Abstract
The application of viscoelastic hemostatic assays (VHAs) (e.g., thromboelastography (TEG) and rotational thromboelastometry (ROTEM)) in orthopedics is in its relative infancy when compared with other surgical fields. Fortunately, several recent studies describe the emerging use of VHAs to quickly and reliably analyze the real-time coagulation and fibrinolytic status in both orthopedic trauma and elective orthopedic surgery. Trauma-induced coagulopathy—a spectrum of abnormal coagulation phenotypes including clotting factor depletion, inadequate thrombin generation, platelet dysfunction, and dysregulated fibrinolysis—remains a potentially fatal complication in severely injured and/or hemorrhaging patients whose timely diagnosis and management are aided by the use of VHAs. Furthermore, VHAs are an invaluable compliment to common coagulation tests by facilitating the detection of hypercoagulable states commonly associated with orthopedic injury and postoperative status. The use of VHAs to identify hypercoagulability allows for an accurate venous thromboembolism (VTE) risk assessment and monitoring of VTE prophylaxis. Until now, the data have been insufficient to permit an individualized approach with regard to dosing and duration for VTE thromboprophylaxis. By incorporating VHAs into routine practice, orthopedic surgeons will be better equipped to diagnose and treat the complete spectrum of coagulation abnormalities faced by orthopedic patients. This work serves as an educational primer and up-to-date review of the current literature on the use of VHAs in orthopedic surgery.
1. Introduction
Orthopedic surgical patients can display a spectrum of coagulopathies, from hypocoagulopathic pelvic fracture trauma patients in shock requiring massive transfusion to postoperative hypercoagulopathic elective arthroplasty patients in need of deep vein thrombosis (DVT) prophylaxis. Until recently, there have been insufficient data to permit an individualized approach with bedside point-of-care precision testing to guide orthopedic surgeons through resuscitation and VTE prophylaxis. However, viscoelastic hemostatic assays (VHAs, including thromboelastography (TEG) and rotational thromboelastometry (ROTEM)) enable the identification and individualized treatment of a dynamic spectrum of coagulation phenotypes. Blood component therapy (BCT) and hemostatic adjunctive therapy (HAT) to address hypocoagulopathy, as well as anticoagulant prophylaxis to address hypercoagulopathy, are both efficiently directed by VHAs utilizing simple algorithms (see Table 1 below).

Table 1.
Simplified algorithm of blood component therapy and hemostatic adjuncts based on the shovel analogy for viscoelastic hemostatic assays [8,14].
An estimated, 25% of severely injured patients arrive in emergency departments with trauma-induced coagulopathy (TIC) [1]; this relatively high incidence of acute hypocoagulopathy in trauma patients has driven an expanded investigation into the use of VHAs. TIC has been reported to be an independent predictor of mortality [2], as well as a risk factor for multisystem organ failure and higher transfusion requirements [3,4,5]. Given the point-of-care nature and fast turn-around time for results, many trauma centers consider VHAs as the standard of care for all severely injured trauma patients on arrival to an emergency department and/or trauma center. There is a large and expanding body of literature describing the utilization of VHAs to better characterize the spectrum of coagulopathy at presentation and the evolving changes through the resuscitation of trauma patients [6].
Utilizing VHAs to guide goal-directed resuscitation and transfusion in trauma patients with coagulopathy may improve patient-centered care and outcomes. A 2016 Cochrane review found that VHA-guided transfusion strategies reduced blood product utilization and improved mortality rates [7]. However, the studies reviewed predominantly involved cardiothoracic surgery patients, highlighting the need for more research in the area of orthopedic injury and polytrauma. Of existing trauma trials, several studies have demonstrated decreased BCT administration and noninferior or improved mortality when using VHA to guide resuscitation [8,9,10,11,12,13]. Randomized controlled trials have demonstrated the use of VHAs to guide BCT and HAT not just in trauma, but also in the management of bleeding patients in the critical care setting [13,14,15,16,17,18]. However, the optimal use of VHAs in resuscitation is not entirely settled: for example, the implementing treatment algorithms for the correction of trauma-induced coagulopathy (ITACTIC) study found no difference in mortality between VHAs and common coagulation tests (CCTs) (e.g., partial thromboplastin time (PTT), prothrombin time (PT), international normalized ratio (INR), platelet count, and fibrinogen level) [19]. The trial investigators detected a lower-than-expected incidence of TIC, although they did identify a mortality benefit in the subgroup of patients with traumatic brain injury. In summary, continued trauma research on the utilization of VHA-guided resuscitation protocols must be implemented.
There is relatively little literature concerning VHAs in orthopedic trauma. From the earliest systematic description of the use of VHA in modern trauma, TEG identified both early hypercoagulability, as well as early hypocoagulability, and transfusion requirements [20]. There was a significant gap of nearly 20 years before the value of VHAs in guiding BCT for hemorrhage in orthopedic trauma pelvic fracture patients was presented [21,22,23]. Beyond its well-described utility in the treatment of hypocoagulopathy, the ability of VHAs to identify hypercoagulopathy has drawn recent attention. More than 85% of injured patients become detectably hypercoagulable by VHA after trauma, and this hypercoagulability is associated with a two-fold increase in the risk of VTE during recovery [24]. VTE rates remain as high as 28% in modern series [25], exceeding 15% even under the most stringent prophylaxis regimens [24], with the presence of orthopedic injury being one of the most influential risk factors. Despite increasing attention paid to VHAs in orthopedic trauma, however, there remains only a single orthopedic-specific review paper on this topic [26].
A barrier to the use of VHA, not just in trauma but in orthopedic surgery, has been an excessive reliance on CCTs to help identify bleeding patterns in patients with musculoskeletal injuries, and a lack of familiarity among orthopedic traumatologists on the indications for and interpretation of these tests. Therefore, a straightforward summary of VHA testing is in order.
1.1. Thromboelastography (TEG)
To perform a first-generation TEG assay (using the TEG 5000 Hemostasis Analyzer: Haemonetics, Braintree, MA, USA), a 0.36 mL citrated sample of whole blood is placed into a heated cup maintained at 37 °C, and citrate anticoagulation is reversed by the addition of calcium. A coagulation activator (such as kaolin for a standard TEG, or tissue factor-containing reagent for a “rapid” TEG) is added to initiate coagulation; “native” coagulation can also be measured when the assay is activated by anticoagulant reversal alone. The sample cup rotates 4.45 degrees every ten seconds, with a concentric pin linked to a sensor suspended in the cup (Figure 1). In viscous, unclotted blood, the pin does not move; however, as a clot begins to form, elastic clot fibers bind the cup to the pin, generating rotational forces on the pin. These forces are transmitted to an electrical transducer, producing a graphical output that represents clot dynamics over time. The five major parameters of TEG are reaction time (R), clot kinetics (K), alpha angle, maximum amplitude (MA), and lysis at 30 min (LY30) [27,28].
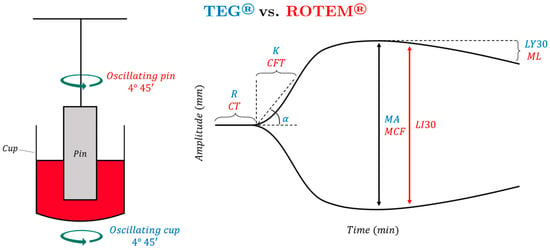
Figure 1.
A 0.36 mL citrated sample of whole blood is placed into a heated cup maintained at 37 °C in which a pin is suspended. In TEG, the cup oscillates while the pin is stationary; in ROTEM, the pin oscillates while the cup remains stationary. In both assays, citrate anticoagulation is reversed with calcium, and a coagulation activation reagent is added; as the blood coagulates, force is detected by the pin and transmitted to an electrical transducer, producing a graphical output that represents clot dynamics over time. Reaction time (R) and clotting time (CT) represent the amount of time until the tracing reaches 2 mm in amplitude. Kinetics (K) and clot formation time (CFT) represent the amount of time between 2 mm of amplitude and when the tracing reaches 20 mm in amplitude. The α-angle is used in both TEG and ROTEM; it represents the angle between the horizontal line and the line between clot initiation and 20 mm amplitude. Maximum amplitude (MA) and maximum clot firmness (MCF) represent the maximum amplitude that the tracing reaches. Lysis at 30 min (LY30) represents the percentage decrease in amplitude 30 min after reaching MA. Clot lysis index (CLI30) represents the percentage of amplitude remaining 30 min after reaching MA. Maximum lysis (ML) refers to the percentage decrease in amplitude relative to the MCF at the end of the run.
The second-generation TEG 6s Hemostasis Analyzer (manufacturer: Haemonetics, Braintree, MA, USA) is a fully automated, cartridge-based test designed for ease of use and reproducibility. It has been cleared by the FDA for use in the trauma setting based on a recent multicenter method comparison trial which included over 500 trauma patients [29]. As opposed to mechanical transduction to sense the viscoelastic parameters of clot formation, the TEG 6s measures changes in resonant frequency of a 1 mL blood samples using an automated cartridge system (thus avoiding errors associated with pipetting by hand as in the earlier TEG 5000 system). As the blood clots, the change in resonant frequency can be used to measure changes in viscoelasticity, generating a tracing and parameters that are conceptually similar, although not directly comparable, to the TEG 5000 legacy cup-and-pin system [30,31].
1.2. Rotational Thromboelastometry (ROTEM)
TEG and ROTEM have similar fundamentals, but in ROTEM, the pin oscillates while the cup remains stationary. The ROTEM tracing is like that of the TEG, but the parameters’ nomenclature and timing of measurement are different (see Figure 1). R coincides with the clotting time (CT), K represents the clot formation time (CFT), and MA represents the maximum clot firmness (MCF). Both analyzers use the alpha angle. ROTEM has multiple parameters to describe fibrinolysis: maximum lysis (ML) refers to the percentage decrease in amplitude relative to the MCF at the end of the run, while the clot lysis index (CLI/CLY/LI) is the ratio of the amplitude of the curve at 30/60 min after reaching MCF [32]. Multiple studies have described the applicability of TEG and ROTEM in cardiac surgery, liver transplantation, trauma resuscitation, and postpartum hemorrhage. There is little disparity between these two tests in the guidance of treatment [20,33,34,35,36,37,38,39].
