Operability of a Resonance-Based Viscoelastic Haemostatic Analyzer in the High-Vibration Environment of Air Medical Transport
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
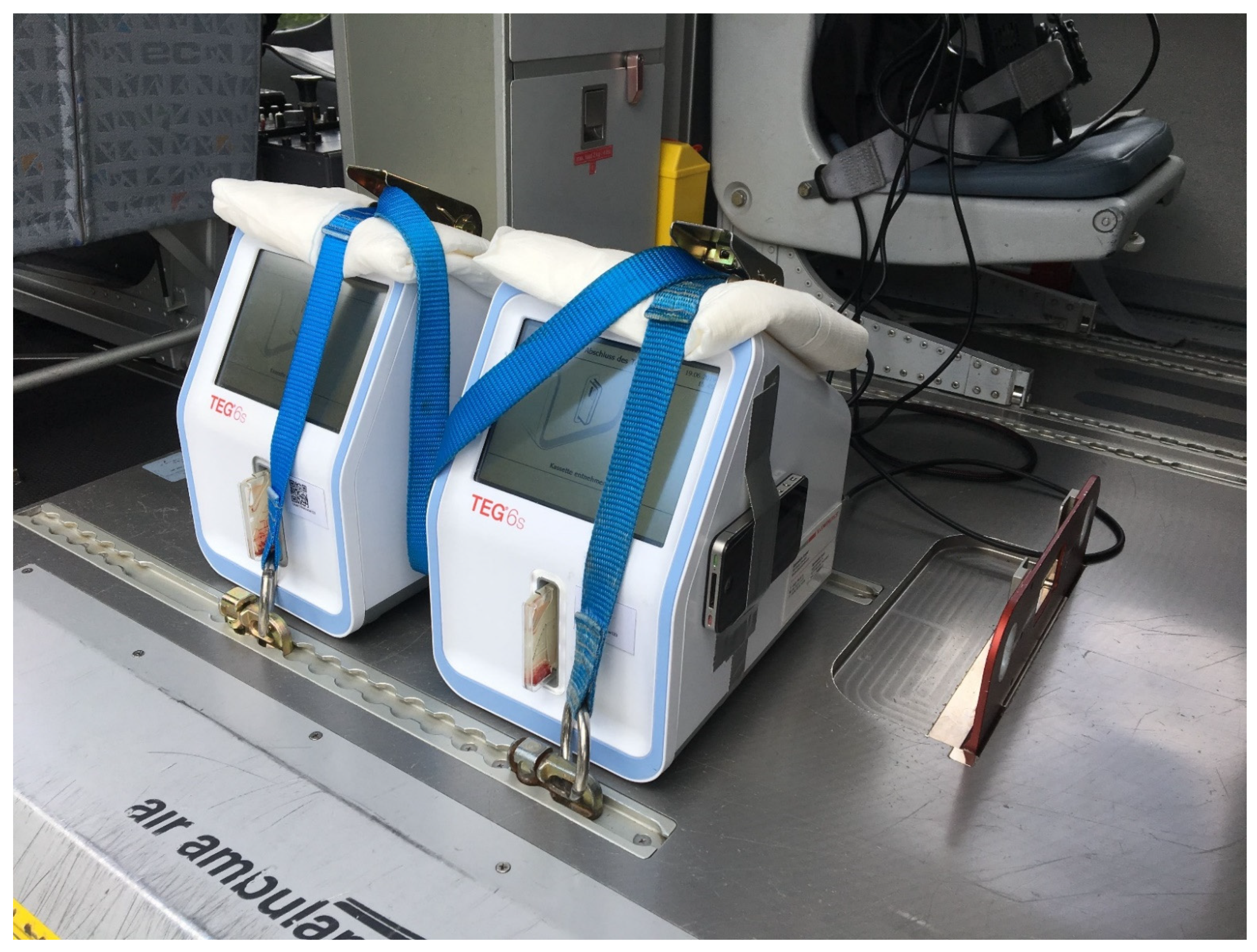
2.2. Viscoelastic Testing
2.3. Vibratometric Measurements
2.4. Statistical Analysis
3. Results
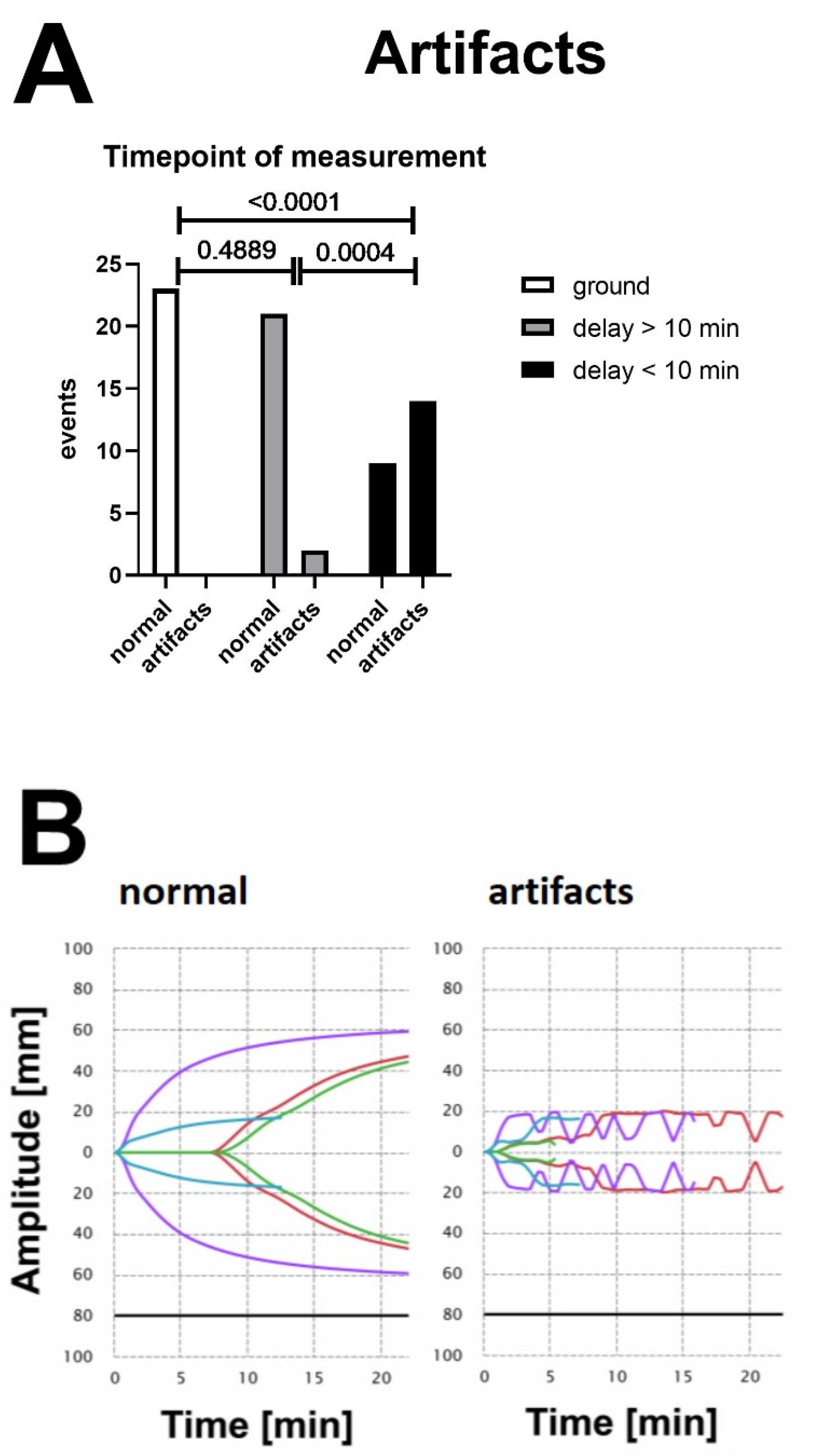
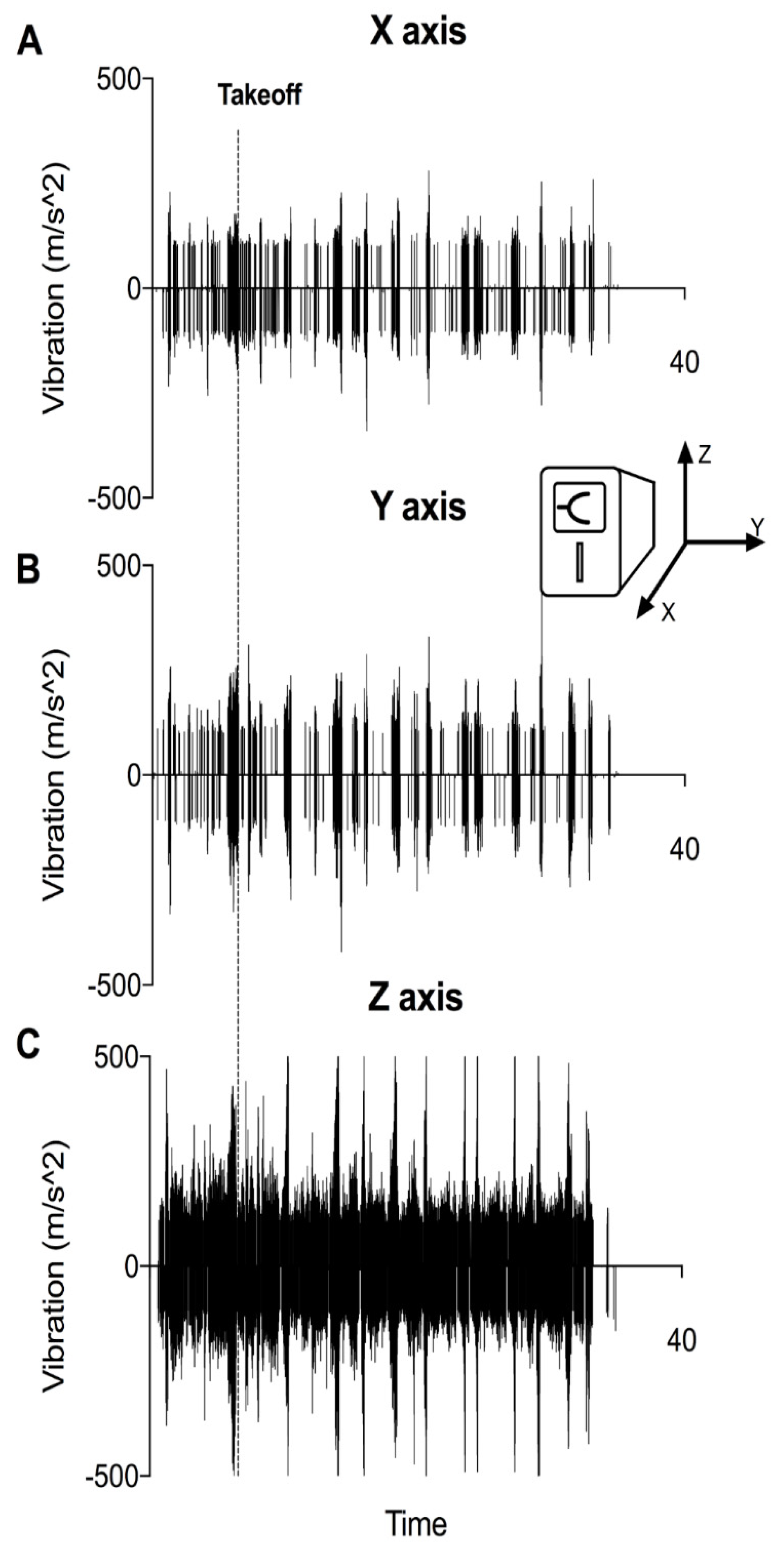
3.1. VHAs Are Exposed to Vibration as Soon as the Engine Is Started
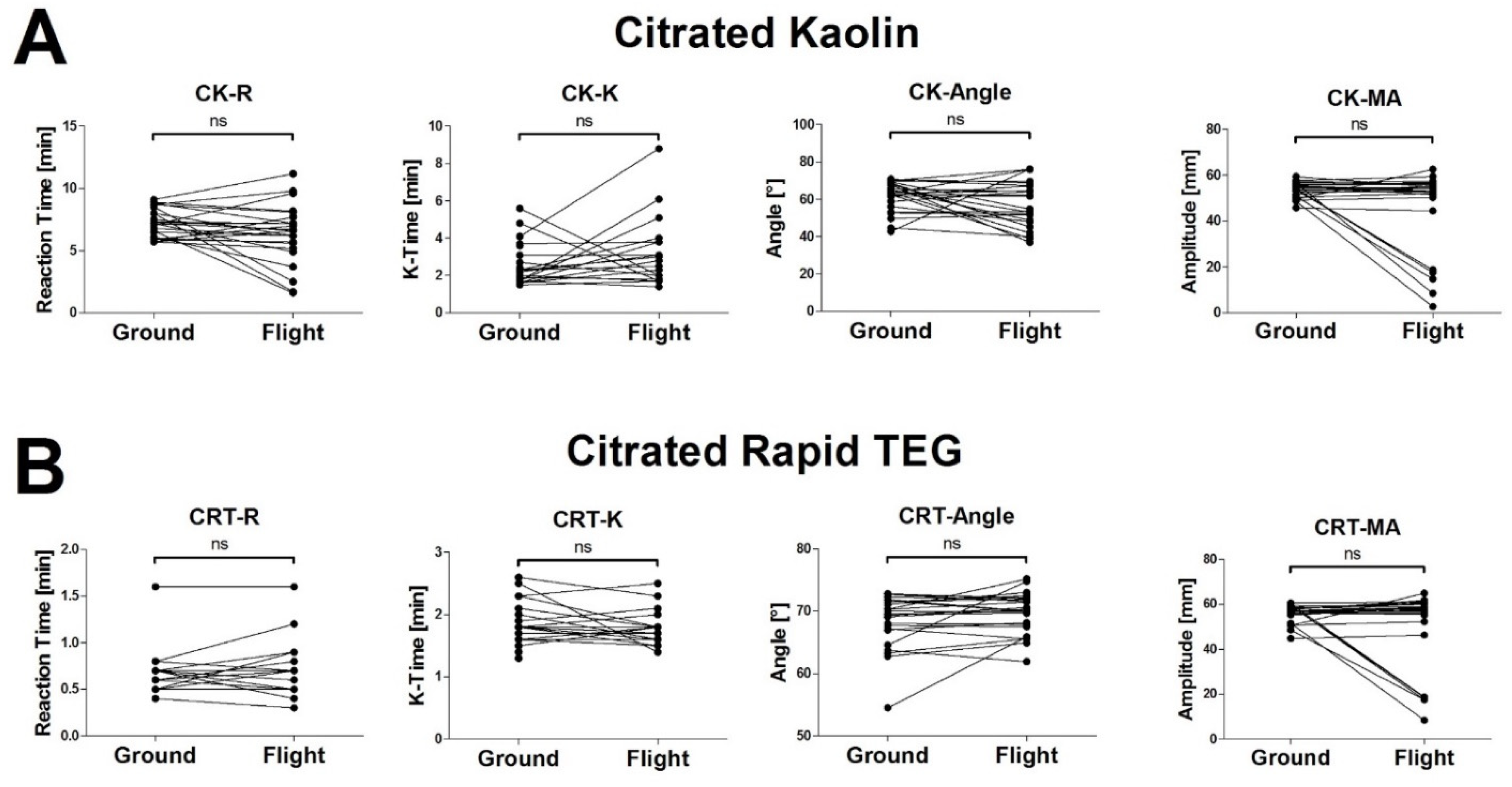
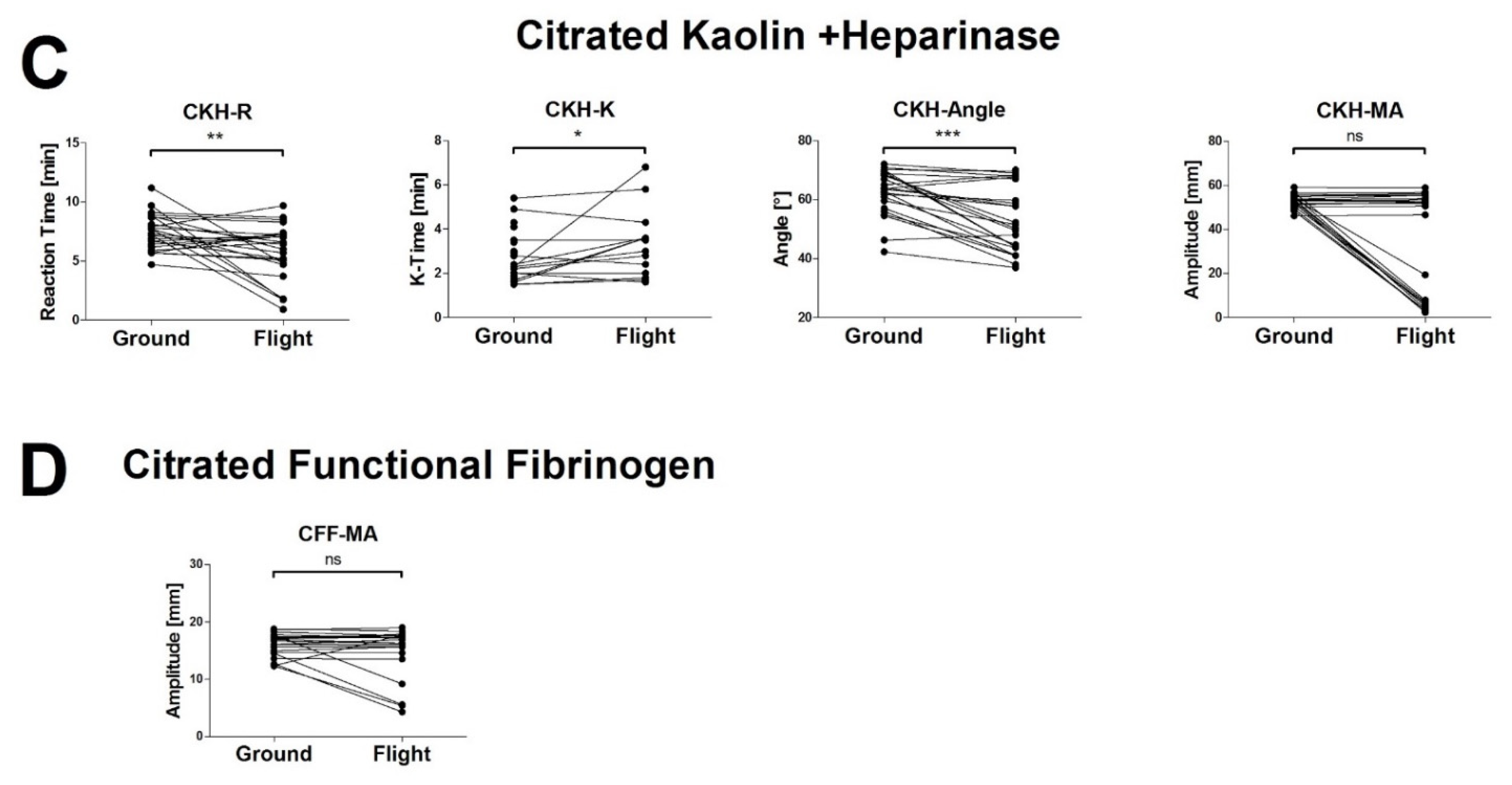
3.2. The High Vibration Environment of Helicopter Operations Alters VHA Results
3.3. Vibration-Induced Artifacts Are Associated with Unreliable Test Results in VHAs
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyeniyi, B.T.; Fox, E.E.; Scerbo, M.; Tomasek, J.S.; Wade, C.E.; Holcomb, J.B. Trends in 1029 trauma deaths at a level 1 trauma center: Impact of a bleeding control bundle of care. Injury 2017, 48, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaua, A. Trauma-induced coagulopathy. Nat. Rev. Dis. Prim. 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Pommer, P.; Oberladstätter, D.; Schlimp, C.J.; Zipperle, J.; Voelckel, W.; Lockie, C.; Osuchowski, M.; Schöchl, H. Multiplate Platelet Function Testing upon Emergency Room Admission Fails to Provide Useful Information in Major Trauma Patients Not on Platelet Inhibitors. J. Clin. Med. 2022, 11, 2578. