Sprint Interval Exercise Improves Cognitive Performance Unrelated to Postprandial Glucose Fluctuations at Different Levels of Normobaric Hypoxia
Abstract
:1. Introduction
2. Materials and Methods
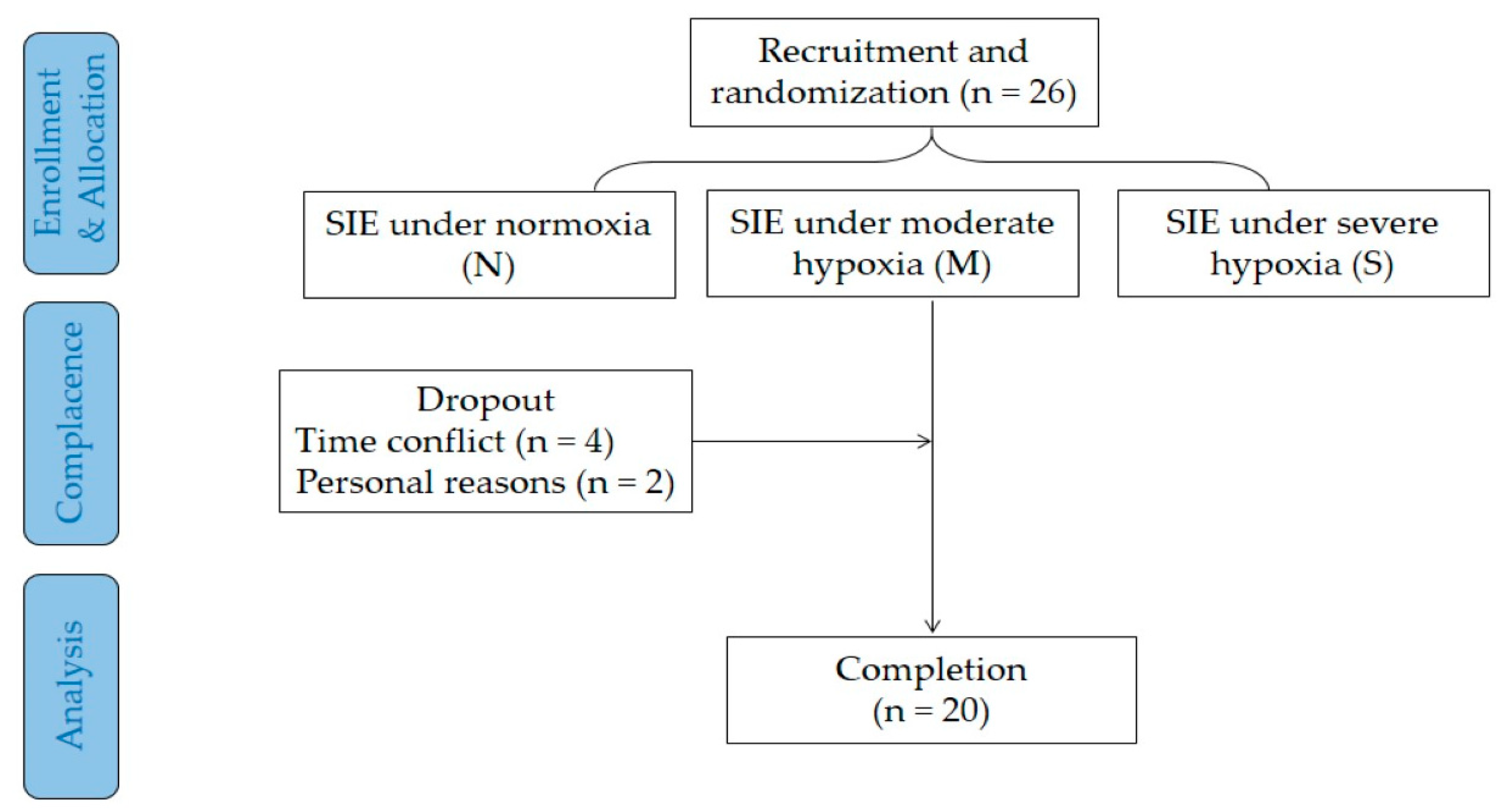
2.1. Participants
2.2. Experimental Design
2.3. Sprint Interval Exercise
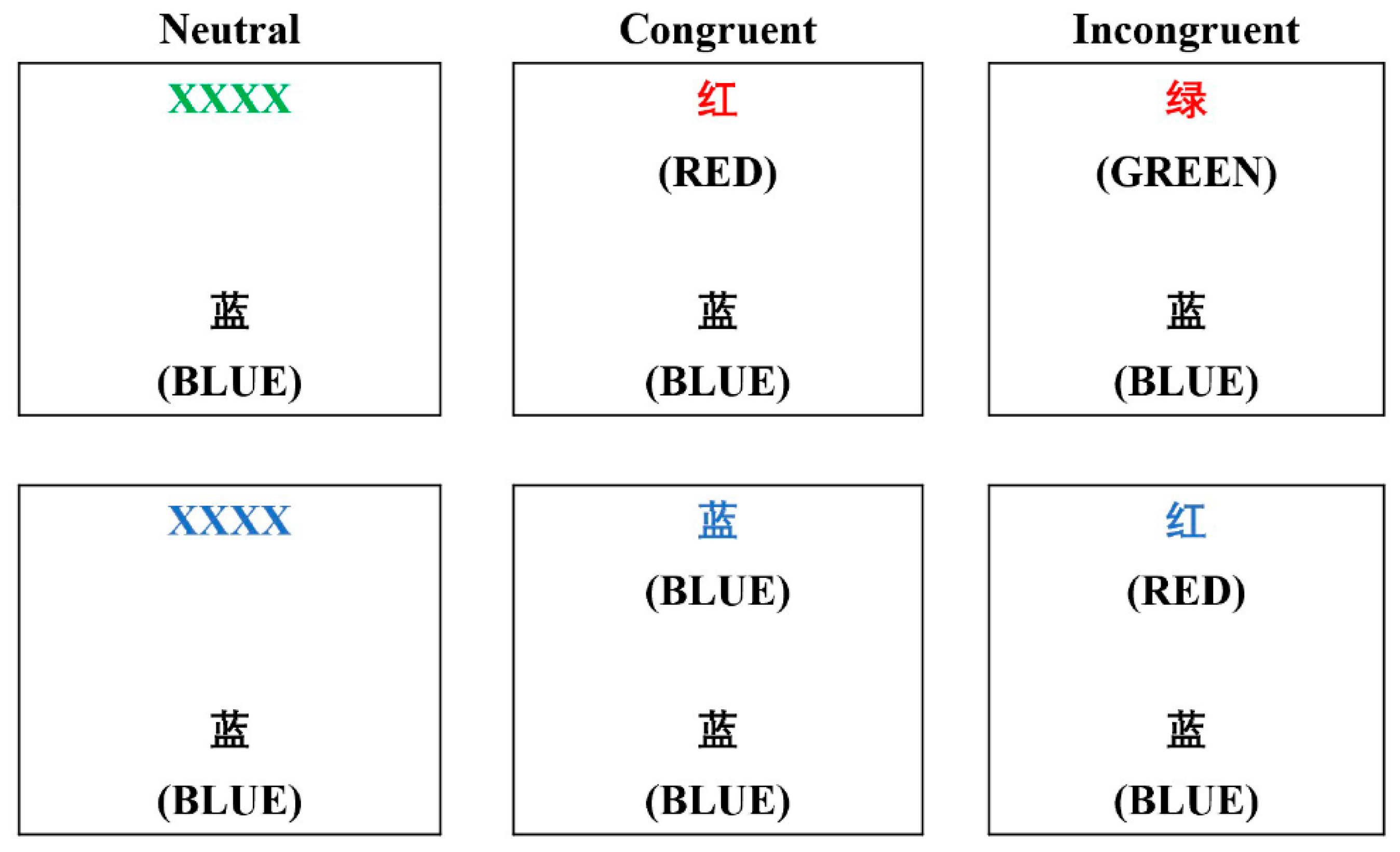
2.4. Cognitive Test
2.5. Blood Glucose Measures
2.6. Physical Activity and Diet
2.7. Statistical Analyses
3. Results
3.1. Daily Activity and Baseline Values
3.2. Exercise Performance and Physiological Responses
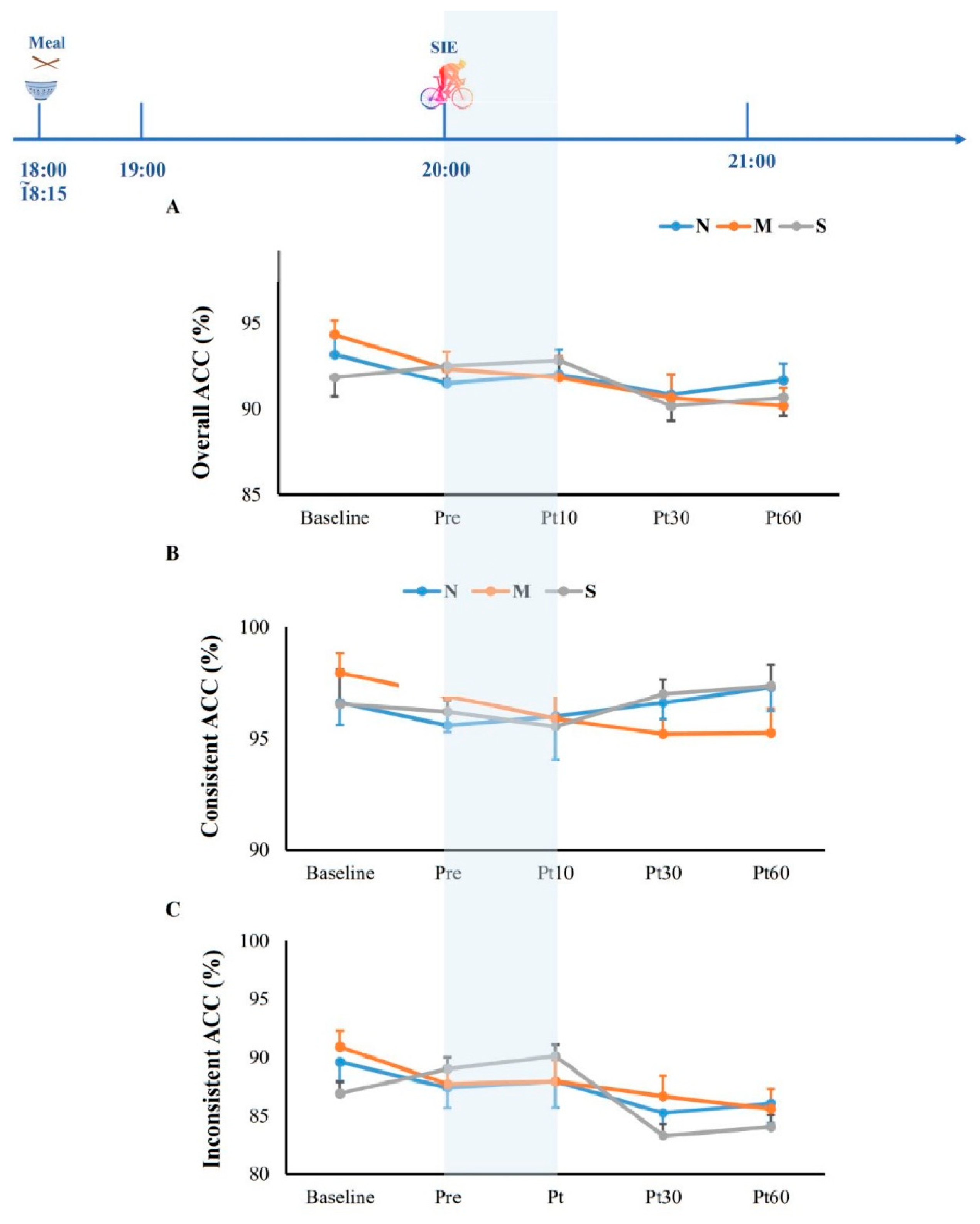
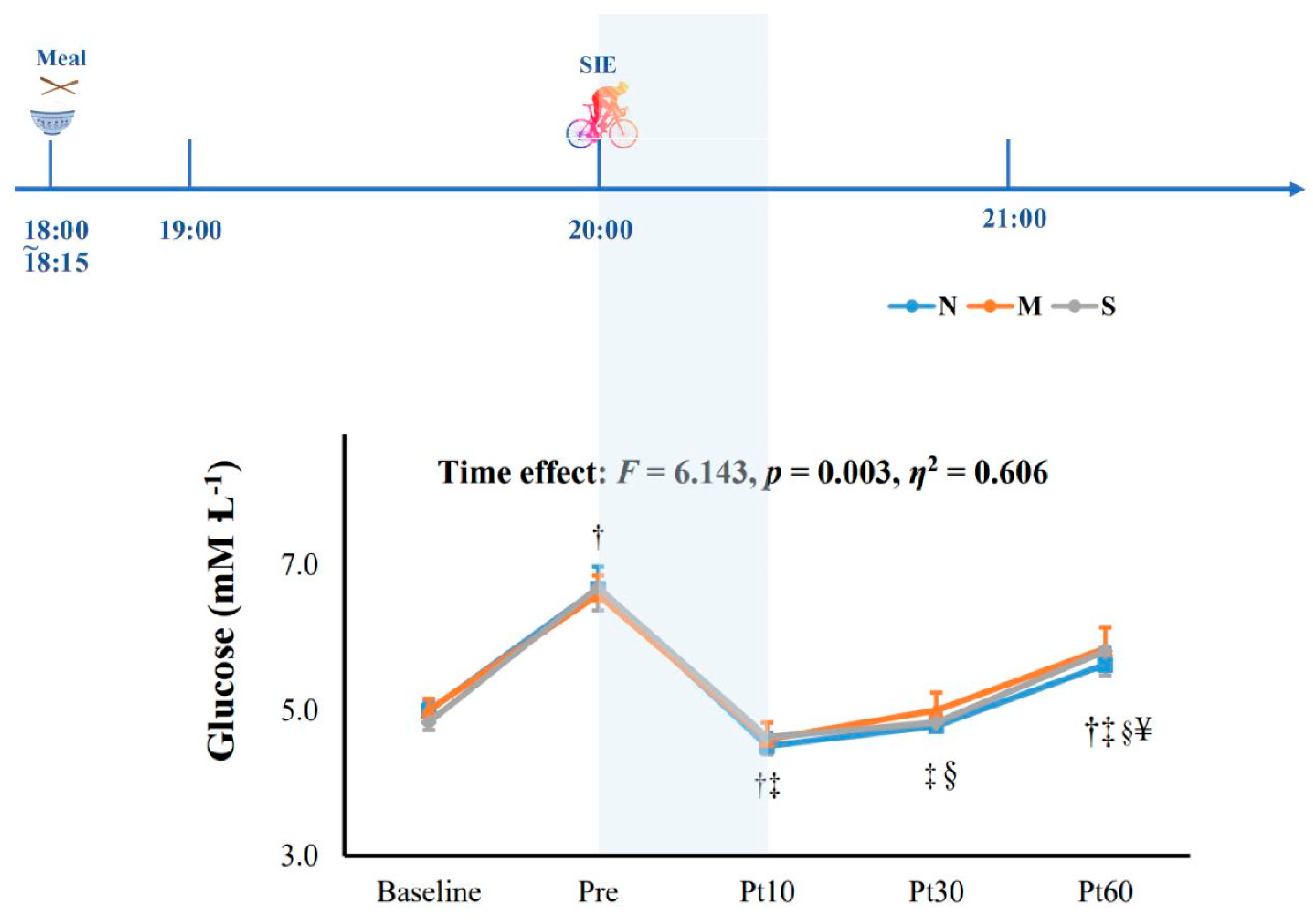
