Retinal Vascular Study Using OCTA in Subjects at High Genetic Risk of Developing Alzheimer’s Disease and Cardiovascular Risk Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Desing
2.2. Ophthalmological Study
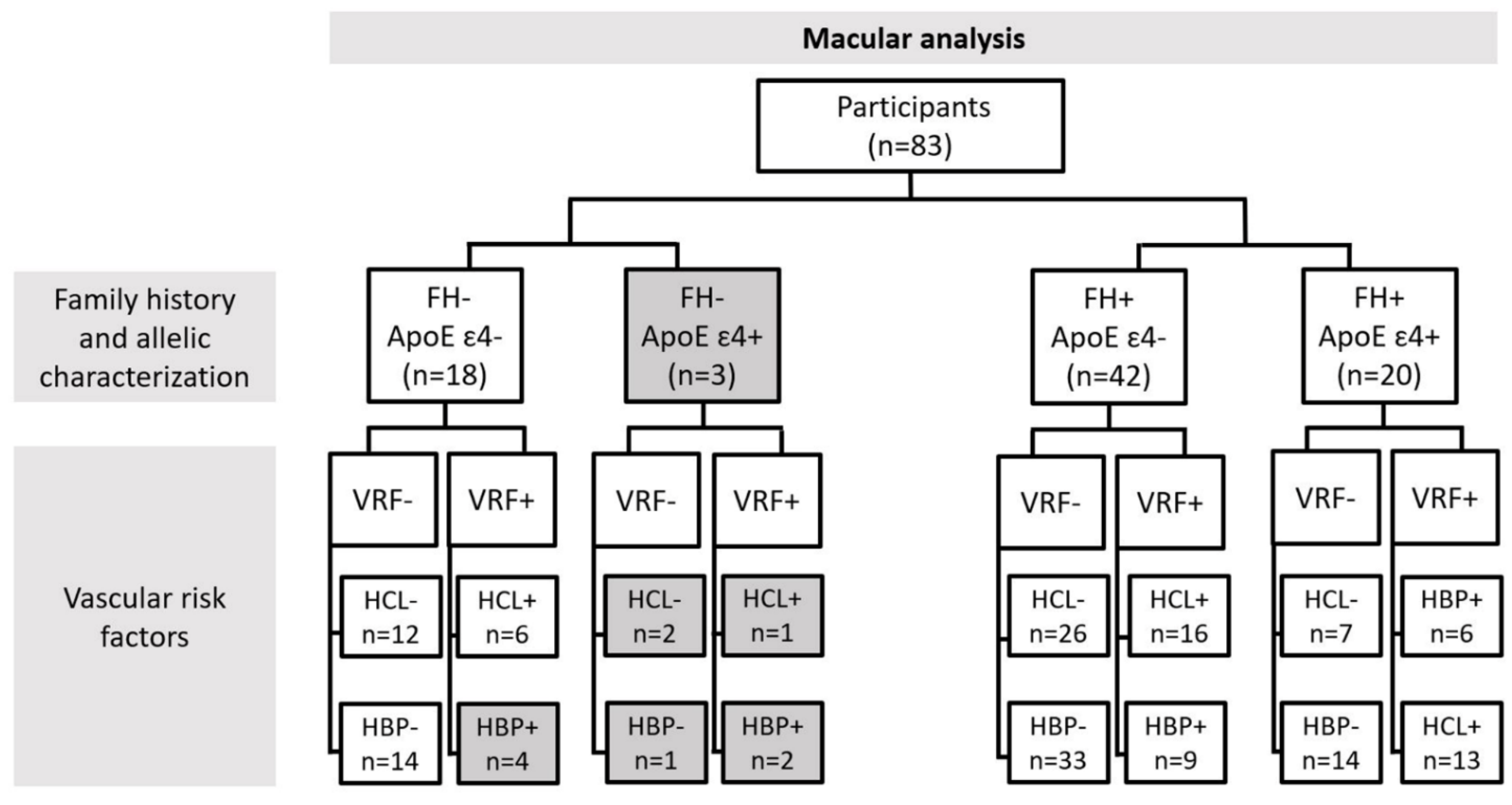
2.3. Classification of Patients for Retinal Vascular Network Analysis
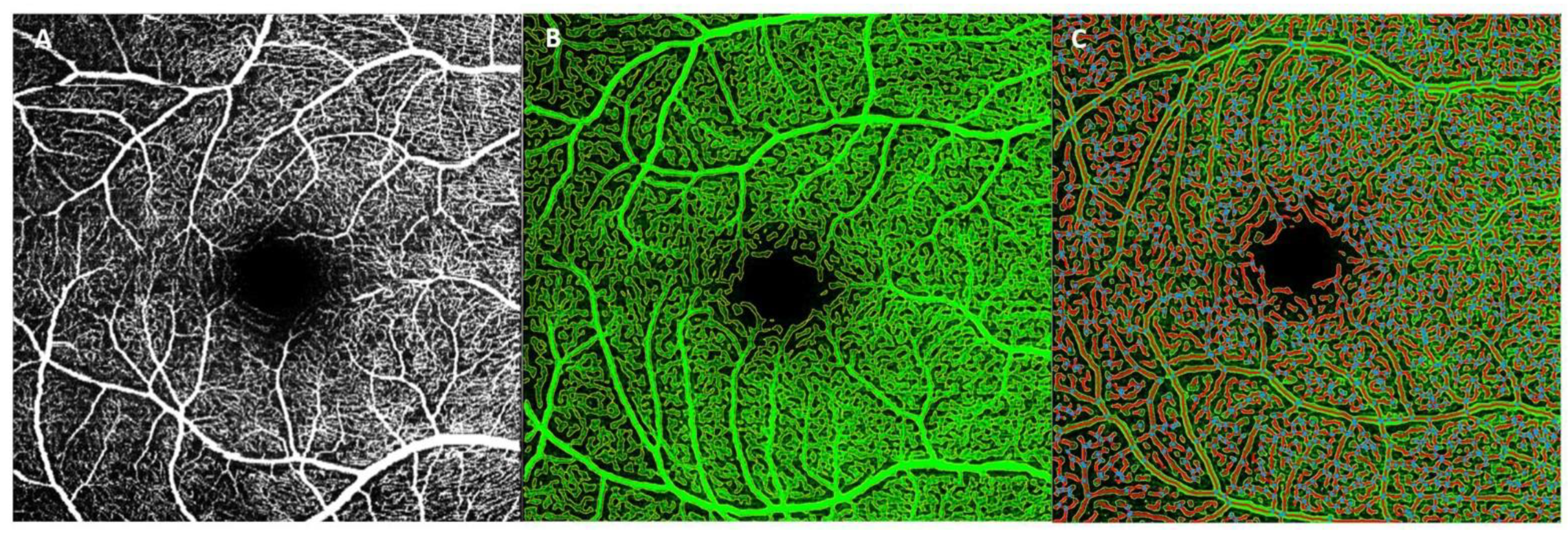
2.4. OCTA Acquisition and Analysis
2.4.1. AngioTool
2.4.2. Erlangen-AngioTool
2.5. ApoE Genotyping
2.6. Statistical Analysis
2.7. Colorimetric Scale
3. Results
3.1. Demography
3.2. Analysis of Retinal Vasculature According to Family History and the Allelic Characterization of ApoE ε4
3.2.1. Macular Analysis
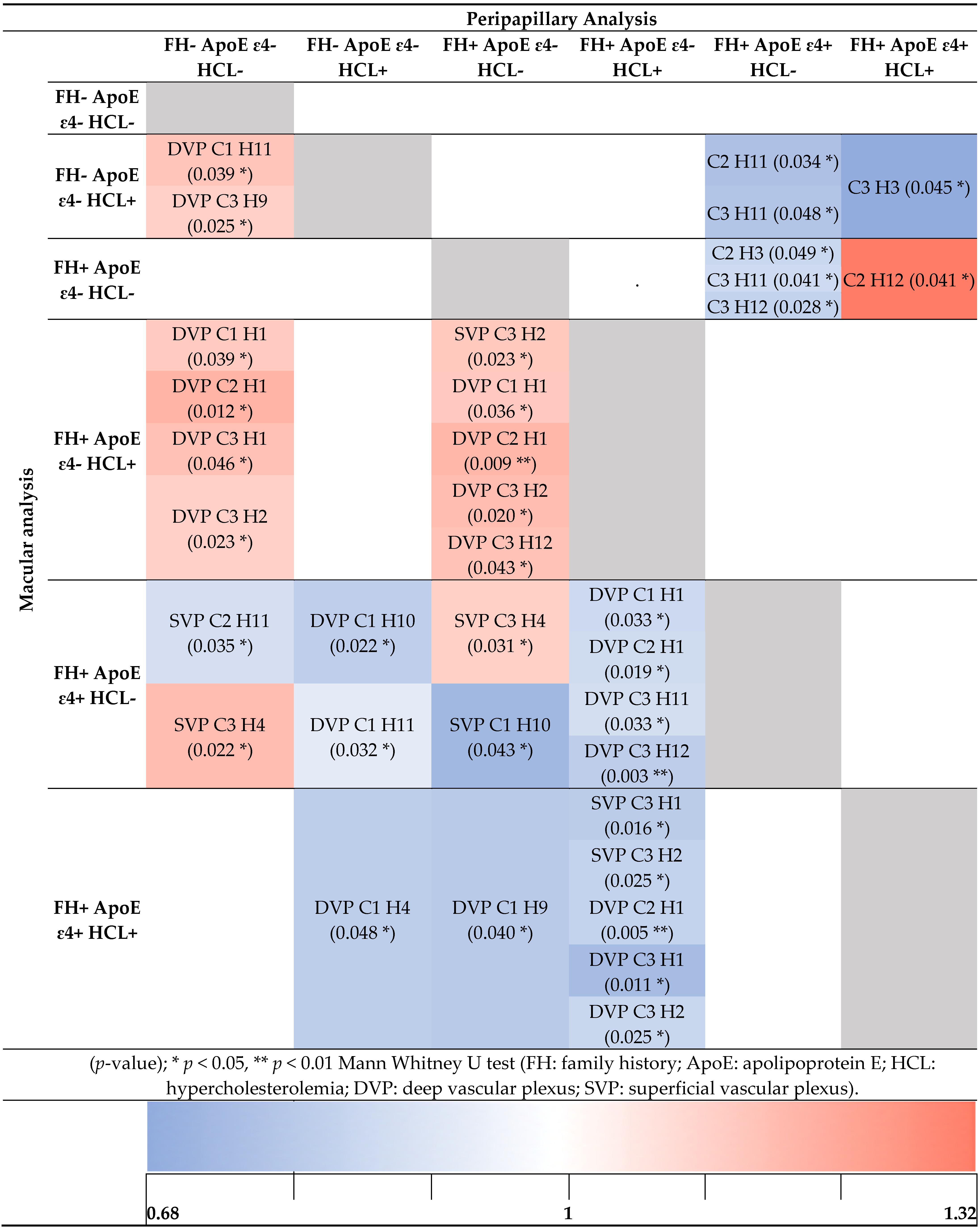
3.2.2. Peripapillary Analysis
3.3. Analysis of Retinal Vasculature According to Family History and the Allelic Characterization of ApoE ε4 in Addition to Hypercholesterolemia
3.3.1. Macular Analysis
3.3.2. Peripapillary Analysis
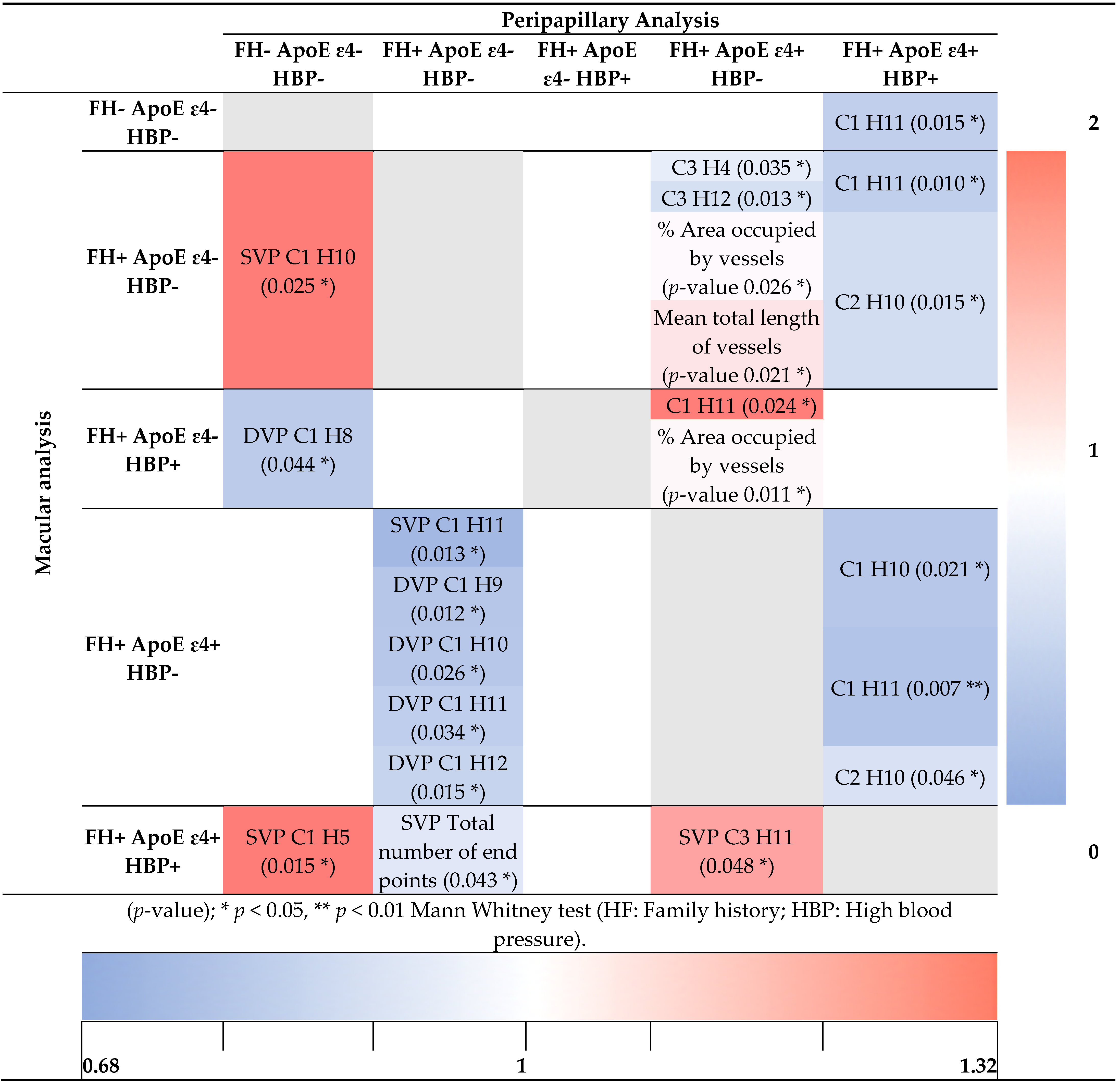
3.4. Analysis of Retinal Vasculature According to Family History and the Allelic Characterization of ApoE ε4 in Addition to High Blood Pressure
3.4.1. Macular Analysis
3.4.2. Peripapillary Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donix, M.; Small, G.W.; Bookheimer, S.Y. Family History and APOE-4 Genetic Risk in Alzheimer’s Disease. Neuropsychol. Rev. 2012, 22, 298–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratiglioni, L.; Ahlbom, A.; Viitanen, M.; Winblad, B. Risk Factors for Late-Onset Alzheimer’s Disease: A Population-Based, Case-control Study. Ann. Neurol. 1993, 33, 258–266. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer’s Disease in Late Onset Families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Imbimbo, C.; Spallazzi, M.; Ferrari-Pellegrini, F.; Villa, A.; Zilioli, A.; Mutti, C.; Parrino, L.; Lazzeroni, D. Heart Rate Variability and Cognitive Performance in Adults with Cardiovascular Risk. Cereb. Circ. Cogn. Behav. 2022, 3, 100136. [Google Scholar] [CrossRef]
- Tai, L.M.; Thomas, R.; Marottoli, F.M.; Koster, K.P.; Kanekiyo, T.; Morris, A.W.J.; Bu, G. The Role of APOE in Cerebrovascular Dysfunction. Acta Neuropathol. 2016, 131, 709–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antes, R.; Ezra-Elia, R.; Weinberger, D.; Solomon, A.; Ofri, R.; Michaelson, D.M. ApoE4 Induces Synaptic and ERG Impairments in the Retina of Young Targeted Replacement ApoE4 Mice. PLoS ONE 2013, 8, e64949. [Google Scholar] [CrossRef]