The ROTEM Sigma is a fully automated test that is more convenient and easier to use than the previous Delta iteration; it provides a cartridge-based system and automated pipetting, but relies on the same cup-and-pin technology used in the ROTEM Delta. Studies comparing the ROTEM Sigma to its Delta predecessor have raised some questions regarding the validity of the reference ranges of the ROTEM Sigma [40,41,42,43].
1.3. Comparative Interpretation of Principle Viscoelastic Hemostatic Assays
In this section, our goal is to compare TEG and ROTEM in a way that will help clinicians better understand both analyzers. TEG and ROTEM have different terminologies, use different activators, and rely on both specific parameter values and the overall pattern recognition for interpretation. This reliance on assay familiarity and pattern recognition for bedside interpretation may be a barrier to a more common utilization of VHAs in orthopedics [30,39,44,45].
Rapid thromboelastography (rTEG) and the EXTEM assay of ROTEM both use an extrinsic cascade-targeted activation reagent, such as tissue factor, to initiate clot formation; thus, rTEG MA corresponds to EXTEM MCF [46]. Similarly, the kaolin TEG and the INTEM assay of ROTEM both use an intrinsic cascade-targeted activation reagent such as kaolin and ellagic acid. Both assays generate parameters describing the clot amplitude at 5 min intervals after R/CT until the graph reaches MA/MCF. These parameters are A5, A10, etc., in rTEG and CA5, CA10, etc., in EXTEM [44]. These values, in addition to the five key parameters described above, allow the real-time assessment of clotting abnormalities, allowing for an early analysis of hemostatic competence and anticipation of necessary blood product therapy (Table 1) [40,45,47,48]. Similarly, the functional fibrinogen MA of TEG and the FIBTEM assay of ROTEM both activate clot formation in the presence of platelet inhibitors, producing a tracing reflective of fibrin-specific contributions to clot formation and allowing the prompt recognition of a deficiency in fibrinogen [49].
A useful analogy for the interpretation of TEG/ROTEM tracings is to compare them to the shape of three shovels (Figure 2). For hemostatic competence or physiologic hemostasis, shovel-shaped tracing has an ideal handle length, blade slope, blade width, and blade tip, shown as the top shovel in Figure 2. The interpretation of the shovel shape and correspondence to ROTEM/TEG parameters is as follows: the CT/R is the length of the handle, the alpha angle is the slope of the blade, MCF/MA is the width of the blade, and CLI60/LY30 is the taper or point of the blade. The extremes of the coagulation spectrum are represented by different shovels, where, for the sake of analogy, the ease of tilling and moving soil corresponds to the ease of moving blood. The hypercoagulable shovel tracing has a short handle and a wide blade with an absent tip (middle shovel in Figure 2), which makes it very difficult for the earth to be broken up and transported. The hypocoagulable shovel tracing has a long handle with a narrow and pointed blade (third shovel in Figure 2), which makes tilling and soil transport less cumbersome but markedly inefficient. In the above examples, by analogy, tilling and soil transport are either difficult (hypercoagulable) or easy but inefficient (hypocoagulable). In summary, in Figure 2, the first shovel represents hemostatic equilibrium, the second shovel represents hypercoagulable disequilibrium, and the third shovel represents hypocoagulable disequilibrium. Goal-directed therapy can be given based on either the corresponding shovel-analogous pattern or the values of the various TEG and ROTEM parameters (Table 1).
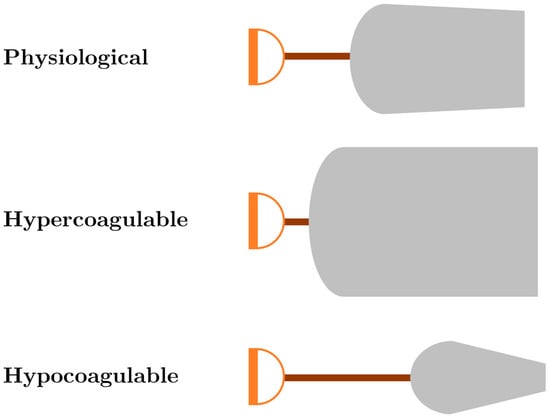
Figure 2.
Shovel analogy of ROTEM/TEG tracings. The first shovel, shown with a normal handle length and moderately wide shovel blade with a slight narrowing of the tip of the blade, represents physiological hemostasis with normal CT/R, alpha angle, MCF/MA, and CLI60/LY30. The second ROTEM/TEG shovel tracing with a very short handle and wide blade with very little tapering of the tip of the blade depicts a tracing with a decreased CT/R and a wide alpha angle, CT/MA, and increased width of the CLI60 with very low LY30. This shovel tracing is indicative of a hypercoagulable/fibrinolytic shutdown phenotype. The third ROTEM/TEG shovel tracing depicts a tracing with a prolonged CT/R, narrow alpha angle, a narrow MCF/MA, and increased lysis represented by the decreased width of CLI60 and the pointed and increased LY30; these parameters are indicative of a hypocoagulopathic/hyperfibrinolytic phenotype. See Table 1 for a treatment algorithm based on this shovel analogy. Abbreviations: clot lysis index at 30/60 min (CLI30/CLI60); clotting time (CT); lysis at 30 min (LY30); maximum amplitude (MA); reaction time (R); rotational thromboelastometry (ROTEM); thromboelastography (TEG).
1.4. Newer Viscoelastic Hemostatic Assays in Orthopedic Patients
The Quantra QPlus System (manufacturer: HemoSonics, LLC; Charlottesville, VA, USA) is a recently developed device approved for use in cardiac and orthopedic surgery which uses ultrasound-induced resonance to assess the dynamic properties of clot formation. The use of Quantra QPlus has been studied in a comparative analysis between Quantra QPlus, ROTEM parameters, and CCTs in a prospective observational study of 277 patients undergoing orthopedic surgery (79 orthopedic patients) or cardiac surgery. Good accuracy and strong inter-parameter correlations were seen between the ROTEM delta and Quantra. The results from receiver-operator curve analysis uncovered specificities and sensitivities in the 80–90% range when Quantra Qplus parameters were used to discriminate ROTEM threshold values commonly used in algorithms for goal-directed BCT for orthopedic patients [50]. With specific regard to coagulation factors such as cryoprecipitate and soluble fibrinogen concentrate, recent studies using Quantra QPlus have shown that fibrin and platelet contributions to clot stiffness measured by the device demonstrated thresholds that allowed for a laboratory-based fibrinogen and platelet threshold assay to guide transfusion decisions in orthopedic patients. Cutoff values were demonstrated for the lowest fibrinogen and platelet thresholds that would trigger the transfusion of cryoprecipitate, soluble fibrinogen, or platelet concentrates [51].
In addition, a new handheld VHA called the viscoelastic coagulation monitoring system (VCM: Entegrion; Durham, NC, USA) operates based on standard thromboelastography principles, but is significantly more portable than either TEG instrument. It has been approved for clinical use in Europe. In one study of patients undergoing orthopedic surgery, VCM parameters were compared to ROTEM delta and NATEM parameters. Specifically, the CT, CFT, alpha angle, A10, A20, MCF and lysis results showed a good correlation between the VCT system and ROTEM and NATEM [52].
As clinical experience with newer, optimized, and more portable VHA devices continues to grow, their clinical integration and optimal usage compared to TEG and ROTEM will be better elucidated.
2. Viscoelastic Hemostatic Assays in Orthopedic Surgery
2.1. Trauma
2.1.1. Pelvic Fractures
There is a sparsity of literature specifically addressing the utilization of VHAs for the guidance of resuscitation in patients with pelvic fractures, which is surprising as this is a quintessential orthopedic injury pattern known to be associated with significant hemorrhage-related morbidity and mortality (Table 2). The high incidence of massive transfusion associated with pelvic fracture has been considered a target area for increased VHA use to guide BCT/HAT and has yielded promising results for improving mortality in polytrauma patients with severe hemorrhage [6,8,13]. Despite literature highlighting the benefit of VHAs to treat massive hemorrhage, there are only four retrospective studies analyzing a total of 522 patients with pelvic fractures and high injury severity scores (Table 2) [22,23,53].

Table 2.
Summary of the literature pertaining to viscoelastic hemostatic assays applied for the treatment of patients with pelvic fractures.
A retrospective study of VHA parameters measured at presentation in a group of 141 patients with traumatic pelvic and or acetabular fracture demonstrated a significant relationship between LY30 on admission, increased packed red blood cells transfused, and increased mortality, suggesting that aggressive BCT/HAT is indicated in pelvic fracture patients with significant fibrinolysis [53]. An additional retrospective review of 131 patients with pelvic trauma revealed that abnormal activated clotting times on admission rTEG was an independent risk factor of death. Specifically, the death rate was 52% in patients with rTEG ACT values >6 min. There was no significant association between CCTs and mortality [54]. These studies highlight the role of VHA in managing traumatic hypocoagulopathy.
However, trauma patients with pelvic fractures often present with hypercoagulability and impaired fibrinolysis (termed “fibrinolytic shutdown”), and less commonly with profound, life-threatening hypocoagulopathy and/or hyperfibrinolysis (HF) [55,56]. In a retrospective study of 210 patients by Nelson et al., 59% of pelvic trauma patients presented fibrinolytic shutdown based upon admission rTEG analysis. The overall incidence of VTE in this group was 11%. It was noted that patients undergoing pelvic external fixation had higher rates of VTE, but details of conversion surgical procedures and pelvic fracture patterns were not included [23].