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.; Fries, D.; Tanaka, K.A.; Asmis, L.; Curry, N.S.; Schöchl, H. Usefulness of standard plasma coagulation tests in the management of perioperative coagulopathic bleeding: Is there any evidence? Br. J. Anaesth. 2015, 114, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöchl, H.; Cotton, B.; Inaba, K.; Nienaber, U.; Fischer, H.; Voelckel, W.; Solomon, C. FIBTEM provides early prediction of massive transfusion in trauma. Crit. Care 2011, 15, R265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, C.; Ranucci, M.; Hochleitner, G.; Schöchl, H.; Schlimp, C.J. Assessing the Methodology for Calculating Platelet Contribution to Clot Strength (Platelet Component) in Thromboelastometry and Thrombelastography. Anesth. Analg. 2015, 121, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Schöchl, H.; Frietsch, T.; Pavelka, M.; Jámbor, C. Hyperfibrinolysis after major trauma: Differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J. Trauma 2009, 67, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Stettler, G.R.; Moore, E.E.; Moore, H.B.; Nunns, G.R.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Redefining postinjury fibrinolysis phenotypes using two viscoelastic assays. J. Trauma Acute Care Surg. 2019, 86, 679–685. [Google Scholar] [CrossRef]
- Maegele, M.; Caspers, M.; Schöchl, H. Viscoelasticity-based treatment of bleeding injuries. Unfallchirurg 2017, 120, 769–785. [Google Scholar] [CrossRef]
- Hochleitner, G.; Sutor, K.; Levett, C.; Leyser, H.; Schlimp, C.J.; Solomon, C. Revisiting Hartert’s 1962 Calculation of the Physical Constants of Thrombelastography. Clin. Appl. Thromb. Hemost. 2017, 23, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Hartert, H. Blood clotting studies with Thrombus stressography; a new Investigation procedure. Klin. Wochenschr. 1948, 26, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Murphy, M.; Dias, J.D. Viscoelastic Hemostatic Assays: Moving from the Laboratory to the Site of Care—A Review of Established and Emerging Technologies. Diagnostics 2020, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, B.; Voelckel, W.; Zipperle, J.; Grottke, O.; Schöchl, H. Comparison between the new fully automated viscoelastic coagulation analysers TEG 6s and ROTEM Sigma in trauma patients: A prospective observational study. Eur. J. Anaesthesiol. 