK.MA | Measures the fibrin formation phase; overall clot strength (mostly driven by platelet count and function as well as fibrin formation) and stability showing platelet and fibrin interacting via GPIIb/IIIa | |
| act.MA | Assesses clot strength without platelet contribution | |
| ADP.MA | Functional component of platelet clot strength derived by ADP-agonist stimulation (for pharmacologic inhibition of ADP pathway using anti-P2Y12 therapies, i.e., clopidogrel, ticagrelor, prasugrel) | |
| AA.MA | Functional component of platelet clot strength derived by AA-agonist stimulation (for pharmacologic inhibition of AA pathway using thromboxane pathway blockers, i.e., aspirin) | |
| ADP.%aggregation | Percentage platelet aggregation rate induced by ADP (calculated from ADP.MA−Fibrin.MA/Thrombin.MA−Fibrin.MA × 100) | |
| AA.%aggregation | Percentage platelet aggregation rate induced by AA (calculated from AA.MA−Fibrin.MA/Thrombin.MA−Fibrin.MA × 100) | |
| ADP.%inhibition | Percentage clot strength change due to platelet function inhibition induced by ADP (calculated from platelet aggregation: [(ADP.MA−Fibrin.MAn)/(Thrombin.MA−Fibrin.MA) × 100] and %inhibition: [100% platelet aggregation]) | |
| AA.%inhibition | Percentage clot strength change due to platelet function inhibition induced by AA (calculated from platelet aggregation: [(AA.MA−Fibrin.MAn)/(Thrombin.MA−Fibrin.MA) × 100] and %inhibition: [100% platelet aggregation]) | |
| LTA | ADP.MPA | ADP-induced maximum platelet aggregation |
| ARADP.LTA | ADP-induced aggregation rate by LTA |
| Study | Drug Intake and Timing of Assay Utilization | TEG® Device Used | Between-Parameter Correlations | Identification of HTPR | Prediction of MACE/Ischemic Risk | Summary | |
|---|---|---|---|---|---|---|---|
| Thromboelastography | LTA | ||||||
| Tang, X F et al. (2015) [24] N = 789 | Loading doses (12 h prior to PCI)—therapy-naïve patients: 300 mg DAPT; patients previously on antiplatelet therapy: 100 mg aspirin, 75 mg clopidogrel Daily maintenance dose following surgery: 100 mg aspirin, 75 mg clopidogrel TEG® assay carried out 6 h after clopidogrel dose | TEG®5000 | Spearman coefficient for ADP.%inhibition vs. ARADP.LTA: r = 0.733, p < 0.001 | HTPR cutoff for ADP.%inhibition (≤32%) found in 36.1% of enrolled subjects HTPR cutoff for ARADP.LTA: (53.2%) found in 29% of subjects | ROC curve analysis AUC, % (95% CI) = 0.684 (0.650–0.716), 0.0001 1-year MACE occurred in 6.7% with and 2.6% without HTPR | ROC curve analysis AUC, % (95% CI) = 0.677 (0.643–0.710), p = 0.0009 1-year MACE occurred in 7.4% and 2.7% without HTPR | Thromboelastography has shown strong performance for detecting low DAPT response/HTPR, with a high sensitivity and specificity for detecting HTPR (similar to LTA); Thromboelastography has also shown comparable performance to LTA in predicting ischemic risk at 6 months and 1 year; The strength of correlations between thromboelastography and LTA parameters varied; the strongest correlation was reported in the largest study and between ADP.%inhibition and ARADP.LTA |
| Cheng, D et al. (2020) [7] N = 110 | Loading dose: aspirin 75 mg, ticagrelor 180 mg Maintenance dose: aspirin 75 mg, ticagrelor 90 mg TEG® and LTA assays were ordered simultaneously (no specific time given) | TEG®5000 | ADP.%aggregation vs. ARADP.LTA: r = 0.5613, p < 0.01 ADP.MA vs. ARADP.LTA: r = 0.5567, p < 0.01 Net ADP.MA vs. ARADP.LTA: r = 0.5836, p < 0.01 | AUCs (95% CI) for ROC curve analysis: * ADP.%aggregation (%) 0.8199 (0.734–0.886); cutoff = 64.6; sensitivity = 82.61; specificity = 80.46 ADP.MA (mm) 0.812 (0.726–0.880); cutoff = 45.6, sensitivity = 78.26; specificity = 81.61 Net ADP.MA (mm) 0.849 (0.768–0.910); cutoff = 26.3; sensitivity = 91.30; specificity = 73.56 | - | ||
| Tang, N et al. (2015) [26] N = 178 | Loading dose (prior to PCI)—therapy-naïve patients: 300 mg clopidogrel; patients previously on antiplatelet therapy: 75 mg clopidogrel Daily maintenance dose following surgery: 100 mg aspirin, 75 mg clopidogrel Blood samples collected 18–24 h post-PCI. | TEG®5000 | - | ADP.MA HTPR defined as >47 mm; positive predictive value = 31.6%, negative predictive value = 91.7% ADP.MPA HTPR defined as >46%; positive predictive value = 33.3%, negative predictive value 97.6% | ADP.MA in patients with MACE vs. those without: 43.5 ± 20.6% vs. 33.0 ± 15.2, p = 0.021 ADP.MA > 47 mm independently predicted 6-month MACE (p = 0.013) | MPA.MA in patients with MACE vs. those without: 52.9 ± 19.2% vs. 29.4 ± 18.7%, p = 0.002 MPA.MA > 46% independently predicted 6-month MACE (p = 0.001) | |
| Li, G et al. (2017) [28] N = 425 | DAPT: aspirin 100 mg/day, clopidogrel 75 mg/day Blood sample taken 3 days after treatment start | TEG®5000 | ADP.%aggregation (11.8%) vs. ARADP.LTA (12.0%): r = 0.351, p = 0.01 | - | - | - | |
| Miao, L et al. (2017) [30] N = 177 | Loading dose for therapy-naïve patients: 300 mg DAPT Daily maintenance dose: 100 mg aspirin, 75 mg clopidogrel Blood sample for TEG® and LTA assay taken one month after PCI | TEG®5000 | Weak correlations between TEG and LTA | Detection rates of low DAPT response: LTA = 30.3%; thromobelastography = 45.5% | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, O.; Hartmann, J.; Tang, Y.-D.; Dias, J. The Use of Thromboelastography in Percutaneous Coronary Intervention and Acute Coronary Syndrome in East Asia: A Systematic Literature Review. J. Clin. Med. 2022, 11, 3652. https://doi.org/10.3390/jcm11133652
Xu O, Hartmann J, Tang Y-D, Dias J. The Use of Thromboelastography in Percutaneous Coronary Intervention and Acute Coronary Syndrome in East Asia: A Systematic Literature Review. Journal of Clinical Medicine. 2022; 11(13):3652. https://doi.org/10.3390/jcm11133652
Chicago/Turabian StyleXu, Ou, Jan Hartmann, Yi-Da Tang, and Joao Dias. 2022. "The Use of Thromboelastography in Percutaneous Coronary Intervention and Acute Coronary Syndrome in East Asia: A Systematic Literature Review" Journal of Clinical Medicine 11, no. 13: 3652. https://doi.org/10.3390/jcm11133652
APA StyleXu, O., Hartmann, J., Tang, Y.-D., & Dias, J. (2022). The Use of Thromboelastography in Percutaneous Coronary Intervention and Acute Coronary Syndrome in East Asia: A Systematic Literature Review. Journal of Clinical Medicine, 11(13), 3652. https://doi.org/10.3390/jcm11133652