VHA is an appealing tool for balancing the resuscitation of both hypo- and hypercoagulopathy in this patient population. A pilot study analyzing the use of platelet mapping (TEG/PM, a modification of the standard TEG assay which evaluates platelet responsiveness) suggested that including TEG/PM may facilitate a reduction in platelet usage and a reduced cost of resuscitation. In this study, the average ratio of packed red blood cells (pRBC): fresh frozen plasma (FFP): platelets was 2.5:1:2.8. This work supports the validity of the so-called “platelet first” resuscitation for those orthopedic trauma patients who require massive transfusion, and further suggests that this may be optimally guided by TEG/PM. This concept is based on military as well as civilian data, which have shown improved mortality for those massive transfusion patients who were given a higher ratio of platelets to packed cells [22,57,58,59].
2.1.2. Long Bone Fractures
Isolated long bone fractures, similar to isolated pelvic fractures, may be associated with abnormalities across the hemostatic spectrum such that these patients can present with both a hypercoagulable state as well as hemorrhage, placing them at risk of both bleeding and early pulmonary emboli after resuscitation [60] (Table 3). One prospective observational study of 250 patients with femoral neck fractures who underwent repair with dynamic hip screw (DHS) or hemiarthroplasty demonstrated no statistical difference between the coagulation and fibrinolysis parameters between patients undergoing hemiarthroplasty and DHS insertion. In addition, no coagulation indices differed between spinal and general anesthesia. However, individual analysis of TEG parameters showed significant and persistent hypercoagulable changes in the postoperative mean K and MA values, but no significant alteration in mean LY30 or LY60. In a subgroup of 70 patients, venography was performed either based on clinical suspicion for deep vein thrombosis (DVT; 20% confirmed) or empirically on postoperative days 7–10 (28% positive for DVT); hypercoagulability on postoperative days 1–7 was significantly associated with DVT [61].

Table 3.
Summary of the literature pertaining to viscoelastic hemostatic assays applied for the treatment of patients with long bone fractures.
TEG and CCTs have also been shown in pooled femur and humerus fractures. In these patients, hypercoagulopathic findings have been described with decreased R and K parameters as well as an increased MA and alpha angle within four hours after fracture and immediately prior to surgery [62]. Although limited, these studies demonstrate the ability of VHA to assess for hypercoagulability as a marker of early risk for VTE during fracture management. Further research is warranted to guide the timing, dosage, and duration of VTE prophylaxis beyond empiric strategies.
2.1.3. VHA-Directed Management of Orthopedic Trauma Patients
The use of VHA during the resuscitation and operative management of orthopedic trauma patients has been shown to help identify and manage hypocoagulability in multiple studies (Table 4). The analysis of TEG MA and ROTEM MCF in 182 trauma patients evaluated in a prospective observational study revealed that early MAs were lower in patients with TIC and that these values for TEG MA and ROTEM MCF allowed differentiation between nontransfused patients, patients who received <10 units of packed RBCs, and patient who required more than 10 RBCs within the first 6 h of admission. In addition, platelet count and fibrinogen concentration revealed significant and superior correlations with the A10s of both TEG MA and the ROTEM MCF [47]. One prospective observational study compared rTEG and functional fibrinogen (FF-TEG) to kaolin TEG among 404 patients with a median ISS of 13. All TEG parameters, except the rTEG MA and the kaolin TEG MA, correlated significantly with mortality in orthopedic patients who had been transfused. The time from the initiation of the VHA assay to A5 and A10 was the lowest for rTEG and the TEG functional fibrinogen compared with the kaolin TEG. It was noted that rTEG A5 reduced the time to result by greater than 50% compared with the rTEG MA [63]. Taken together, these two studies suggest that the A5 and A10 for both the TEG MA and the ROTEM MCF may allow a faster assessment of the orthopedic patient’s hemostatic integrity than waiting for a completed TEG MA and ROTEM MCF. In a large study of 402 patients with trauma injury severity score (ISS) >15, including those with orthopedic injury, 132 patients at admission demonstrated hyperfibrinolysis (HF), as measured by FIBTEM. Using a multivariable Cox regression analysis, FIBTEM-HF at admission was independently associated with inpatient mortality (29/132 patients expired, 22%), highlighting the important role of VHA in the early detection and treatment of fibrinolysis—a state which is otherwise invisible to CCTs alone [64].

Table 4.
Summary of the literature pertaining to viscoelastic hemostatic assay-guided goal-directed therapy for orthopedic patients.
Importantly, although VHA detected hypocoagulability and can guide BCT and/or HAT, it also identifies hypercoagulability, which may influence thromboprophylaxis management (Table 4). Tsantes et al. studied preoperative and postoperative ROTEM values for 198 patients with fractured hips compared to age-matched controls. There was no detectable difference between the fracture and control patients based on CCTs. On the other hand, the ROTEM EXTEM parameters CT, CFT, MCF, and alpha angle, as well as the INTEM parameters CT, CFT, A10, MCF, and alpha angle, were significantly hypercoagulable in orthopedic fracture patients compared to age-matched controls. In addition, INTEM CT and CFT significantly decreased, while INTEM MCF, A10, and alpha angle significantly increased postoperatively, reflecting the exacerbation of existing hypercoagulability by fracture fixation. Thus, a hypercoagulable state following hip fractures and their operative repair can be detected by ROTEM soon after the initial trauma, which is not uncovered by conventional coagulation assays [65].
Among patient identifiable risk factors associated with hypercoagulability as determined by VHAs, age and gender have been specifically analyzed within the orthopedic population. In a study of patients undergoing orthopedic surgery for fracture repair, age was weakly correlated with increased hypercoagulability with all TEG parameters studied. This correlation was stronger for men than for women, and only R was significantly correlated with age when women were separately analyzed. In optimized regression models, age remained an independent predictor of hypercoagulable R, K, and alpha parameters. Therefore, age may need to be taken into account when interpreting TEG parameters and managing hypercoagulability in the elderly orthopedic trauma population [66].
2.2. Viscoelastic Hemostatic Assays in the Orthopedic Subspecialties
2.2.1. Arthroplasty
The diagnosis of coagulation abnormalities and their perioperative management during elective arthroplasty is also facilitated by the use of VHAs (Table 5). With respect to the use of VHAs to prospectively identify hypocoagulability as a risk factor for bleeding complications, one 75-patient observational study evaluating ecchymosis after TKA identified that a hypercoagulable alpha angle and coagulation index (CI: a calculated summary parameter taking into account TEG R, K, angle, and MA) were protective against the incidence of postoperative ecchymosis, which occurred in 25/75 (33%) patients [62]. In this group, TEG CI was an important prognostic tool for predicting operative blood loss, and the change in the TEG CI allowed the prediction of significant blood loss and the development of ecchymosis after TKA [67].

Table 5.
Summary of the literature pertaining to viscoelastic hemostatic assays applied to patients undergoing arthroplasty.
However, similar to orthopedic trauma scenarios, hypercoagulability was markedly common after arthroplasty. In one study, 20 patients underwent general anesthesia versus 32 receiving spinal anesthesia for THA. TEG demonstrated a progressively increased hypercoagulable MA postoperatively. The TEG also demonstrated intraoperative hypercoagulability that was temporary in patients receiving spinal anesthesia. As expected and reported by others, CCTs were normal in all patients in the pre- and postoperative periods except for an increase in fibrinogen concentration at day five postoperation. Despite VTE prophylaxis, patients following THA are in a hypercoagulable state, as measured by both TEG and by CCT fibrinogen measurement. It is proposed that these patients may benefit from more optimal anticoagulation and or additional perioperative hemostatic monitoring using VHAs [68].
The effect of thromboprophylaxis to mitigate this hypercoagulability has also been investigated using VHAs (Table 5). One study identified 90 patients undergoing elective unilateral arthroplasty surgery of the hip or knee followed by 10-day enoxaparin thromboprophylaxis. At day 9 postoperation, 34/90 (37.8%) of patients were found to be hypercoagulable. Most patients (26/34 patients, 76.5%) demonstrated a mixed hypercoagulable state with features of platelet hyperactivity. It was postulated that serial TEG analysis allowed the identification of hypercoagulopathic patients at risk for VTE following total hip and knee replacement, and that platelet hyperactivity may represent a specifically important factor in orthopedic patients’ hypercoagulable state [69].
The use of antifibrinolytic tranexamic acid (TXA) has been suggested to contribute to the hypercoagulable perioperative state in elective orthopedic surgery. One study evaluated the effect of TXA in primary hip arthroplasty (THA) and TKA, finding that multiple doses of TXA contributed to a hypercoagulable state, as demonstrated by shortened R times on postoperative days one and three for both THA and TKA. CCTs, as expected in cases of hypercoagulability, demonstrated no change. However, this prothrombotic state did not contribute to an increased incidence of VTE when patients were provided appropriate thromboprophylaxis [70]. On the other hand, a similar study of the effect of TXA demonstrated significant reductions in total blood loss, units of packed red blood cell transfusion, and overall transfusion rates without an increase in VTE complications. It was found that TEG analysis did not change with the administration of TXA in this group of patients. Specifically, 86 patients received a single dose of TXA in the standard 15 milligrams per kilogram IV dose compared to 88 controls without TXA, and TEG parameters and CCTs were collected on the day prior to surgery and on the first and seventh postoperative days. There was no difference in any TEG parameters and CCTs between the two groups. However, total blood loss and drain blood loss in the TXA group were significantly lower than in the control arthroplasty group [71]. A small study of 23 patients demonstrated that TXA was associated with a selective increase in inflammatory markers following TKA. Specifically, in the small group of 23 patients of whom 12 received TXA before and after surgery, the levels of the inflammatory mediators monocyte chemotactic protein (MCP)-1, tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 significantly increased compared to non-TXA patients, which was further amplified postoperatively. The authors also describe little change in the viscoelastic clot strength or fibrinolysis caused by TXA in this small group of patients [72]. Overall, additional work remains to be conducted to fully characterize the role and mechanisms of TXA with respect to coagulation and fibrinolysis in orthopedic patients, but VHA remains a useful tool to identify and manage any potential TXA-associated hypercoagulability.