2019, 36, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.D.; Moore, E.E.; Walsh, M.; Thomas, S.; Callcut, R.A.; Kornblith, L.Z.; Schreiber, M.; Ekeh, A.P.; Singer, A.J.; Lottenberg, L.; et al. A comparison between the TEG 6s and TEG 5000 analyzers to assess coagulation in trauma patients. J. Trauma Acute Care Surg. 2020, 88, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Schenk, B.; Görlinger, K.; Treml, B.; Tauber, H.; Fries, D.; Niederwanger, C.; Oswald, E.; Bachler, M. A comparison of the new ROTEM® sigma with its predecessor, the ROTEMdelta. Anaesthesia 2019, 74, 348–356. [Google Scholar] [CrossRef]
- Pham, H.P.; Azad, A.; Gounlong, J.; Gutierrez, J.; Mikrut, K.; Miller, J.L.; Wool, G.D. Comparison of Viscoelastic Testing by Rotational Torsion and Harmonic Resonance Methods. Am. J. Clin. Pathol. 2021, 156, 818–828. [Google Scholar] [CrossRef]
- Chandel, A.; Desai, M.; King, C.S.; Patolia, S.; Raja, A.I.; Singh, R.; Dalton, H.J. Agreement Between the TEG 6s and TEG 5000 Analyzers in Extracorporeal Membrane Oxygenation. ASAIO J. 2022, 68, 73–78. [Google Scholar] [CrossRef]
- Lloyd-Donald, P.; Churilov, L.; Cheong, B.; Bellomo, R.; McCall, P.R.; Mårtensson, J.; Glassford, N.; Weinberg, L. Assessing TEG6S reliability between devices and across multiple time points: A prospective thromboelastography validation study. Sci. Rep. 2020, 10, 7045. [Google Scholar] [CrossRef]
