Anaesthesia-Relevant Disease Manifestations and Perianaesthetic Complications in Patients with Mucolipidosis—A Retrospective Analysis of 44 Anaesthetic Cases in 12 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Patients
2.2. Data Acquisition
2.3. Statistics
3. Results
3.1. Data Acquisition and Quality
3.2. Patient Characteristics
3.2.1. Patient Baseline Characteristics
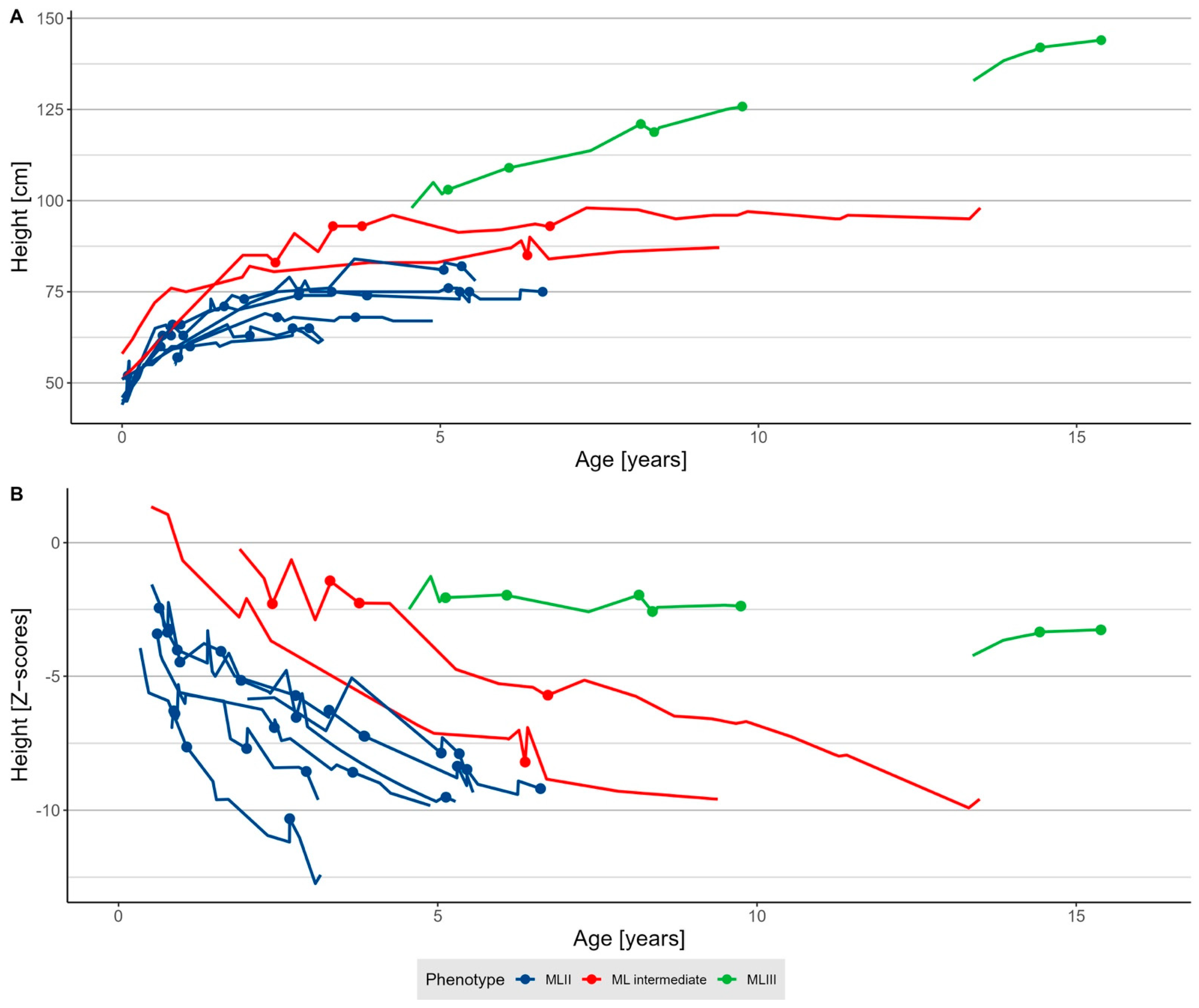
3.2.2. Growth
3.2.3. Upper Airway and Respiratory Tract Pathologies
3.2.4. Cardiovascular Manifestations
3.2.5. Gastrointestinal Manifestations
3.2.6. Spine Disease
3.3. Characteristics of Anaesthesia Procedures
3.3.1. Frequency of Anaesthesias and Technical Information
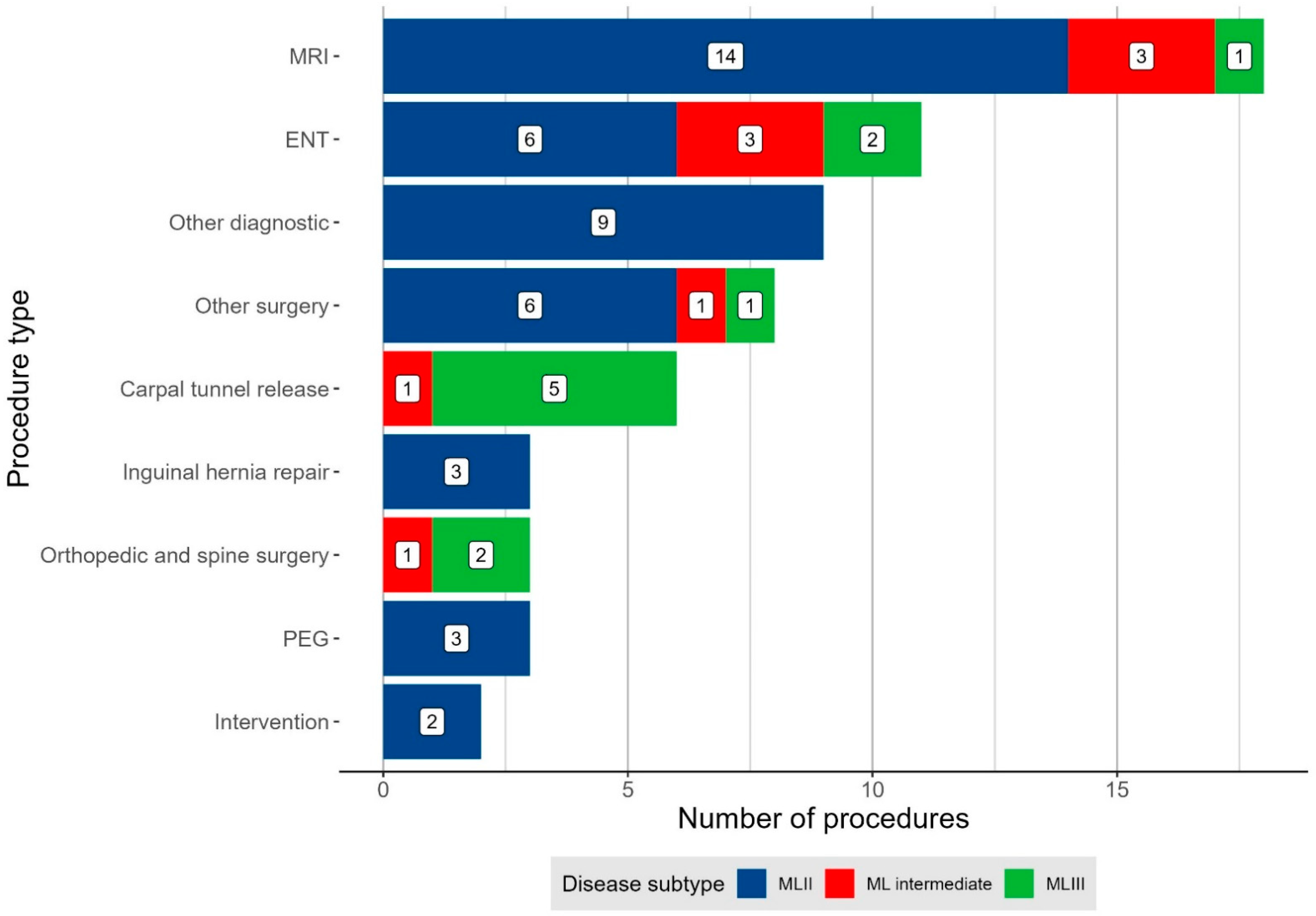
3.3.2. Indication for Anaesthesia
3.4. Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiede, S.; Storch, S.; Lubke, T.; Henrissat, B.; Bargal, R.; Raas-Rothschild, A.; Braulke, T. Mucolipidosis II is caused by mutations in GNPTA encoding the alpha/beta GlcNAc-1-phosphotransferase. Nat. Med. 2005, 11, 1109–1112. [Google Scholar] [CrossRef]
- Braulke, T.; Bonifacino, J.S. Sorting of lysosomal proteins. Biochim. Biophys. Acta 2009, 1793, 605–614. [Google Scholar] [CrossRef]
- Kollmann, K.; Pohl, S.; Marschner, K.; Encarnacao, M.; Sakwa, I.; Tiede, S.; Poorthuis, B.J.; Lübke, T.; Müller-Loennies, S.; Storch, S.; et al. Mannose phosphorylation in health and disease. Eur. J. Cell Biol. 2010, 89, 117–123. [Google Scholar] [CrossRef]
- Khan, S.A.; Tomatsu, S.C. Mucolipidoses Overview: Past, Present, and Future. Int. J. Mol. Sci. 2020, 21, 6812. [Google Scholar] [CrossRef]
- Dogterom, E.J.; Wagenmakers, M.; Wilke, M.; Demirdas, S.; Muschol, N.M.; Pohl, S.; van der Meijden, J.C.; Rizopoulos, D.; van der Ploeg, A.T.; Oussoren, E. Mucolipidosis type II and type III: A systematic review of 843 published cases. Genet. Med. Off. J. Am. Coll. Med. Genet. 2021, 23, 2047–2056. [Google Scholar] [CrossRef]
- Cathey, S.S.; Leroy, J.G.; Wood, T.; Eaves, K.; Simensen, R.J.; Kudo, M.; Stevenson, R.E.; Friez, M.J. Phenotype and genotype in mucolipidoses II and III alpha/beta: A study of 61 probands. J. Med. Genet. 2010, 47, 38–48. [Google Scholar] [CrossRef]
- Oussoren, E.; van Eerd, D.; Murphy, E.; Lachmann, R.; van der Meijden, J.C.; Hoefsloot, L.H.; Verdijk, R.; Ruijter, G.J.G.; Maas, M.; Hollak, C.E.M.; et al. Mucolipidosis type III, a series of adult patients. J. Inherit. Metab. Dis. 2018, 41, 839–848. [Google Scholar] [CrossRef]