VHA allows for sensitive study of other nonhemostatic aspects of intraoperative conduct on coagulation as well (Table 5). In one study of 22 patients, VHAs were utilized to investigate the effect of surgical pain on coagulation and fibrinolysis related to tourniquet use during TKA. TEG MA levels were noted to rise after tourniquet inflation only in patients undergoing general anesthesia; however, after deflation, MA values in both general and epidural anesthesia increased significantly. Fibrinolysis parameters did not change in either group during tourniquet inflation, but increased by 160% in both groups after touniquet deflation. This study suggests that surgical or tourniquet pain or both may impact coagulation, and epidural anesthesia was a useful technique to prevent hypercoagulopathy [73]. Topcu et al. studied the TEG effects of different resuscitative solutions comprising either Ringer’s lactate, 6% hydroxyethyl starch, or 4% succinylated gelatin solutions on the perioperative coagulability of orthopedic patients. This study demonstrated that all three solutions changed the coagulability in all patients, but these coagulation changes remained within normal limits [74]. However, it is concerning that overzealous intraoperative nonblood fluid management can contribute to potential perioperative coagulopathies with the most concerning being a dilutional hypocoagulopathy in the bleeding orthopedic surgical patient.
Importantly for implementation, variability between venous and arterial samples is known for several frequently monitored resuscitation markers, such as lactate. Venous and arterial variability in VHA parameters was studied using the INTEM, EXTEM, and FIBTEM assays of ROTEM in 52 patients at the time of arterial line insertion, intraoperatively, and postoperatively. There was no change in any parameters between venous and arterial samples, demonstrating that VHA assessment was consistent across both venous and arterial blood sampling [75].
2.2.2. Spine Surgery
The diagnosis of coagulation abnormalities and their perioperative management during elective spine surgery is also facilitated by the use of VHAs (Table 6). Specifically, a group of 80 patients undergoing lumbar spine fusion surgery were divided into aspirin-treated and aspirin-naïve groups, and analyzed via TEG. Correlation analysis showed no significant differences between aspirin-treated and aspirin-naïve groups in any assayed TEG parameter. This absence of dysfunction in aspirin-treated patients undergoing fusion suggests that the relaxation of the restriction of aspirin therapy to approximately 2–3 days prior to surgery rather than the standard seven days may be safe from a bleeding perspective [76].

Table 6.
Summary of the literature pertaining to viscoelastic hemostatic assays applied to patients undergoing spine surgery.
In a prospective cohort study evaluating the effect of different resuscitative fluids, the hemostatic competence of 66 spine fusion patients randomly assigned to receive gelatin solution, hydroxyethyl starch, or Ringer’s lactate solution was assessed. Plasma obtained prior to the induction of anesthesia and at the end of surgery was assayed for markers of fibrinolysis, and exposed to recombinant tissue plasminogen activator (tPA) in vitro followed by modified ROTEM analysis to sensitively assay for fibrin clot strength. While markers of fibrinolytic activity did not differ between groups, the tPA-modified ROTEM assay revealed more tPA-resistant clot formation in patients managed with crystalloid as opposed to colloid fluids, suggesting a subtle coagulopathy related to clot instability in patients receiving colloids [77]. In a similar study by the same researchers using the same patient population, modified ROTEM was used to correlate the changes in alpha angle, clot firmness, and fibrinogen polymerization, as manifested by the FIBTEM assay. The FIBTEM alpha angle and MCF were decreased in patients receiving hydroxyethyl starch, followed by lower magnitude decreases with gelatin, and the least reduction seen with Ringer’s lactate. In addition, 13 patients in the colloid groups but none in the crystalloid group required fibrinogen concentrate to maintain borderline FIBTEM clot firmness. Further, the activities of FVII, FVIII, FIX, and von Willebrand factor activity were significantly decreased in the colloid groups. The authors concluded that intraoperative dilutional coagulopathy is largely a function of decreased fibrin polymerization, which may be exacerbated by colloid administration compared to crystalloids [78].
Because high perioperative blood loss has been noted in spine and spinal deformity surgery along with other areas of orthopedic surgery, methods to reduce intraoperative bleeding include the use of cell savage, new surgical techniques, the substitution of coagulation factors, antifibrinolytics, desmopressin, induced hypertension, and the avoidance of hypothermia. The use of VHAs to guide anticoagulation in the perioperative period has been suggested and thoroughly reviewed elsewhere by Oliver Theusinger and Donat Spahn [79].
2.2.3. VHA-Guided Operative Conduct in Elective Orthopedic Surgery
The use of VHA to guide BCT and other elements of operative conduct has been shown to help identify and manage hypocoagulability during elective orthopedic surgery, similar to its utility in orthopedic trauma (Table 7). One large retrospective study evaluated the utility of TEG-guided BCT compared to CCT-guided BCT among 480 patients undergoing orthopedic surgery, finding that TEG-guided BCT was associated with lower overall transfusion volumes of RBC, FFP, platelets, and cryoprecipitate. In addition, the TEG-guided BCT patients were found to have shorter hospital stays compared to the CCT-guided group. This suggests that the TEG guidance of intraoperative BCT during elective orthopedic surgery is associated with optimized blood product usage and potentially improved outcomes overall [80]. The ability of CCTs versus ROTEM to identify perioperative coagulopathy in patients who underwent major elective orthopedic surgery was evaluated in 40 patients undergoing elective THA, femur fracture fixation, and spinal surgery. ROTEM analysis was compared to routine CCTs and thrombin generation profiles. Their findings demonstrated a correlation between a lower platelet count and ROTEM MCF in bleeding patients. At baseline, a significantly lower platelet count and FIBTEM MCF were observed in patents with excessive bleeding. Thrombocytopenia and a low fibrinogen activity were also associated with intraoperative bleeding in these patients. It was therefore proposed that ROTEM identified hypocoagulopathic phenotypes in patients receiving major elective orthopedic surgery [81]. In a prospective observational trial, perioperative coagulation monitoring and transfusion data were analyzed for 70 patients undergoing general orthopedic surgery. Of the 23 patients who were transfused, there was a reduction in FIBTEM below the 7 mm threshold. In addition, preoperative CT was also prolonged in the transfusion group when compared to the nontransfused group, identifying ROTEM parameters predictive of and associated with bleeding that may serve as useful targets to optimize BCT [82].

Table 7.
Summary of the literature pertaining to viscoelastic hemostatic assays applied to the intraoperative conduct of patients undergoing elective orthopedic surgery.
One study specifically evaluated the effect of intraoperative autotransfusion via cell salvage in 25 hip arthroplasty patients. ROTEM analysis as well as CCTs and cytokine levels were evaluated prior to surgery and within one hour of reinfusion of 300 cc or more of salvaged whole blood (average of 460 cc). ROTEM analysis demonstrated normal clot formation at baseline and no parameter significantly changed following reinfusion. In addition, CCTs were all normal in these patients. However, MCP-1 but no other cytokine levels were elevated after reinfusion. Therefore, the reinfusion of salvaged whole blood did not appear to alter the hemostatic integrity of these patients when assayed by either ROTEM or CCTs [83].
Similarly to the findings on the management of orthopedic trauma identified above (Table 4), the use of VHA during elective orthopedic surgery may allow for the optimization of nonhemostatic aspects of intraoperative conduct as well (Table 7). For example, one study evaluated the effect of various anesthetic and paralyzing agents on hemostatic integrity, identifying that supratherapeutic doses of sugammadex were associated with reduced clot strength as determined by TEG [84].
2.3. Prediction and Prevention of Venous Thromboembolism
The area where VHAs have found the greatest utility in the field of orthopedics is in the prediction and prevention of VTE. In a major study of 349 patients at eight level 1 European trauma centers, VTE incidence was found in 58% of patients sustaining major trauma who were screened by venography and who were not administered chemical thromboprophylaxis. Of 182 patients with pelvic and/or long bone lower extremity fractures, 69% developed VTE, with multivariate analysis demonstrating femur or tibia fracture as an independent risk factor for VTE [85]. Increased ISS scores have also demonstrated an increased odds ratio of developing VTE [86,87]. In addition, a delay in fracture fixation of four days or more post-trauma results in a three-fold increased risk of DVT in the trauma population [88]. Through their ability to identify and monitor hypercoagulability, VHAs offer a unique and singular opportunity to reduce the incidence of VTE postoperatively for orthopedic trauma patients (Table 8).

Table 8.
Summary of the literature pertaining to viscoelastic hemostatic assays applied to the prediction and prevention of venous thromboembolism among orthopedic operative patients.
In a systematic review of 31 studies, 17 studies cited an elevated MA as a significant predictor of VTE among 6348 total patients. A subsequent subcohort meta-analysis comprising 3180 patients from five studies demonstrated the ability of an MA >65 mm to predict VTE. However, there were inconsistencies in the cut-off MA value to define hypercoagulability in those patients who developed VTE. Other studies, however, have demonstrated that an MA >65 mm is a useful threshold for VHA prediction in orthopedic trauma patients [89]. Specifically, a group of 1818 patients stratified by extremity abbreviated injury severity scores (AIS) of 2+ or <2 were shown to have an odds ratio of developing a VTE of 3.66 for an MA >65 mm and a ratio of 6.7 for an MA >72 mm [90]. The use of surveillance ultrasound in orthopedic trauma patients to determine the incidence of postoperative VTE has been studied in prospective observational studies. Specifically, 983 patients, of whom 684 received both an admission TEG and surveillance ultrasound during admission, revealed that a VTE was diagnosed in 14.5% (99/684) of these patients. Of these 684 patients who received both a surveillance ultrasound and a TEG at index admission, 582 or 85.1% demonstrated hypercoagulable findings. Those patients with hypercoagulable findings had a much higher rate of lower extremity VTE than those who did not have a hypercoagulopathic TEG (15.6% versus 8%). After adjustment for relevant covariates, multivariate analysis demonstrated that a hypercoagulable admission TEG was associated with VTE with an odds ratio of 2.41 [24]. An additional retrospective study over 13 months evaluated 354 patients with peritrochanteric and femoral neck fractures who underwent hemiarthroplasty or cephalomedullary nailing. ROTEM parameters were evaluated for correlation with the hypercoagulable state and symptomatic VTE. ROTEM analysis was performed within hours of injury and also on the second postoperative day. The diagnosis of VTE was made by CT pulmonary angiography or vascular ultrasound, which was performed only in symptomatic patients. No routine screening exams were performed. Specific ROTEM parameters were compared between symptomatic and asymptomatic VTE. Preoperative MCF was found to be higher and postoperative CFT lower in patients with subsequent clinically proven VTE than in those patients without VTE. Based on this study, ROTEM provided a high level of accuracy in the detection of postoperative hypercoagulability after orthopedic surgery that was predictive of later symptomatic VTE [91].