- Meledeo, M.A.; Peltier, G.C.; McIntosh, C.S.; Voelker, C.R.; Bynum, J.A.; Cap, A.P. Functional stability of the TEG 6s hemostasis analyzer under stress. J. Trauma Acute Care Surg. 2018, 84, S83–S88. [Google Scholar] [CrossRef]
- Scott, R.; Burns, B.; Ware, S.; Oud, F.; Miller, M. The reliability of thromboelastography in a simulated rotary wing environment. Emerg. Med. J. 2018, 35, 739–742. [Google Scholar] [CrossRef]
- Boyé, M.; Boissin, J.; Poyat, C.; Pasquier, P.; Martinaud, C. Evaluation of the altitude impact on a point-of-care thromboelastography analyzer measurement: Prerequisites for use in airborne medical evacuation courses. Eur. J. Trauma Emerg. Surg. 2022, 48, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.; Donohue, A.; McCullough, J.; Winearls, J. Viscoelastic haemostatic assays in aeromedical transport. Emerg. Med. Australas. 2020, 32, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.D.; Haney, E.I.; Mathew, B.A.; Lopez-Espina, C.G.; Orr, A.W.; Popovsky, M.A. New-Generation Thromboelastography: Comprehensive Evaluation of Citrated and Heparinized Blood Sample Storage Effect on Clot-Forming Variables. Arch. Pathol. Lab. Med. 2017, 141, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, J.H.; Richards, J.E.; Morrison, J.J.; Galvagno, S.M.; Tanaka, K.A.; Madurska, M.J.; Rock, P.; Scalea, T.M.; Mazzeffi, M.A. Viscoelastic Signals for Optimal Resuscitation in Trauma: Kaolin Thrombelastography Cutoffs for Diagnosing Hypofibrinogenemia (VISOR Study). Anesth. Analg. 2019, 129, 1482–1491. [Google Scholar] [CrossRef]