- Velho, R.V.; Harms, F.L.; Danyukova, T.; Ludwig, N.F.; Friez, M.J.; Cathey, S.S.; Filocamo, M.; Tappino, B.; Güneş, N.; Tüysüz, B.; et al. The lysosomal storage disorders mucolipidosis type II, type III alpha/beta, and type III gamma: Update on GNPTAB and GNPTG mutations. Hum. Mutat. 2019, 40, 842–864. [Google Scholar]
- Ammer, L.S.; Oussoren, E.; Muschol, N.M.; Pohl, S.; Rubio-Gozalbo, M.E.; Santer, R.; Stuecker, R.; Vettorazzi, E.; Breyer, S.R. Hip Morphology in Mucolipidosis Type II. J. Clin. Med. 2020, 9, 728. [Google Scholar] [CrossRef]
- Ammer, L.S.; Dohrmann, T.; Muschol, N.M.; Lang, A.; Breyer, S.R.; Ozga, A.K.; Petzoldt, M. Disease Manifestations in Mucopolysaccharidoses and Their Impact on Anaesthesia-Related Complications—A Retrospective Analysis of 99 Patients. J. Clin. Med. 2021, 10, 3518. [Google Scholar] [CrossRef]
- Dohrmann, T.; Muschol, N.M.; Sehner, S.; Punke, M.A.; Haas, S.A.; Roeher, K.; Breyer, S.; Koehn, A.F.; Ullrich, K.; Zöllner, C.; et al. Airway management and perioperative adverse events in children with mucopolysaccharidoses and mucolipidoses: A retrospective cohort study. Paediatr. Anaesth. 2020, 30, 181–190. [Google Scholar] [CrossRef]
- Scott-Warren, V.L.; Walker, R. Perioperative management of patients with Mucolipidosis II and III: Lessons from a case series. Paediatr. Anaesth. 2021, 31, 260–267. [Google Scholar] [CrossRef]
- Mahfouz, A.K.; George, G. Anesthesia for gingivectomy and dental extractions in a child with I-cell disease—A case report. Middle East J. Anaesthesiol. 2011, 21, 121–124. [Google Scholar]
- Mahfouz, A.K.; George, G.; Al-Bahlani, S.S.; Al Nabhani, M.Z. Difficult intubation management in a child with I-cell disease. Saudi J. Anaesth. 2010, 4, 105–107. [Google Scholar] [CrossRef]
- Mallen, J.; Highstein, M.; Smith, L.; Cheng, J. Airway management considerations in children with I-cell disease. Int. J. Pediatric Otorhinolaryngol. 2015, 79, 760–762. [Google Scholar] [CrossRef]