- Antes, R.; Salomon-Zimri, S.; Beck, S.; Garrido, M.; Livnat, T.; Maharshak, I.; Kadar, T.; Seeliger, M.; Weinberger, D.; Michaelson, D. VEGF Mediates ApoE4-Induced Neovascularization and Synaptic Pathology in the Choroid and Retina. Curr. Alzheimer Res. 2015, 12, 323–334. [Google Scholar] [CrossRef]
- Katsimpris, A.; Karamaounas, A.; Sideri, A.M.; Katsimpris, J.; Georgalas, I.; Petrou, P. Optical Coherence Tomography Angiography in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Eye 2021, 1–8. [Google Scholar] [CrossRef]
- Patton, N.; Aslam, T.; MacGillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal Vascular Image Analysis as a Potential Screening Tool for Cerebrovascular Disease: A Rationale Based on Homology between Cerebral and Retinal Microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.L.; Ong, Y.T.; Ikram, M.K.; Ong, S.Y.; Li, X.; Hilal, S.; Catindig, J.A.S.; Venketasubramanian, N.; Yap, P.; Seow, D.; et al. Microvascular Network Alterations in the Retina of Patients with Alzheimer’s Disease. Alzheimer’s Dement. 2014, 10, 135–142. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The Retina as a Window to the Brain—From Eye Research to CNS Disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Leurgans, S.E.; Wang, Z.; Wilson, R.S.; Bennett, D.A.; Schneider, J.A. Cerebral Amyloid Angiopathy Pathology and Cognitive Domains in Older Persons. Ann. Neurol. 2011, 69, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, W.R.; Thore, C.R. Review: Cerebral Microvascular Pathology in Ageing and Neurodegeneration. Neuropathol. Appl. Neurobiol. 2011, 37, 56–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of Amyloid Plaques in Retinas from Alzheimer’s Patients and Noninvasive in Vivo Optical Imaging of Retinal Plaques in a Mouse Model. Neuroimage 2011, 54, S204–S217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Koronyo, Y.; Rentsendorj, A.; Regis, G.C.; Sheyn, J.; Fuchs, D.-T.; Kramerov, A.A.; Ljubimov, A.V.; Dumitrascu, O.M.; Rodriguez, A.R.; et al. Identification of Early Pericyte Loss and Vascular Amyloidosis in Alzheimer’s Disease Retina. Acta Neuropathol. 2020, 139, 813–836. [Google Scholar] [CrossRef] [Green Version]