- Gill, M. The TEG®6s on Shaky Ground? A Novel Assessment of the TEG®6s Performance under a Challenging Condition. J. Extra Corpor. Technol. 2017, 49, 26–29. [Google Scholar]
- Sperry, J.L.; Guyette, F.X.; Brown, J.B.; Yazer, M.H.; Triulzi, D.J.; Early-Young, B.J.; Adams, P.W.; Daley, B.J.; Miller, R.S.; Harbrecht, B.G.; et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N. Engl. J. Med. 2018, 379, 315–326. [Google Scholar] [CrossRef]
- Innerhofer, P.; Fries, D.; Mittermayr, M.; Innerhofer, N.; von Langen, D.; Hell, T.; Gruber, G.; Schmid, S.; Friesenecker, B.; Lorenz, I.H.; et al. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): A single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017, 4, e258–e271. [Google Scholar] [CrossRef]
- Ziegler, B.; Bachler, M.; Haberfellner, H.; Niederwanger, C.; Innerhofer, P.; Hell, T.; Kaufmann, M.; Maegele, M.; Martinowitz, U.; Nebl, C.; et al. Efficacy of prehospital administration of fibrinogen concentrate in trauma patients bleeding or presumed to bleed (FIinTIC): A multicentre, double-blind, placebo-controlled, randomised pilot study. Eur. J. Anaesthesiol. 