- Edmiston, R.; Wilkinson, S.; Jones, S.; Tylee, K.; Broomfield, A.; Bruce, I.A. I-Cell Disease (Mucolipidosis II): A Case Series from a Tertiary Paediatric Centre Reviewing the Airway and Respiratory Consequences of the Disease. JIMD Rep. 2019, 45, 1–8. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Ammer, L.S.; Pohl, S.; Breyer, S.R.; Aries, C.; Denecke, J.; Perez, A.; Petzoldt, M.; Schrum, J.; Müller, I.; Muschol, N.M. Is hematopoietic stem cell transplantation a therapeutic option for mucolipidosis type II? Mol. Genet. Metab. Rep. 2021, 26, 100704. [Google Scholar] [CrossRef]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; Von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Mon. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- White, K.K.; Bompadre, V.; Goldberg, M.J.; Bober, M.B.; Cho, T.J.; Hoover-Fong, J.E.; Irving, M.; Mackenzie, W.G.; Kamps, S.E.; Raggio, C.; et al. Best practices in peri-operative management of patients with skeletal dysplasias. Am. J. Med. Genet. Part A 2017, 173, 2584–2595. [Google Scholar] [CrossRef]
- Dangel, J.H. Cardiovascular changes in children with mucopolysaccharide storage diseases and related disorders—Clinical and echocardiographic findings in 64 patients. Eur. J. Pediatrics 1998, 157, 534–538. [Google Scholar] [CrossRef]
- Carboni, E.; Sestito, S.; Lucente, M.; Morrone, A.; Zampini, L.; Chimenz, R.; Ceravolo, M.D.; De Sarro, R.; Ceravolo, G.; Calabrò, M.P. Dilated cardiomyopathy in mucolipidosis type 2. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. 2), 71–77. [Google Scholar]
- Tabone, L.; Caillaud, C.; Amaddeo, A.; Khirani, S.; Michot, C.; Couloigner, V.; Brassier, A.; Cormier-Daire, V.; Baujat, G.; Fauroux, B. Sleep-disordered breathing in children with mucolipidosis. Am. J. Med. Genet. Part A 2019, 179, 1196–1204. [Google Scholar] [CrossRef]
- Peters, M.E.; Arya, S.; Langer, L.O.; Gilbert, E.F.; Carlson, R.; Adkins, W. Narrow trachea in mucopolysaccharidoses. Pediatr. Radiol. 1985, 15, 225–228. [Google Scholar] [CrossRef]
- Poore, T.S.; Prager, J.; Weinman, J.P.; Larson, A.; Houin, P. Tracheal and lower airway changes in a patient with mucolipidosis type II. Pediatr. Pulmonol. 2020, 55, 1843–1845. [Google Scholar] [CrossRef]
- Nakaoka, S.; Kondo, H.; Matsuoka, K.; Shibuya, T.; Otomo, T.; Hamada, Y.; Sakamoto, K.; Ozono, K.; Sakai, N. Mucolipidosis Ⅱ and III with neurological symptoms due to spinal cord compression. Brain Dev. 2021, 43, 867–872. [Google Scholar] [CrossRef]