However, not all studies have identified as clear a causal link between hypercoagulability and subsequent VTE. A differential population-based study used TEGs to test the hypothesis and confirm the clinical observation that the incidence of VTE was lower in Asian patients than in the Western population. The incidence of VTE was studied in 101 patients who had THA or TKA or surgeries for hip fractures without chemoprophylaxis for DVT. TEG was adopted to predict the presence of a hypercoagulable state and predict the occurrence of VTE. The incidence of VTE in this population was 7%. Of the patients who developed VTE, 6/7 had hip fractures while one had a TKA. Preoperative TEG was hypercoagulable in only 1/7 patients with VTEs but was also hypercoagulable in 37 of the remaining 94 patients without VTE. The incidence of DVT in the study population was sufficiently high to recommend some form of DVT prophylaxis in this Asian population following hip and knee surgery. However, TEG assessment preoperatively did not predict the risk of VTE [92]. These results are supported by a recent meta-analysis including more than 8000 postoperative patients across several surgical disciplines, which identified common hypercoagulability in postoperative patients but did not find that TEG-based MA was a significant predictor of VTE [89]. While work remains to be conducted to identify specific VHA parameters and patient factors predictive of VTE in orthopedic patients, overall, VHA-based hypercoagulability appears to be a significant risk factor for VTE.
Many initial studies of TEG in orthopedic surgery involved the use of TEG to monitor anticoagulant effects. In a group of 52 patients undergoing TKA who received prophylactic warfarin and epidural anesthesia, daily INR values and TEG parameters were compared with preoperative values. The threshold for the removal of the epidural catheter was on POD2 if the INR was ≤2. If the INR was persistently elevated >2 on POD2, warfarin was held until the INR dropped ≤2 prior to catheter removal. On the day of epidural catheter removal, the INR was elevated (mean INR, 1.5), while the TEG parameters K, MA, alpha angle, and CI remained normal in those patients who received low-dose warfarin DVT prophylaxis [93]. With respect to LMWH chemoprophylaxis, anti-Xa levels as a marker for enoxaparin efficacy were compared to TEG parameters for the ability to predict VTE prophylaxis effectiveness. In a prospective study of 24 patients who had unilateral TKA or THA, the mean R time and K time in TEG correlated with the anticipated peak and trough levels of enoxaparin and serum anti-Xa levels. It was shown that the R time and K time on postoperative day 3 indicated an exaggerated response to enoxaparin, and TEG was suggested as a test that could potentially monitor the degree of anticoagulation required to provide effective VTE prophylaxis for patients administered low-molecular-weight heparins [94].
The use of VHAs to direct VTE prophylaxis has traditionally involved the use of LMWH for orthopedic patients. However, recent evidence suggests that novel oral anticoagulants (NOACs, including direct thrombin inhibitors and Factor Xa inhibitors) may be efficacious and more cost-effective than LMWH for VTE prophylaxis after orthopedic surgery [95]. Whether VHAs can be used to monitor NOAC-based prophylaxis is an active area of investigation. One proof-of-concept study investigated the in vitro effect of varying concentrations of dabigatran (a direct thrombin inhibitor) on whole blood, demonstrating that dabigatran prolonged the TEG R time, and correlated well with validated thrombin inhibitor tests such as the Hemoclot thrombin inhibtor assay (manufacturer: HYPHEN BioMed; Neuville-sur-Oise, France) and ecarin clotting time [96]. Another proof-of-concept study including the Xa inhibitors rivaroxaban and apixaban in addition to dabigatran showed that the in vitro addition of these agents to healthy volunteer blood samples led to the prolongation of both the R time and the maximum rate of thrombin generation, demonstrating the feasibility of monitoring NOAC therapy with TEG [97]. In a prospective observational study of 188 patients undergoing elective THA or TKA who were administered either a 40 mg daily subcutaneous dose of enoxaparin or 10 mg oral dose of rivaroxaban, pre- and postoperative ROTEM parameters were analyzed and compared to thrombin generation and coagulation activation markers. Rivaroxaban increased the extrinsic ROTEM CT more robustly than enoxaparin, and resulted in impaired clot initiation and propagation in thrombin generation studies as well as lower plasma markers of clot formation. Interestingly, the levels of the anticoagulant antithrombin III were reduced after enoxparin treatment but preserved after rivaroxaban treatment, suggesting the preservation of natural anticoagulants as a mechanism for more efficacious VTE prevention compared to enoxaparin [98].
2.4. Future Study
The current literature surrounding the use of VHAs in orthopedic surgery has provided a foundation, but there are gaps in the literature that need to be addressed. There remains a necessity for longitudinal studies following orthopedic patient outcomes with and without the use of VHAs to guide management. Additionally, studies to assess the prognostic value of VHAs in both elective and nonelective orthopedic surgery patients may allow for proactive and beneficial changes in patient management.
2.5. Summary of Principles for VHA Use in Orthopedic Patients
This review has summarized data supporting three key assertions:
VHAs optimize the resuscitation and intraoperative management of hypocoagulability and hyperfibrinolysis in both orthopedic trauma and elective surgical patients.
VHAs also detect clinically significant hypercoagulability and impaired fibrinolysis, which are otherwise undetectable by CCTs.
The VHA-based identification and management of hypercoagulability has significant implications for risk stratification and chemoprophylaxis management of VTE prophylaxis in orthopedic patients.
3. Conclusions
Orthopedic coagulopathy consists of a spectrum of phenotypes that are multifactorial in relation to patient factors, injury patterns, the conduct of resuscitation and surgical procedures, and perioperative VTE prophylaxis strategies. The last 20 years have seen a dramatic growth of bedside VHA use for the management of both hypo- and hypercoagulopathies, with orthopedic surgery being relatively late to the game. There has been an expansion of the routine use of these tests in the management of not just trauma, but also in all postsurgical, obstetrical, and medical intensive care units where severe bleeding is often encountered. Since major hemorrhage is not an uncommon finding in orthopedic trauma, the same rigor of monitoring these patients at the bedside during the initial stages of resuscitation now requires that orthopedic traumatologists adapt and familiarize themselves with the use of VHAs. Beyond orthopedic trauma alone, there is also a profound proof of concept that VTE prophylaxis should entail a VHA-driven individualized approach for dosing and duration as compared to the antiquated empiric strategy. The future use of VHAs in orthopedic trauma and elective surgery must become more commonplace. It is imperative that current and future orthopedic surgeons are educated on the applied science, rationale, and methodical use of VHAs as they relate to BCT transfusions, the administration of HAT products, the pre-operative assessment and management of patients taking anticoagulants, and the management of VTE prophylaxis. Future studies are necessary to further expand the routine acceptance and use of VHAs over CCTs, which will only begin with a widespread acceptance of VHAs by the orthopedic surgical community.