- Querques, G.; Borrelli, E.; Sacconi, R.; De Vitis, L.; Leocani, L.; Santangelo, R.; Magnani, G.; Comi, G.; Bandello, F. Functional and Morphological Changes of the Retinal Vessels in Alzheimer’s Disease and Mild Cognitive Impairment. Sci. Rep. 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Hosari, S.; Hohberger, B.; Theelke, L.; Sari, H.; Lucio, M.; Mardin, C.Y. OCT Angiography: Measurement of Retinal Macular Microvasculature with Spectralis II OCT Angiography-Reliability and Reproducibility. Ophthalmologica 2020, 243, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A Computational Tool for Quantitative Analysis of Vascular Networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.S.; Jia, Y.; Zhang, M.; Su, J.P.; Liu, G.; Hwang, T.S.; Bailey, S.T.; Huang, D. Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT27–OCT36. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.P.; Grewal, D.S.; Thompson, A.C.; Polascik, B.W.; Dunn, C.; Burke, J.R.; Fekrat, S. Retinal Microvascular and Neurodegenerative Changes in Alzheimer’s Disease and Mild Cognitive Impairment Compared with Control Participants. Ophthalmol. Retin. 2019, 3, 489–499. [Google Scholar] [CrossRef]
- Jiang, H.; Wei, Y.; Shi, Y.; Wright, C.B.; Sun, X.; Gregori, G.; Zheng, F.; Vanner, E.A.; Lam, B.L.; Rundek, T.; et al. Altered Macular Microvasculature in Mild Cognitive Impairment and Alzheimer Disease. J. Neuro-Ophthalmol. 2018, 38, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Choi, E.Y.; Kim, M.; Lee, H.K.; Byeon, S.H. Changes in Retinal Microvasculature and Retinal Layer Thickness in Association with Apolipoprotein E Genotype in Alzheimer’s Disease. Sci. Rep. 2021, 11, 1847. [Google Scholar] [CrossRef]
- Grewal, D.S.; Polascik, B.W.; Hoffmeyer, G.C.; Fekrat, S. Assessment of Differences in Retinal Microvasculature Using OCT Angiography in Alzheimer’s Disease: A Twin Discordance Report. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 440–444. [Google Scholar] [CrossRef]
- Chalkias, E.; Chalkias, I.-N.; Bakirtzis, C.; Messinis, L.; Nasios, G.; Ioannidis, P.; Pirounides, D. Differentiating Degenerative from Vascular Dementia with the Help of Optical Coherence Tomography Angiography Biomarkers. Healthcare 2022, 10, 539. [Google Scholar] [CrossRef]
- O’bryhim, B.E.; Apte, R.S.; Kung, N.; Coble, D.; Van Starven, G.P. Association of Preclinical Alzheimer Disease with Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018, 136, 1242–1248. [Google Scholar] [CrossRef]