- Scarpa, M.; Almássy, Z.; Beck, M.; Bodamer, O.; Bruce, I.A.; De Meirleir, L.; Guffon, N.; Guillén-Navarro, E.; Hensman, P.; Jones, S.; et al. Mucopolysaccharidosis type II: European recommendations for the diagnosis and multidisciplinary management of a rare disease. Orphanet J. Rare Dis. 2011, 6, 72. [Google Scholar] [CrossRef]
- Wooten, W.I., 3rd; Muhlebach, M.S.; Muenzer, J.; Loughlin, C.E.; Vaughn, B.V. Progression of Polysomnographic Abnormalities in Mucolipidosis II (I-Cell Disease). J. Clin. Sleep Med. 2016, 12, 1695–1696. [Google Scholar] [CrossRef][Green Version]
- Alegra, T.; Sperb-Ludwig, F.; Guarany, N.R.; Ribeiro, E.M.; Lourenco, C.M.; Kim, C.A.; Valadares, E.R.; Galera, M.F.; Acosta, A.X.; Horovitz, D.D.G.; et al. Clinical Characterization of Mucolipidoses II and III: A Multicenter Study. J. Pediatric Genet. 2019, 8, 198–204. [Google Scholar] [CrossRef]
- Pruszczynski, B.; Mackenzie, W.G.; Rogers, K.; White, K.K. Spinal Cord Injury After Extremity Surgery in Children with Thoracic Kyphosis. Clin. Orthop. Relat. Res. 2015, 473, 3315–3320. [Google Scholar] [CrossRef]
- Farley, C.W.; Curt, B.A.; Pettigrew, D.B.; Holtz, J.R.; Dollin, N.; Kuntz, C., IV. Spinal cord intramedullary pressure in thoracic kyphotic deformity: A cadaveric study. Spine 2012, 37, E224–E230. [Google Scholar] [CrossRef]
- Habre, W.; Disma, N.; Virag, K.; Becke, K.; Hansen, T.G.; Jöhr, M.; Leva, B.; Morton, N.S.; Vermeulen, P.M.; Zielinska, M.; et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): A prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir. Med. 2017, 5, 412–425. [Google Scholar] [CrossRef]
- Kurth, C.D.; Tyler, D.; Heitmiller, E.; Tosone, S.R.; Martin, L.; Deshpande, J.K. National pediatric anesthesia safety quality improvement program in the United States. Anesth. Analg. 2014, 119, 112–121. [Google Scholar] [CrossRef]
- Lee, J.J.; Lim, B.G.; Lee, M.K.; Kong, M.H.; Kim, K.J.; Lee, J.Y. Fiberoptic intubation through a laryngeal mask airway as a management of difficult airwary due to the fusion of the entire cervical spine—A report of two cases. Korean J. Anesthesiol. 2012, 62, 272–276. [Google Scholar] [CrossRef]
| Characteristic | Overall, N = 12 | MLII, N = 7 | MLII/III, N = 2 | MLIII, N = 3 |
|---|---|---|---|---|
| Demographics | ||||
| Male sex 2 | 6 (50) | 4 (57) | 1 (50) | 1 (33) |
| Minimum age at anaesthesia 1 | 1.7 (0.1–19.0) | 0.9 (0.1–5.1) | 4.4 (2.4–6.4) | 14.4 (5.1–19.0) |