2021, 38, 348–357. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zipperle, J.; Ziegler, B.; Schöchl, H.; Voelckel, W.; Schlimp, C.J.; Oberladstätter, D. Operability of a Resonance-Based Viscoelastic Haemostatic Analyzer in the High-Vibration Environment of Air Medical Transport. J. Clin. Med. 2022, 11, 3630. https://doi.org/10.3390/jcm11133630
Zipperle J, Ziegler B, Schöchl H, Voelckel W, Schlimp CJ, Oberladstätter D. Operability of a Resonance-Based Viscoelastic Haemostatic Analyzer in the High-Vibration Environment of Air Medical Transport. Journal of Clinical Medicine. 2022; 11(13):3630. https://doi.org/10.3390/jcm11133630
Chicago/Turabian StyleZipperle, Johannes, Bernhard Ziegler, Herbert Schöchl, Wolfgang Voelckel, Christoph J. Schlimp, and Daniel Oberladstätter. 2022. "Operability of a Resonance-Based Viscoelastic Haemostatic Analyzer in the High-Vibration Environment of Air Medical Transport" Journal of Clinical Medicine 11, no. 13: 3630. https://doi.org/10.3390/jcm11133630
APA StyleZipperle, J., Ziegler, B., Schöchl, H., Voelckel, W., Schlimp, C. J., & Oberladstätter, D. (2022). Operability of a Resonance-Based Viscoelastic Haemostatic Analyzer in the High-Vibration Environment of Air Medical Transport. Journal of Clinical Medicine, 11(13), 3630. https://doi.org/10.3390/jcm11133630