- Salobrar-Garcia, E.; Méndez-Hernández, C.; de Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Fernández-Albarral, J.A.; Rojas, P.; Wang, S.; García-Feijoo, J.; Gil, P.; et al. Ocular Vascular Changes in Mild Alzheimer’s Disease Patients: Foveal Avascular Zone, Choroidal Thickness, and ONH Hemoglobin Analysis. J. Pers. Med. 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- López-Cuenca, I.; de Hoz, R.; Alcantara-Rey, C.; Salobrar-García, E.; Elvira-Hurtado, L.; Fernández-Albarral, J.A.; Barabash, A.; Ramírez-Toraño, F.; de Frutos-Lucas, J.; Salazar, J.J.; et al. Foveal Avascular Zone and Choroidal Thickness Are Decreased in Subjects with Hard Drusen and without High Genetic Risk of Developing Alzheimer’s Disease. Biomedicines 2021, 9, 638. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E.; Bohl, J. Staging of Alzheimer-Related Cortical Destruction. Eur. Neurol. 1993, 33, 403–408. [Google Scholar] [CrossRef]
- Ohm, T.G.; Müller, H.; Braak, H.; Bohl, J. Close-Meshed Prevalence Rates of Different Stages as a Tool to Uncover the Rate of Alzheimer’s Disease-Related Neurofibrillary Changes. Neuroscience 1995, 64, 209–217. [Google Scholar] [CrossRef]
- López-Cuenca, I.; de Hoz, R.; Salobrar-García, E.; Elvira-Hurtado, L.; Rojas, P.; Fernández-Albarral, J.A.; Barabash, A.; Salazar, J.J.; Ramírez, A.I.; Ramírez, J.M. Macular Thickness Decrease in Asymptomatic Subjects at High Genetic Risk of Developing Alzheimer’s Disease: An OCT Study. J. Clin. Med. 2020, 9, 1728. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.; Kanagasingam, Y.; Sohrabi, H.; Vignarajan, J.; Bourgeat, P.; Salvado, O.; Villemagne, V.; Rowe, C.C.; Lance MacAulay, S.; Szoeke, C.; et al. Retinal Vascular Biomarkers for Early Detection and Monitoring of Alzheimer’s Disease. Transl. Psychiatry 2013, 3, e233. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Q.; Tao, R.; Lu, H.; Xiao, Z.; Zheng, L.; Ding, D.; Ding, S.; Ma, Y.; Lu, Z.; et al. Decreased Retinal Vascular Density in Alzheimer’s Disease (AD) and Mild Cognitive Impairment (MCI): An Optical Coherence Tomography Angiography (OCTA) Study. Front. Aging Neurosci. 2021, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Feke, G.T.; Hyman, B.T.; Stern, R.A.; Pasquale, L.R. Retinal Blood Flow in Mild Cognitive Impairment and Alzheimer’s Disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2015, 1, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Liu, Y.; Wei, Y.; Shi, Y.; Wright, C.B.; Sun, X.; Rundek, T.; Baumel, B.S.; Landman, J.; Wang, J. Impaired Retinal Microcirculation in Patients with Alzheimer’s Disease. PLoS ONE 2018, 13, e0192154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulut, M.; Kurtuluş, F.; Gözkaya, O.; Erol, M.K.; Cengiz, A.; Akldan, M.; Yaman, A. Evaluation of Optical Coherence Tomography Angiographic Findings in Alzheimer’s Type Dementia. Br. J. Ophthalmol. 