| Maximum age at anaesthesia 1 | 5.9 (0.8–19.1) | 3.7 (0.8–6.6) | 6.5 (6.4–6.7) | 15.4 (8.4–19.0) |
| Medical history | ||||
| Number of anaesthesias 1 | 4 (1–11) | 4 (2–11) | 2 (1–4) | 3 (1–5) |
| HSCT 2 | 1 (8) | 1 (14) | - | - |
| History of recurrent infections 2 | 2 (17) | 2 (29) | - | 1 (33) |
| Upper airway and respiratory tract pathology | ||||
| Craniofacial dysmorphia 2 | 10 (83) | 7 (100) | 2 (100) | 1 (33) |
| Gingival hyperplasia 2 | 9 (75) | 7 (100) | 2 (100) | - |
| Macroglossia 2 | 6 (50) | 5 (71) | 1 (50) | - |
| Tonsil hyperplasia 2 | 6 (50) | 2 (29) | 2 (100) | 2 (67) |
| Sleep apnoea 2 | ||||
| No | 3 (25) | 1 (14) | - | 2 (67) |
| Yes | 5 (42) | 2 (29) | 2 (100) | 1 (33) |
| NIV-treated | 4 (33) | 4 (57) | - | - |
| Obstructive lung disease 2 | ||||
| Yes | 5 (42) | 4 (57) | - | 1 (33) |
| Antiobstructive medication | 2 (17) | 1 (14) | 1 (50) | - |
| Thorax deformities 2 | ||||
| Pectus carinatum | 5 (42) | 4 (57) | 1 (50) | - |
| Narrow thorax | 1 (8.3) | 1 (14) | - | |
| Cardiovascular manifestations | ||||
| Severity of cardiac pathologies 2 | ||||
| Mild | 3 (25) | 1 (14) | 1 (50) | 1 (33) |
| Moderate | 3 (25) | 2 (29) | - | 1 (33) |
| Severe | 5 (42) | 4 (57) | 1 (50) | - |
| Cardiac pathology 2 | ||||
| Valve insufficiency | 9 (75) | 5 (71) | 2 (100) | 2 (67) |
| ASD type II | 5 (42) | 5 (71) | - | - |
| LVH | 4 (33) | 4 (57) | - | - |
| PFO | 2 (17) | 2 (29) | - | - |
| PA stenosis | 2 (17) | 2 (29) | - | - |
| Heart failure | 2 (17) | 1 (14) | 1(50) | |
| LV dilatation | 1 (8) | 1 (14) | - | - |
| Hypertension | 1 (8) | 1 (14) | - | - |
| Tachycardia | 1 (8) | - | - | 1 (33) |
| Gastrointestinal manifestations | ||||
| Organomegaly 2 | ||||
| Hepatomegaly | 4 (33) | 4 (57) | - | - |
| Hepatosplenomegaly | 1 (8) | 1 (14) | - | - |
| Dysphagia 2 | ||||
| Yes | 4 (33) | 3 (43) | 1 (50) | - |
| Gastric tube | 1 (8) | 1 (14) | - | - |
| Spine disease | ||||
| Short neck 2 | 12 (100) | 7 (100) | 2 (100) | 3 (100) |
| Cervical spinal stenosis2 | ||||
| Stenosis | 5 (42) | 4 (57) | 1 (50) | - |
| Stenosis + myelopathy | 1 (8) | 1 (14) | - | - |
| State after surgical decompression | 1 (8) | - | 1 (50) | - |
| Cervical spinal instability 2 | 6 (50) | 4 (57) | 2 (100) | - |
| Spinal deformities 2 | ||||
| Thoracolumbar kyphosis | 5 (42) | 4 (57) | 1 (50) | - |
| Lumbar hyperlordosis | 1 (8) | - | - | 1 (33) |
| Kyphoscoliosis | 1 (8) | - | 1(50) | - |