Author Contributions
Conceptualization, C.N.M., J.S., J.E.S., J.D. and A.P.; writing—original draft preparation, J.S., J.E.S., J.D., A.P., E.E.P. and D.G.; writing—review and editing, C.N.M., J.S., J.D., A.P., M.A., E.C., M.M., J.L., S.L., R.S. and M.E.K.; visualization, C.N.M., J.S., J.E.S., J.D., A.P. and M.E.K.; supervision, J.S. and J.E.S.; project administration, J.S. and J.E.S.; funding acquisition, C.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
M.E.K. receives funding from the National Institutes of Health, grant number K08 GM-138812, outside the submitted work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| A5 | amplitude at 5 min |
| A10 | amplitude at 10 min |
| A20 | amplitude at 20 min |
| ACT | activated clotting time |
| AIS | abbreviated injury severity score |
| BCT | blood component therapy |
| CA5 | clot amplitude at 5 min |
| CA10 | clot amplitude at 10 min |
| CCT | common coagulation test |
| CFT | clot formation time |
| CI | coagulation index |
| CL30 | clot lysis index at 30 min |
| CLI60 | clot lysis index at 60 min |
| CT | clotting time |
| DDAVP | desmopressin |
| DHS | dynamic hip screw |
| DVT | deep vein thrombosis |
| ECT | ecarin clotting time |
| ELISA | enzyme-linked immunosorbent assay |
| FCS | fibrinogen contribution to clot stiffness |
| FF | functional fibrinogen |
| FFP | fresh frozen plasma |
| GA | general anesthesia |
| HAT | hemostatic adjunct therapy |
| HES | hydroxyethyl starch |
| HF | hyperfibrinolysis |
| IL | interleukin |
| INR | international normalized ratio |
| ISS | injury severity score |
| ITACTIC | implementing treatment algorithms for the correction of trauma-induced coagulopathy |
| K | clot kinetics |
| k-TEG | kaolin thromboelastography |
| LMWH | low molecular weight heparin |
| LY30 | lysis at 30 min |
| MA | maximum amplitude |
| MAff | maximum amplitude functional fibrinogen |
| MCF | maximum clot firmness |
| MCP | monocyte chemotactic protein |
| ML | maximum lysis |
| MRTG | maximum rate of thrombus generation |
| NATEM | native thromboelastometry |
| NOAC | novel oral anticoagulant |
| PAI-1 | plasminogen activator inhibitor-1 |
| PBM | precision-based medicine |
| PCC | prothrombin complex concentrate |
| PCS | platelet contribution to clot stiffness |
| PE | pulmonary embolism |
| POD | postoperative day |
| pRBC | packed red blood cells |
| PT | prothrombin time |
| PTT | partial thromboplastin time |
| R | reaction time |
| RL | Ringer’s lactate |
| ROTEM | rotational thromboelastography |
| rTEG | rapid thromboelastography |
| TAT | thrombin–antithrombin complex |
| TDR | thrombodynamic ratio |
| TEG | thromboelastography |
| TEG/PM | thromboelastography with platelet mapping |
| THA | total hip arthroplasty |
| TIC | trauma-induced coagulopathy |
| TKA | total knee arthroplasty |
| TMRTG | time to maximum rate of thrombus generation |
| TNF | tumor necrosis factor |
| tPA | tissue plasminogen activator |
| TXA | tranexamic acid |
| VCM | viscocoagulation monitoring |
| VCT | viscocoagulation testing |
| VHA | viscoelastic hemostatic assay |
| VTE | venous thromboembolism |
References
- Brohi, K.; Singh, J.; Heron, M.; Coats, T. Acute traumatic coagulopathy. J. Trauma Acute Care Surg. 2003, 54, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J.B.A.; Lynn, M.; McKenney, M.G.; Cohn, S.M.; Murtha, M. Early Coagulopathy Predicts Mortality in Trauma. J. Trauma Acute Care Surg. 2003, 55, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Brohi, K.; Cohen, M.J.; Davenport, R. Acute coagulopathy of trauma: Mechanism, identification and effect. Curr. Opin. Crit. Care 2007, 13, 680–685. [Google Scholar] [CrossRef]
- Niles, S.E.; McLaughlin, D.F.; Perkins, J.G.; Wade, C.E.; Li, Y.; Spinella, P.C.; Holcomb, J.B. Increased Mortality Associated with the Early Coagulopathy of Trauma in Combat Casualties. J. Trauma Acute Care Surg. 2008, 64, 1459–1465, discussion 63–65. [Google Scholar] [CrossRef] [PubMed]
- Maegele, M.; Lefering, R.; Yucel, N.; Tjardes, T.; Rixen, D.; Paffrath, T. Early Coagulopathy in mulitple injury: An analysis from the German Trauma Registry on 8724 patients. Injury 2007, 38, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Pierce, B. Trauma-induced coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef]
- Wikkelsø, A.; Wetterslev, J.; Møller, A.M.; Afshari, A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst. Rev. 2016, 2016, CD007871. [Google Scholar] [CrossRef]
- Gonzalez, E.; Moore, E.E.; Moore, H.B.; Chapman, M.P.; Chin, T.L.; Ghasabyan, A.; Wohlauer, M.V.; Barnett, C.C.; Bensard, D.D.; Biffl, W.L.; et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann. Surg. 2016, 263, 1051–1059. [Google Scholar] [CrossRef]
- Johansson, P.I. Goal-directed hemostatic resuscitation for massively bleeding patients: The Copenhagen concept. Transfus. Apher. Sci. 2010, 43, 401–405. [Google Scholar] [CrossRef]
- Nardi, G.; Agostini, V.; Rondinelli, B.; Russo, E.; Bastianini, B.; Bini, G.; Bulgarelli, S.; Cingolani, E.; Donato, A.; Gambale, G.; et al. Trauma-induced coagulopathy: Impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit. Care 2015, 19, 83. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Minei, K.M.; Scerbo, M.L.; Radwan, Z.A.; Wade, C.E.; Kozar, R.A.; Gill, B.S.; Albarado, R.; McNutt, M.K.; Khan, S.; et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: Experience with 1974 consecutive trauma patients. Ann. Surg. 2012, 256, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Schöchl, H.; Nienaber, U.; Hofer, G.; Voelckel, W.; Jambor, C.; Scharbert, G.; Kozek-Langenecker, S.; Solomon, C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM®)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit. Care 2010, 14, R55. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Bugaev, N.; Como, J.J.; Golani, G.; Freeman, J.J.; Sawhney, J.S.; Vatsaas, C.J.; Yorkgitis, B.K.; Kreiner, L.A.; Garcia, N.M.; Aziz, H.A.; et al. Thromboelastography and rotational thromboelastometry in bleeding patients with coagulopathy: Practice management guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2020, 89, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Thomas, S.; Kwaan, H.; Aversa, J.; Anderson, S.; Sundararajan, R.; Zimmer, D.; Bunch, C.; Stillson, J.; Draxler, D.; et al. Modern methods for monitoring hemorrhagic resuscitation in the United States: Why the delay? J. Trauma Acute Care Surg. 2020, 89, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Schöchl, H.; Maegele, M.; Solomon, C.; Görlinger, K.; Voelckel, W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 15. [Google Scholar] [CrossRef]
- Görlinger, K.; Pérez-Ferrer, A.; Dirkmann, D.; Saner, F.; Maegele, M.; Calatayud, Á.A.P.; Kim, T.-Y. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J. Anesthesiol. 2019, 72, 297–322. [Google Scholar] [CrossRef]
- Vorweg, M.; Hanke, A.; Görlinger, K.; Lier, H. Thromboelastometry guided therapy of severe bleeding. Hämostaseologie 2013, 33, 51–61. [Google Scholar] [CrossRef]
- Baksaas-Aasen, K.; Gall, L.S.; Stensballe, J.; Juffermans, N.P.; Curry, N.; Maegele, M.; Brooks, A.; Rourke, C.; Gillespie, S.; Murphy, J.; et al. Viscoelastic Haemostatic Assay Augmented Protocols for Major Trauma Haemorrhage (ITACTIC): A Randomised, Controlled Trial. Intensive Care Med. 2020, 47, 49–59. [Google Scholar] [CrossRef]
- Kaufmann, C.R.; Dwyer, K.M.; Crews, J.D.; Dols, S.J.; Trask, A.L. Usefulness of Thrombelastography in Assessment of Trauma Patient Coagulation. J. Trauma Acute Care Surg. 1997, 42, 716–722. [Google Scholar] [CrossRef]
- Mauffrey, C.; Cuellar, D.; Pieracci, F.; Hak, D.; Hammerberg, E.; Stahel, P.; Burlew, C.C. Strategies for the management of haemorrhage following pelvic fractures and associated trauma-induced coagulopathy. Bone Jt. J. 2014, 96, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Mamczak, C.N.; Maloney, M.; Fritz, B.; Boyer, B.; Thomas, S.; Evans, E.; Ploplis, V.A.; Castellino, F.J.; McCollester, J.; Walsh, M. Thromboelastography in Orthopaedic Trauma Acute Pelvic Fracture Resuscitation: A Descriptive Pilot Study. J. Orthop. Trauma 2016, 30, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.T.; Coleman, J.R.; Carmichael, H.; Mauffrey, C.; Vintimilla, D.R.; Samuels, J.M.; Sauaia, A. High rate of fibrinolytic shutdown and venous thromboembolism in patients with severe pelvic fracture. J. Surg. Res. 2020, 246, 182–189. [Google Scholar] [CrossRef]
- Brill, J.B.; Badiee, J.; Zander, A.L.; Wallace, J.D.; Lewis, P.R.; Sise, M.J.; Bansal, V.; Shackford, S.R. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. J. Trauma Acute Care Surg. 2017, 83, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Van, P.Y.; Cho, S.D.; Underwood, S.J.; Morris, M.; Watters, J.M.; Schreiber, M.A. Thrombelastography Versus AntiFactor Xa Levels in the Assessment of Prophylactic-Dose Enoxaparin in Critically Ill Patients. J. Trauma Acute Care Surg. 2009, 66, 1509–1517, discussion 15–17. [Google Scholar] [CrossRef]
- Hagedorn, J.C.; Bardes, J.M.; Paris, C.L.; Lindsey, R.W. Thromboelastography for the Orthopaedic Surgeon. J. Am. Acad. Orthop. Surg. 2019, 27, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Luddington, R.J. Thrombelastography/thromboelastometry. Clin. Lab. Haematol. 2005, 27, 81–90. [Google Scholar] [CrossRef]
- Thakur, M.; Ahmed, A.B. A review of thromboelastography. Int. J. Periop. Ultrasound Appl. Technol. 2012, 1, 25–29. [Google Scholar] [CrossRef]
- Multipurpose System for In Vitro Coagulation Studies; US Food & Drug Administration: Silver Spring, MD, USA, 2019.