2018, 102, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Zhou, N.; Knoll, B.M.; Samra, S.; Ward, M.R.; Weintraub, S.; Fawzi, A.A. Parafoveal Vessel Loss and Correlation between Peripapillary Vessel Density and Cognitive Performance in Amnestic Mild Cognitive Impairment and Early Alzheimer’s Disease on Optical Coherence Tomography Angiography. PLoS ONE 2019, 14, e0214685. [Google Scholar] [CrossRef]
- Kotliar, K.E.; Vilser, W.; Nagel, E.; Lanzl, I.M. Retinal Vessel Reaction in Response to Chromatic Flickering Light. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 377–392. [Google Scholar] [CrossRef]
- Van De Kreeke, J.A.; Nguyen, H.T.; Konijnenberg, E.; Tomassen, J.; Den Braber, A.; Ten Kate, M.; Yaqub, M.; Van Berckel, B.; Lammertsma, A.A.; Boomsma, D.I.; et al. Optical Coherence Tomography Angiography in Preclinical Alzheimer’s Disease. Br. J. Ophthalmol. 2020, 104, 157–161. [Google Scholar] [CrossRef]
- Sousa, D.C.; Leal, I.; Moreira, S.; Dionísio, P.; Abegão Pinto, L.; Marques-Neves, C. Hypoxia Challenge Test and Retinal Circulation Changes–a Study Using Ocular Coherence Tomography Angiography. Acta Ophthalmol. 2018, 96, e315–e319. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.M.; Kwan, J.; Malek-Ahmadi, M.; Maarouf, C.L.; Kokjohn, T.A.; Belden, C.; Sabbagh, M.N.; Beach, T.G.; Roher, A.E. Morphological and Pathological Evolution of the Brain Microcirculation in Aging and Alzheimer’s Disease. PLoS ONE 2012, 7, e36893. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Murphy, O.; Caldito, N.G.; Calabresi, P.A.; Saidha, S. Emerging Applications of Optical Coherence Tomography Angiography (OCTA) in Neurological Research. Eye Vis. 2018, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- De Jong, G.I.; De Vos, R.A.I.; Jansen Steur, E.N.H.; Luiten, P.G.M. Cerebrovascular Hypoperfusion: A Risk Factor for Alzheimer’s Disease? Ann. N. Y. Acad. Sci. 1997, 826, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N.; Pax, A.B. Increased Collagen Content of Cerebral Microvessels in Alzheimer’s Disease. Brain Res. 1995, 705, 349–352. [Google Scholar] [CrossRef]