| Characteristic | Overall, N = 44 | MLII, N = 30 | MLII/III, N = 5 | MLIII, N = 9 |
|---|---|---|---|---|
| Cases | ||||
| Age (years) 1 | 3.3 (0.1; 19.1) | 2.2 (0.1; 6.6) | 3.8 (2.4; 6.7) | 9.7 (5.1; 19.1) |
| Weight (Z-score) 1 | −3.6 (−13.1; 0.3) | −4.8 (−13.1; −0.6) | −1.7 (−4.6; −0.7) | −0.8 (−2.5; −0.3) |
| Height (Z-score) 1 | −5.7 (−10.3; −1.4) | −6.6 (−10.3; −2.4) | −2.3 (−8.2; −1.4) | −2.37 (−3.3; −2.0) |
| Present respiratory infection 2 | 6 (14) | 4 (13) | 2 (40) | - |
| ASA score 2 | ||||
| 2 | 8 (18) | - | 1 (20) | 7 (78) |
| 3 | 32 (73) | 27 (90) | 3 (60) | 2 (22) |
| 4 | 4 (9) | 3 (10) | 1 (20) | 0 (0) |
| Procedural information | ||||
| Number of procedures during anaesthesia 2 | 1.0 (1.0; 6.0) | 1.0 (1.0; 6.0) | 2.0 (1.0; 3.0) | 1.0 (1.0; 2.0) |
| Duration (minutes) 1 | 120 (55; 405) | 122 (55; 270) | 270 (90; 405) | 105 (60; 240) |
| Postoperative ICU care 2 | 24 (56) | 19 (63) | 5 (100) | - |
| Technical information | ||||
| Type of anaesthesia 2 | ||||
| Standby | 2 (5) | 1 (3) | - | 1 (11) |
| Sedation | 7 (16) | 7 (23) | - | - |
| Regional only | 2 (5) | 1 (3) | - | 1 (11) |
| General anaesthesia | 33 (75) | 21 (70) | 5 (100) | 7 (78) |
| Total intravenous anaesthesia | 29 (66) | 17(57) | 5 (100) | 7 (78) |
| Balanced anaesthesia | 4 (9) | 4 (13) | - | - |
| Induction of anaesthesia | ||||
| Intravenous (Propofol) | 34 (85) | 25 (89) | 3 (60) | 6 (86) |
| Inhalative (Sevoflurane) | 6 (15) | 3 (11) | 2 (40) | 1 (14) |
| Primary airway approach 2 | ||||
| No airway | 11 (25) | 9 (30) | - | 2 (22) |
| Laryngeal mask | 9 (20) | 5 (17) | - | 4 (44) |
| Tracheal intubation | 24 (55) | 16 (53) | 5 (100) | 3 (33) |
| Direct laryngoscopy | 3 (7) | 2 (7) | 1 (20) | - |
| Videolaryngoscopy | 8 (18) | 5 (17) | - | 3 (33) |
| FOI-SGA | 13 (30) | 9 (30) | 4 (80) | - |
| Characteristic, n (%) | Overall, N = 44 | MLII, N = 30 | MLII/III, N = 5 | MLIII, N = 9 |
|---|---|---|---|---|
| Anaesthesias with at least one complication | 12 (27) | 8 (27) | 3 (60) | 1 (11) |
| Difficult airway management | 6 (14) | 5 (17) | 1 (20) | - |
| Difficult facemask ventilation | 5 (14) | 4 (18) | 1 (20) | - |
| Difficult laryngeal mask airway | 1 (4) | 1 (6.2) | - | - |
| Difficult tracheal intubation | 6 (23) | 5 (29) | 1 (20) | - |
| Respiratory complications | 8 (18) | 6 (20) | 2 (40) | - |