- Curry, N.S.; Davenport, R.; Pavord, S.; Mallett, S.V.; Kitchen, D.; Klein, A.A.; Maybury, H. The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br. J. Haematol. 2018, 182, 789–806. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Tantry, U.S.; Monroe, A.L.; Muresan, A.A.; Brunner, N.E.; Lopez-Espina, C.G.; Delmenico, P.R.; Cohen, E.; Raviv, G.; et al. First report of the point-of-care TEG: A technical validation study of the TEG-6S system. Platelets 2016, 27, 642–649. [Google Scholar] [CrossRef]
- Hartmann, J.; Murphy, M.; Dias, J.D. Viscoelastic Hemostatic Assays: Moving from the Laboratory to the Site of Care—A Review of Established and Emerging Technologies. Diagnostics 2020, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Shore-Lesserson, L.; Manspeizer, H.E.; DePerio, M.; Francis, S.; Vela-Cantos, F.; Ergin, M.A. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth. Analg. 1999, 88, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Spalding, G.J.; Hartrumpf, M.; Sierig, T.; Oesberg, N.; Kirschke, C.G.; Albes, J.M. Cost reduction of perioperative coagulation management in cardiac surgery: Value of ‘bedside’ thrombelastography (ROTEM). Eur. J. Cardiothorac. Surg. 2007, 31, 1052–1057. [Google Scholar] [CrossRef]
- Kang, Y.G.; Martin, D.J.; Marquez, J.; Lewis, J.H.; Bontempo, F.A.; Shaw, B.W.; Starzl, T.E.; Winter, P.M. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth. Analg. 1985, 64, 888–896. [Google Scholar] [CrossRef]
- Trzebicki, J.; Flakiewicz, E.; Kosieradzki, M.; Blaszczyk, B.; Kołacz, M.; Jureczko, L.; Pacholczyk, M.; Chmura, A.; Lagiewska, B.; Lisik, W.; et al. The use of thromboelastometry in the assessment of hemostasis during orthotopic liver transplantation reduces the demand for blood products. Ann. Transplant. 2010, 15, 19–24. [Google Scholar] [PubMed]
- Snegovskikh, D.; Souza, D.; Walton, Z.; Dai, F.; Rachler, R.; Garay, A.; Snegovskikh, V.V.; Braveman, F.R.; Norwitz, E.R. Point-of-care viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J. Clin. Anesthesia 2018, 44, 50–56. [Google Scholar] [CrossRef]
- Sankarankutty, A.; Nascimento, B.; da Luz, L.T.; Rizoli, S. TEG® and ROTEM® in trauma: Similar test but different results? World J. Emerg. Surg. 2012, 7, S3. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef]
- Veigas, P.V.; Callum, J.; Rizoli, S.; Nascimento, B.; da Luz, L.T. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 114. [Google Scholar] [CrossRef]
- Gillissen, A.; Akker, T.V.D.; Caram-Deelder, C.; Henriquez, D.D.C.A.; Bloemenkamp, K.W.M.; Eikenboom, J.; van der Bom, J.G.; de Maat, M.P.M. Comparison of thromboelastometry by ROTEM® Delta and ROTEM® Sigma in women with postpartum haemorrhage. Scand. J. Clin. Lab. Investig. 2019, 79, 32–38. [Google Scholar] [CrossRef]
- Schenk, B.; Görlinger, K.; Treml, B.; Tauber, H.; Fries, D.; Niederwanger, C.; Oswald, E. A comparison of the new ROTEM® sigma with its predecessor, the ROTEMdelta. Anaesthesia 2019, 74, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Field, A.; Poole, T.; Bamber, J. ROTEM® sigma reference range validity. Anaesthesia 2019, 74, 1063. [Google Scholar] [CrossRef] [PubMed]
- Volod, O.; Bunch, C.M.; Zackariya, N.; Moore, E.E.; Moore, H.B.; Kwaan, H.C.; Neal, M.D.; Al-Fadhl, M.D.; Patel, S.S.; Wiarda, G.; et al. Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. J. Clin. Med. 2022, 11, 860. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.; Stanworth, S.; Curry, N.; Woolley, T.; Cooper, C.; Ukoumunne, O.; Zhelev, Z.; Hyde, C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst. Rev. 2015, 2, CD010438. [Google Scholar] [CrossRef] [PubMed]
- Schöchl, H.; Voelckel, W.; Grassetto, A.; Schlimp, C.J. Practical application of point-of-care coagulation testing to guide treatment decisions in trauma. J. Trauma Acute Care Surg. 2013, 74, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.S.P.; Meyer, M.A.S.; Sørensen, A.M.; Rasmussen, L.S.; Hansen, M.B.; Holcomb, J.B.; Cotton, B.A.; Wade, C.E.; Ostrowski, S.R.; Johansson, P.I. Thrombelastography and rotational thromboelastometry early amplitudes in 182 trauma patients with clinical suspicion of severe injury. J. Trauma Acute Care Surg. 2014, 76, 682–690. [Google Scholar] [CrossRef]
- Baumann, H.M.; Klinkspoor, J.H.; Goslings, J.C.; Juffermans, N.P.; Wirtz, M.R. Viscoelastic Testing in Trauma. Semin. Thromb. Hemost. 2017, 43, 375–385. [Google Scholar] [CrossRef]
- Solomon, C.; Schöchl, H.; Ranucci, M.; Schlimp, C. Can the Viscoelastic Parameter α-Angle Distinguish Fibrinogen from Platelet Deficiency and Guide Fibrinogen Supplementation? Anesth. Analg. 2015, 121, 289–301. [Google Scholar] [CrossRef]
- Groves, D.S.; Welsby, I.J.; Naik, B.I.; Tanaka, K.; Hauck, J.N.; Greenberg, C.S.; Winegar, D.A.; Viola, F. Multicenter Evaluation of the Quantra QPlus System in Adult Patients Undergoing Major Surgical Procedures. Anesth. Analg. 2020, 130, 899–909. [Google Scholar] [CrossRef]
- Naik, B.I.; Tanaka, K.; Sudhagoni, R.G.; Viola, F. Prediction of hypofibrinogenemia and thrombocytopenia at the point of care with the Quantra® QPlus® System. Thromb. Res. 2020, 197, 88–93. [Google Scholar] [CrossRef]
- Brearton, C.; Rushton, A.; Parker, J.; Martin, H.; Hodgson, J. Performance Evaluation of a New Point of Care Viscoelastic Coagulation Monitoring System in Major Abdominal, Orthopaedic and Vascular Surgery. Platelets 2020, 31, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Bostian, P.A.; Ray, J.J.; Karolcik, B.A.; Bramer, M.A.; Wilson, A.; Dietz, M.J. Thromboelastography is predictive of mortality, blood transfusions, and blood loss in patients with traumatic pelvic fractures: A retrospective cohort study. Eur. J. Trauma Emerg. Surg. 2020, 48, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kane, I.; Ong, A.; Orozco, F.R.; Post, Z.D.; Austin, L.S.; Radcliff, K.E. Thromboelastography Predictive of Death in Trauma Patients. Orthop. Surg. 2015, 7, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B.; Moore, E.E.; Neal, M.D.; Sheppard, F.R.; Kornblith, L.Z.; Draxler, D.F.; Walsh, M.; Medcalf, R.L.; Cohen, M.J.; Cotton, B.A.; et al. Fibrinolysis Shutdown in Trauma: Historical Review and Clinical Implications. Anesth. Analg. 2019, 129, 762–773. [Google Scholar] [CrossRef]
- Moore, H.; Moore, E.; Gonzalez, E.; Chapman, M.; Chin, T.; Silliman, C.; Banerjee, A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma Acute Care Surg. 2014, 77, 811–817, discussion 7. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs. a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef]
- McCurdy, M.; Spilger-Liew Walsh, M. Mortality and ratio of blood products used in patients with severe trauma. JAMA 2015, 313, 2077. [Google Scholar] [CrossRef]
- Spinella, P.C.; Wade, C.E.; Blackbourne, L.H.; Borgman, M.A.; Zarzabal, L.A.; Du, F.; Perkins, J.G.; Maegele, M.; Schreiber, M.; Hess, J.R.; et al. The Association of Blood Component Use Ratios with the Survival of Massively Transfused Trauma Patients With and Without Severe Brain Injury. J. Trauma Acute Care Surg. 2011, 71, S343–S352. [Google Scholar] [CrossRef]
- Coleman, J.J.; Zarzaur, B.L.; Katona, C.W.; Plummer, Z.J.; Johnson, L.S.; Fecher, A.; O’Rear, J.M.; Feliciano, D.V.; Rozycki, G.S. Factors Associated with Pulmonary Embolism Within 72 Hours of Admission after Trauma: A Multicenter Study. J. Am. Coll. Surg. 2015, 220, 731–736. [Google Scholar] [CrossRef]
- Wilson, D.; Cooke, E.; McNally, M.; Wilson, H.; Yeates, A.; Mollan, R. Changes in coagulability as measured by thrombelastography following surgery for proximal femoral fracture. Injury 2001, 32, 765–770. [Google Scholar] [CrossRef]
- Liu, C.; Guan, Z.; Xu, Q.; Zhao, L.; Song, Y.; Wang, H. Relation of thromboelastography parameters to conventional coagulation tests used to evaluate the hypercoagulable state of aged fracture patients. Medicine 2016, 95, e3934. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.H.; Meyer, M.A.S.; Meyer, A.S.P.; Gaarder, T.; Naess, P.A.; Stensballe, J.; Ostrowski, S.R.; Johansson, P.I. Thrombelastography early amplitudes in bleeding and coagulopathic trauma patients: Results from a multicenter study. J. Trauma Acute Care Surg. 2018, 84, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-J.; Park, S.-W.; Bae, B.-K.; Lee, S.-H.; Choi, H.J.; Ahn, T.Y.; Goh, T.S.; Lee, M.J.; Yeom, S.R.; Park, S.J. FIBTEM Improves the Sensitivity of Hyperfibrinolysis Detection in Severe Trauma Patients: A Retrospective Study Using Thromboelastometry. Sci. Rep. 2020, 10, 6980. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Trikoupis, I.G.; Papadopoulos, D.V.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Savvidou, O.D.; Kontogeorgakos, V.A.; Piovani, D.; Kriebardis, A.G.; et al. Higher coagulation activity in hip fracture patients: A case-control study using rotational thromboelastometry. Int. J. Lab. Hematol. 2020, 43, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.F.J. Changes in Thrombelastograph Variables Associated with Aging. Anesth. Analg. 2004, 99, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.-L.; Shi, Z.-J.; Zhang, Y. The Application of Thromboelastography in Understanding and Management of Ecchymosis after Total Knee Arthroplasty. J. Arthroplast. 2018, 33, 3754–3758. [Google Scholar] [CrossRef]