- Nobili, F.; Frisoni, G.B.; Portet, F.; Verhey, F.; Rodriguez, G.; Caroli, A.; Touchon, J.; Calvini, P.; Morbelli, S.; De Carli, F.; et al. Brain SPECT in Subtypes of Mild Cognitive Impairment. J. Neurol. 2008, 255, 1344–1353. [Google Scholar] [CrossRef]
- Henkind, P. Radial Peripapillary Capillaries of the Retina. I. Anatomy: Human and Comparative. Br. J. Ophthalmol. 1967, 51, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.P.; Robbins, C.B.; Lee, J.M.; Soundararajan, S.; Stinnett, S.S.; Agrawal, R.; Plassman, B.L.; Lad, E.M.; Whitson, H.; Grewal, D.S.; et al. Longitudinal Analysis of the Retina and Choroid in Cognitively Normal Individuals at Higher Genetic Risk for Alzheimer Disease. Ophthalmol. Retina. 2022. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.F.; Robbins, M.A.; Budge, M.M.; Abhayaratna, W.P.; Dore, G.A.; Elias, P.K. Arterial Pulse Wave Velocity and Cognition with Advancing Age. Hypertension 2009, 53, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Pase, M.P.; Herbert, A.; Grima, N.A.; Pipingas, A.; O’Rourke, M.F. Arterial Stiffness as a Cause of Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Intern. Med. J. 2012, 42, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Vos, S.J.B.; Verhey, F.; Frölich, L.; Kornhuber, J.; Wiltfang, J.; Maier, W.; Peters, O.; Rüther, E.; Nobili, F.; Morbelli, S.; et al. Prevalence and Prognosis of Alzheimer’s Disease at the Mild Cognitive Impairment Stage. Brain 2015, 138, 1327–1338. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, K.-I.; Taniwaki, Y.; Utsunomiya, H.; Taniwaki, T. Cerebral Blood Flow Reduction Associated with Orientation for Time in Amnesic Mild Cognitive Impairment and Alzheimer Disease Patients. J. Neuroimaging 2014, 24, 590–594. [Google Scholar] [CrossRef]
- Katusic, Z.S.; Austin, S.A. Endothelial Nitric Oxide: Protector of a Healthy Mind. Eur. Heart J. 2014, 35, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Golzan, S.M.; Goozee, K.; Georgevsky, D.; Avolio, A.; Chatterjee, P.; Shen, K.; Gupta, V.; Chung, R.; Savage, G.; Orr, C.F.; et al. Retinal Vascular and Structural Changes Are Associated with Amyloid Burden in the Elderly: Ophthalmic Biomarkers of Preclinical Alzheimer’s Disease. Alzheimer’s Res. Ther. 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, J.M.; Salazar, J.J.; De, R.; Rojas, B.; Gallego, B.I.; Ramrez, A.I.; Trivio, A. Choroidal Vessel Wall: Hypercholesterolaemia-Induced Dysfunction and Potential Role of Statins. In Current Basic and Pathological Approaches to the Function of Muscle Cells and Tissues-from Molecules to Humans; Sugi, H., Ed.; IntechOpen: London, UK, 2012; pp. 255–298. [Google Scholar]