| Cardiocirculatory complications | - | - | - | - |
| Other complications | 3 (7) | 1 (3) | 1 (20) | 1 (11) |
| Pat. | Sex | Subtype | Year | Age | Procedure | Airway Management | Anaesthesia Procedure | Event Categories | Detailed Descriptions |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | MLII | 2020 | 3 years | PEG exchange | VL → FOI-LM | TIVA: Propofol, Remifentanil | DiffAir, RESP | Videolaryngoscopy: failed, C/L 4 view; FOI-LM: passage of 4.0 tube failed, successful placement of 3.5 uncuffed tube; tube exchange because of significant air leak (3.5 cuffed tube via exchange catheter); hypoxemia with SpO2 75%; unplanned ICU admission |
| 1 | Female | MLII | 2019 | 2 years | MRI, BERA | No airway | Sedation: Propofol, Esketamine | RESP | Fever, bronchopneumonia treated with i.v. antibiotics |
| 1 | Female | MLII | 2017 | 10 months | MRI, lumbar puncture | No airway → LM → VL → FOI-LM | Sedation: Propofol, Esketamine | DiffAir, RESP, other | Sedation: failed due to insufficient spontaneous breathing; difficult face mask ventilation; tracheal intubation: failed (conventional and videolaryngoscopy VL: C/L 3; hypoxemia and consecutive severe bradycardia requiring CPR; LM (rescue manoeuvre) facilitated oxygenation, ROSC, massive hypercapnia; FOI-LM: finally successful; postoperative respiratory insufficiency with prolonged ventilation on ICU; sepsis on ICU |
| 2 | Male | MLII/III | 2008 | 6 years | ENT surgery | FOI-LM | TIVA: Propofol, Sufentanil | RESP | Postoperative tube dislocation into the main bronchus with atelectasis |
| 3 | Male | MLIII | 2020 | 8 years | hip osteotomy | VL | Sevoflurane, TIVA: Propofol, Remifentanil | Other | Postoperative fever |
| 4 | Male | MLII | 2021 | 2 years | ENT surgery | FOI-LM → VL → FOI | TIVA: Propofol, Remifentanil | DiffAir, RESP | Impossible face mask ventilation; FOI-LM: failed due to secretion and unfavourable angle; VL failed (C/L 3). Finally, tracheal intubation was secured by oral FOI. Hypoxemia with minimal SpO2 58% |
| 5 | Male | MLII | 2017 | 1 year | MRI | FOI-LM | TIVA: Propofol, Remifentanil | RESP | Hypoxemia with minimal SpO2 of 70% during intubation |
| 5 | Male | MLII | 2016 | 11 months | ENT surgery | DL | TIVA: Propofol, Sufentanil | DiffAir | Impossible face mask ventilation, LM; tracheal intubation via direct laryngoscopy |