- Lloyd-Donald, P.; Lee, W.-S.; Liu, G.-M.; Bellomo, R.; McNicol, L.; Weinberg, L. Thromboelastography in elective total hip arthroplasty. World J. Orthop. 2021, 12, 555–564. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Z.; Dai, W.; Shi, P.; Luo, L.; Zhang, C. Changes of thrombelastography in patients undergoing elective primary total knee and total hip replacement with low molecular heparin prophylaxis. J. Orthop. Surg. Res. 2014, 9, 52. [Google Scholar] [CrossRef]
- Wu, X.-D.; Chen, Y.; Tian, M.; He, Y.; Tao, Y.-Z.; Xu, W.; Cheng, Q.; Chen, C.; Liu, W.; Huang, W. Application of thrombelastography (TEG) for safety evaluation of tranexamic acid in primary total joint arthroplasty. J. Orthop. Surg. Res. 2019, 14, 214. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Sun, M.-J.; Pan, S.; Rui, M.; Zhao, F.-C.; Zha, G.-C.; Pang, Y.; Zheng, X.; Guo, K.-J. Intravenous administration of tranexamic acid in total hip arthroplasty does not change the blood coagulopathy: A prospective thrombelastography analysis. J. Orthop. Surg. 2020, 28, 2309499020959516. [Google Scholar] [CrossRef]
- Grant, A.L.; Letson, H.L.; Morris, J.L.; McEwen, P.; Hazratwala, K.; Wilkinson, M.; Dobson, G.P. Tranexamic acid is associated with selective increase in inflammatory markers following total knee arthroplasty (TKA): A pilot study. J. Orthop. Surg. Res. 2018, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Kohro, S.; Yamakage, M.; Arakawa, J.; Kotaki, M.; Omote, T.; Namiki, A. Surgical/tourniquet pain accelerates blood coagulability but not fibrinolysis. Br. J. Anaesth. 1998, 80, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Topçu, I.; Çivi, M.; Öztürk, T.; Keleş, G.; Çoban, S.; Yentür, E.; Okçu, G. Evaluation of hemostatic changes using thromboelastography after crystalloid or colloid fluid administration during major orthopedic surgery. Braz. J. Med Biol. Res. 2012, 45, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Oswald, E.; Finsterwalder, T.; Innerhofer, N.; Haas, T.; Mittermayr, M.; Strohmaier, S.; Innerhofer, P. Comparison of arterial versus venous parameters of Rotational thromboelastometry and multiple platelet function analyzer: Results of a pilot study. Scand. J. Clin. Lab. Investig. 2013, 73, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.; Zhang, S.; Liu, S.; Yuan, J.; Wang, C.; Miao, X.; Lin, X.; Li, J.; Shi, Z. Perioperative Coagulation Profile with Thromboelastography in Aspirin-Treated Patients Undergoing Posterior Lumbar Fusion. Pain Physician 2020, 23, E619–E628. [Google Scholar]
- Mittermayr, M.; Streif, W.; Haas, T.; Fries, D.; Velik-Salchner, C.; Klingler, A.; Innerhofer, P. Effects of colloid and crystalloid solutions on endogenous activation of fibrinolysis and resistance of polymerized fibrin to recombinant tissue plasminogen activator added ex vivo. Br. J. Anaesth. 2008, 100, 307–314. [Google Scholar] [CrossRef][Green Version]
- Mittermayr, M.; Streif, W.; Haas, T.; Fries, D.; Velik-Salchner, C.; Klingler, A.; Oswald, E.; Bach, C.; Schnapka-Koepf, M.; Innerhofer, P. Hemostatic Changes After Crystalloid or Colloid Fluid Administration During Major Orthopedic Surgery: The Role of Fibrinogen Administration. Anesth. Analg. 2007, 105, 905–917. [Google Scholar] [CrossRef]
- Theusinger, O.M.; Spahn, D.R. Perioperative blood conservation strategies for major spine surgery. Best Pract. Res. Clin. Anaesthesiol. 2015, 30, 41–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Zhang, Y.; Yu, L.; Zhang, K. Thromboelastogram-Guided Transfusion Therapy Reduces Blood-Component Transfusion and Improves Coagulation Function during Orthopedic Surgery. J. Nanomater. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- Spiezia, L.; Vasques, F.; Behr, A.U.; Campello, E.; Maggiolo, S.; Berizzi, A.; Gavasso, S.; Woodhams, B.; Biancari, F.; Simioni, P. Perioperative coagulation assessment of patients undergoing major elective orthopedic surgery. Intern. Emerg. Med. 2016, 11, 793–801. [Google Scholar] [CrossRef]
- Hanke, A.A.; Bartlau, J.; Flöricke, F.; Przemeck, M.; Horstmann, H.; Weber-Spickschen, T.S.; Sieg, L.; Schumacher, C. Coagulation monitoring and transfusion in major non-emergency orthopaedic surgery—An observational study. J. Orthop. 2020, 22, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Froessler, B.; Weber, I.; Hodyl, N.A.; Saadat-Gilani, K. Dynamic changes in clot formation determined using thromboelastometry after reinfusion of unwashed anticoagulated cell-salvaged whole blood in total hip arthroplasty. Blood Transfus. 2015, 13, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.O.; Kim, Y.S.; Chang, H.W.; Kim, H.; Lim, B.G.; Lee, M. In vitro investigation of the effects of exogenous sugammadex on coagulation in orthopedic surgical patients. BMC Anesthesiol. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Geerts, W.H.; Code, K.I.; Jay, R.M.; Chen, E.; Szalai, J.P. A Prospective Study of Venous Thromboembolism after Major Trauma. N. Engl. J. Med. 1994, 331, 1601–1606. [Google Scholar] [CrossRef]
- Cotton, B.A.; Minei, K.M.; Radwan, Z.A.; Matijevic, N.; Pivalizza, E.; Podbielski, J.; Wade, C.E.; Kozar, R.A.; Holcomb, J.B. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J. Trauma Acute Care Surg. 2012, 72, 1470–1477. [Google Scholar] [CrossRef]
- Stawicki, S.P.; Grossman, M.D.; Cipolla, J.; Hoff, W.S.; Hoey, B.A.; Wainwright, G.; Reed III, J.F. Deep venous thrombosis and pulmonary embolism in trauma patients: An overstatement of the problem? Am. Surg. 2005, 71, 387–391. [Google Scholar] [CrossRef]
- Nathens, A.B.; McMurray, M.K.; Cuschieri, J.; Durr, E.A.; Moore, E.E.; Bankey, P.E.; Freeman, B.; Harbrecht, B.G.; Johnson, J.L.; Minei, J.P.; et al. The Practice of Venous Thromboembolism Prophylaxis in the Major Trauma Patient. J. Trauma Acute Care Surg. 2007, 62, 557–563. [Google Scholar] [CrossRef]
- Brown, W.; Lunati, M.; Maceroli, M.; Ernst, A.; Staley, C.; Johnson, R.; Schenker, M. Ability of Thromboelastography to Detect Hypercoagulability: A Systematic Review and Meta-Analysis. J. Orthop. Trauma 2020, 34, 278–286. [Google Scholar] [CrossRef]
- Gary, J.L.; Schneider, P.S.; Galpin, M.; Radwan, Z.; Munz, J.W.; Achor, T.S.; Cotton, B.A. Can thrombelastography predict venous thromboembolic events in patients with severe extremity trauma? J. Orthop. Trauma 2016, 30, 294–298. [Google Scholar]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Piovani, D.; Kriebardis, A.G.; Gialeraki, A.; Nikolopoulos, G.K.; et al. Rotational Thromboelastometry Findings Are Associated with Symptomatic Venous Thromboembolic Complications after Hip Fracture Surgery. Clin. Orthop. Relat. Res. 2021. ahead of print. [Google Scholar] [CrossRef]
- Parameswaran, A.; Krishnamoorthy, V.P.; Oommen, A.T.; Jasper, A.; Korula, R.J.; Nair, S.C.; Poonnoose, P.M. Is pre-operative assessment of coagulation profile with Thrombelastography (TEG) useful in predicting venous thromboembolism (VTE) following orthopaedic surgery? J. Clin. Orthop. Trauma 2016, 7, 225–229. [Google Scholar] [CrossRef]
- Hepner, D.L.; Concepcion, M.; Bhavani-Shankar, K. Coagulation Status Using Thromboelastography in Patients Receiving Warfarin Prophylaxis and Epidural Analgesia. J. Clin. Anesth. 2002, 14, 405–410. [Google Scholar] [CrossRef]
- Klein, S.M.; Slaughter, T.F.; Vail, P.T.; Ginsberg, B.; El-Moalem, H.E.; Alexander, R.; D’Ercole, F.; Greengrass, R.A.; Perumal, T.T.; Welsby, I.; et al. Thromboelastography as a perioperative measure of anticoagulation resulting from low molecular weight heparin: A comparison with anti-Xa concentrations. Anesth. Analg. 2000, 91, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chang, D.; Chui, J.; Cao, J.; Negus, J. The efficacy and cost-effectiveness of enoxaparin versus rivaroxaban in the prevention of venous thromboembolism following total hip or knee arthroplasty: A meta-analysis. J. Orthop. 2022, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Solbeck, S.; Ostrowski, S.R.; Stensballe, J.; Johansson, P.I. Thrombelastography detects dabigatran at therapeutic concentrations in vitro to the same extent as gold-standard tests. Int. J. Cardiol. 2016, 208, 14–18. [Google Scholar] [CrossRef]
- Dias, J.D.; Norem, K.; Doorneweerd, D.D.; Thurer, R.L.; Popovsky, M.A.; Omert, L.A. Use of Thromboelastography (TEG) for Detection of New Oral Anticoagulants. Arch. Pathol. Lab. Med. 2015, 139, 665–673. [Google Scholar] [CrossRef]
- Oswald, E.; Velik-Salchner, C.; Innerhofer, P.; Tauber, H.; Auckenthaler, T.; Ulmer, H.; Streif, W. Results of rotational thromboelastometry, coagulation activation markers and thrombin generation assays in orthopedic patients during thromboprophylaxis with rivaroxaban and enoxaparin: A prospective cohort study. Blood Coagul. Fibrinolysis 2015, 26, 136–144. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).