- Sun, C.; Wang, J.J.; Mackey, D.A.; Wong, T.Y. Retinal Vascular Caliber: Systemic, Environmental, and Genetic Associations. Surv. Ophthalmol. 2009, 54, 74–95. [Google Scholar] [CrossRef]
- Ikram, M.K.; Witteman, J.C.M.; Vingerling, J.R.; Breteler, M.M.B.; Hofman, A.; De Jong, P.T.V.M. Retinal Vessel Diameters and Risk of Hypertension. Hypertension 2006, 47, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, A.; Pillai, J.A.; Bermel, R.; Bonner-Jackson, A.; Rae-Grant, A.; Fernandez, H.; Bena, J.; Jones, S.E.; Leverenz, J.B.; Srivastava, S.K.; et al. Outer Retinal Assessment Using Spectral-Domain Optical Coherence Tomography in Patients With Alzheimer’s and Parkinson’s Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2768–2777. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi Falavarjani, K.; Al-Sheikh, M.; Akil, H.; Sadda, S.R. Image Artefacts in Swept-Source Optical Coherence Tomography Angiography. Br. J. Ophthalmol. 2017, 101, 564–568. [Google Scholar] [CrossRef]
- Holmen, I.C.; Konda, M.S.; Pak, J.W.; McDaniel, K.W.; Blodi, B.; Stepien, K.E.; Domalpally, A. Prevalence and Severity of Artifacts in Optical Coherence Tomographic Angiograms. JAMA Ophthalmol. 2020, 138, 119–126. [Google Scholar] [CrossRef]
- Shahlaee, A.; Samara, W.A.; Hsu, J.; Say, E.A.T.; Khan, M.A.; Sridhar, J.; Hong, B.K.; Shields, C.L.; Ho, A.C. In Vivo Assessment of Macular Vascular Density in Healthy Human Eyes Using Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2016, 165, 39–46. [Google Scholar] [CrossRef]
- Gadde, S.G.K.; Anegondi, N.; Bhanushali, D.; Chidambara, L.; Yadav, N.K.; Khurana, A.; Roy, A.S. Quantification of Vessel Density in Retinal Optical Coherence Tomography Angiography Images Using Local Fractal Dimension. Investig. Ophthalmol. Vis. Sci. 2016, 57, 246–252. [Google Scholar] [CrossRef] [PubMed]


| Macular analysis | ||||||||
| Software | Plexus | Ring | Sector | FH- ApoE ɛ4- | FH+ ApoE ɛ4- | FH+ ApoE ɛ4+ | FH- ApoE ɛ4- vs. FH+ ApoE ɛ4+ | FH+ ApoE ɛ4- vs. FH+ ApoE ɛ4+ |
| (n = 18) | (n= 42) | (n = 20) | p-value | |||||
| EA-Tool | DVP | C3 | H12 | 23.3 (18.4–27.4) | 25.2 (21.7–27.4) | 20.6 (18.2–25.6) | 0.313 | 0.041 * |
| Peripapillary analysis | ||||||||
| Software | Plexus | Ring | Sector | FH- ApoE ɛ4- | FH+ ApoE ɛ4- | FH+ ApoE ɛ4+ | FH- ApoE ɛ4- vs. FH+ ApoE ɛ4+ | FH+ ApoE ɛ4- vs. FH+ ApoE ɛ4+ |