| 6 | Female | MLII | 2014 | 7 months | Quinton catheter implantation | DL → other | Balanced: Propofol, Sufentanil, Sevoflurane | DiffAir | Difficult intubation; conventional laryngoscopy C/L 3; successful intubation with a rigid bronchoscope |
| 7 | Female | MLII/III | 2015 | 6 years | ENT surgery, MRI | FOI-LM | TIVA: Propofol, Sufentanil | Other | Postoperative fever |
| 7 | Female | MLII/III | 2012 | 3 years | Atlanto-occipital decompression | DL → VL → other | Sevoflurane TIVA: Propofol, Sufentanil | DiffAir, RESP | Difficult mask ventilation; VL: C/L 3; intubation with a McCoy blade and rigid bronchoscope, hypoxemia |
| 8 | Male | MLII | 2021 | 5 years | ENT surgery, MRI, other diagnostics | VL | TIVA: Propofol, Remifentanil | RESP | Postextubation airway obstruction with severe hypoxemia (SpO2 8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammer, L.S.; Muschol, N.M.; Santer, R.; Lang, A.; Breyer, S.R.; Sasu, P.B.; Petzoldt, M.; Dohrmann, T. Anaesthesia-Relevant Disease Manifestations and Perianaesthetic Complications in Patients with Mucolipidosis—A Retrospective Analysis of 44 Anaesthetic Cases in 12 Patients. J. Clin. Med. 2022, 11, 3650. https://doi.org/10.3390/jcm11133650
Ammer LS, Muschol NM, Santer R, Lang A, Breyer SR, Sasu PB, Petzoldt M, Dohrmann T. Anaesthesia-Relevant Disease Manifestations and Perianaesthetic Complications in Patients with Mucolipidosis—A Retrospective Analysis of 44 Anaesthetic Cases in 12 Patients. Journal of Clinical Medicine. 2022; 11(13):3650. https://doi.org/10.3390/jcm11133650
Chicago/Turabian StyleAmmer, Luise Sophie, Nicole Maria Muschol, René Santer, Annika Lang, Sandra Rafaela Breyer, Phillip Brenya Sasu, Martin Petzoldt, and Thorsten Dohrmann. 2022. "Anaesthesia-Relevant Disease Manifestations and Perianaesthetic Complications in Patients with Mucolipidosis—A Retrospective Analysis of 44 Anaesthetic Cases in 12 Patients" Journal of Clinical Medicine 11, no. 13: 3650. https://doi.org/10.3390/jcm11133650
APA StyleAmmer, L. S., Muschol, N. M., Santer, R., Lang, A., Breyer, S. R., Sasu, P. B., Petzoldt, M., & Dohrmann, T. (2022). Anaesthesia-Relevant Disease Manifestations and Perianaesthetic Complications in Patients with Mucolipidosis—A Retrospective Analysis of 44 Anaesthetic Cases in 12 Patients. Journal of Clinical Medicine, 11(13), 3650. https://doi.org/10.3390/jcm11133650