| (n = 24) | (n= 44) | (n = 30) | p-value | |||||
| AngioTool | % Area occupied by vessels | 47.1 (43.7–48.6) | 47.5 (45.2–49.1) | 45.8 (44.2–47.2) | 0.423 | 0.031 * | ||
| EA-Tool | EA-Tool | C3 | H3 | 17.2 (12.0–22.6) | 16.9 (13.1–27.4) | 23.2 (17.4–30.5) | 0.021* | 0.073 |
| H4 | 16.0 (11.3–31.3) | 19.6 (13.9–31.1) | 23.9 (20.3–30.7) | 0.030 * | 0.049 * | |||
| H10 | 22.1 (17.5–26.2) | 24.1 (17.6–27.9) | 27.2 (20.7–31.8) | 0.037 * | 0.151 | |||
| H11 | 23.9 (17.4–27.6) | 24.0 (15.1–32.0) | 27.9 (23.2–37.9) | 0.031 * | 0.057 | |||
| H12 | 22.8 (14.1–32.5) | 17.8 (10.6–29.6) | 31.5 (16.4–39.1) | 0.280 | 0.023 * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cuenca, I.; Salobrar-García, E.; Sánchez-Puebla, L.; Espejel, E.; García del Arco, L.; Rojas, P.; Elvira-Hurtado, L.; Fernández-Albarral, J.A.; Ramírez-Toraño, F.; Barabash, A.; et al. Retinal Vascular Study Using OCTA in Subjects at High Genetic Risk of Developing Alzheimer’s Disease and Cardiovascular Risk Factors. J. Clin. Med. 2022, 11, 3248. https://doi.org/10.3390/jcm11113248
López-Cuenca I, Salobrar-García E, Sánchez-Puebla L, Espejel E, García del Arco L, Rojas P, Elvira-Hurtado L, Fernández-Albarral JA, Ramírez-Toraño F, Barabash A, et al. Retinal Vascular Study Using OCTA in Subjects at High Genetic Risk of Developing Alzheimer’s Disease and Cardiovascular Risk Factors. Journal of Clinical Medicine. 2022; 11(11):3248. https://doi.org/10.3390/jcm11113248
Chicago/Turabian StyleLópez-Cuenca, Inés, Elena Salobrar-García, Lidia Sánchez-Puebla, Eva Espejel, Lucía García del Arco, Pilar Rojas, Lorena Elvira-Hurtado, José A. Fernández-Albarral, Federico Ramírez-Toraño, Ana Barabash, and et al. 2022. "Retinal Vascular Study Using OCTA in Subjects at High Genetic Risk of Developing Alzheimer’s Disease and Cardiovascular Risk Factors" Journal of Clinical Medicine 11, no. 11: 3248. https://doi.org/10.3390/jcm11113248
APA StyleLópez-Cuenca, I., Salobrar-García, E., Sánchez-Puebla, L., Espejel, E., García del Arco, L., Rojas, P., Elvira-Hurtado, L., Fernández-Albarral, J. A., Ramírez-Toraño, F., Barabash, A., Salazar, J. J., Ramírez, J. M., de Hoz, R., & Ramírez, A. I. (2022). Retinal Vascular Study Using OCTA in Subjects at High Genetic Risk of Developing Alzheimer’s Disease and Cardiovascular Risk Factors. Journal of Clinical Medicine, 11(11), 3248. https://doi.org/10.3390/jcm11113248