3.3. Cognitive Function and Blood Glucose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costigan, S.; Eather, N.; Plotnikoff, R.; Hillman, C.; Lubans, D. High intensity interval training on cognitive and mental health in adolescents. J. Sci. Med. Sport 2017, 20, e108–e109. [Google Scholar] [CrossRef]
- Gibala, M.J.; McGee, S.L. Metabolic Adaptations to Short-Term High-Intensity Interval Training: A Little Pain for a Lot of Gain? Exerc. Sport Sci. Rev. 2008, 36, 6. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; McKenzie, D.C.; Haykowsky, M.J.; Taylor, A.; Shoemaker, P.; Ignaszewski, A.P.; Chan, S.Y. Effectiveness of High-Intensity Interval Training for the Rehabilitation of Patients With Coronary Artery Disease. Am. J. Cardiol. 2005, 95, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Sloth, M.; Sloth, D.; Overgaard, K.; Dalgas, U. Effects of sprint interval training on VO2max and aerobic exercise performance: A systematic review and meta-analysis: Effects of Sprint Interval Training on VO2max. Scand. J. Med. Sci. Sports 2013, 23, e341–e352. [Google Scholar] [CrossRef]
- Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Brooks, D.; Timón, R.; Olcina, G.; Brazo-Sayavera, J. Effects training in hypoxia on cardiometabolic parameters in obese people: A systematic review of randomized controlled trial. Aten. Primaria. 2019, 51, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Hobbins, L.G.; Hunter, S.; Gaoua, N.; Girard, O. Normobaric hypoxic conditioning to maximize weight loss and ameliorate cardio-metabolic health in obese populations: A systematic review. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R251–R264. [Google Scholar] [CrossRef] [Green Version]
- Kong, Z.; Sun, S.; Liu, M.; Shi, Q. Short-Term High-Intensity Interval Training on Body Composition and Blood Glucose in Overweight and Obese Young Women. J. Diabetes Res. 2016, 2016, 4073618. [Google Scholar] [CrossRef]
- Kong, Z.; Shi, Q.; Nie, J.; Tong, T.K.; Song, L.; Yi, L.; Hu, Y. High-Intensity Interval Training in Normobaric Hypoxia Improves Cardiorespiratory Fitness in Overweight Chinese Young Women. Front. Physiol. 2017, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Brazo-Sayavera, J.; Timón, R.; González-Custodio, A.; Olcina, G. Repeated sprint in hypoxia as a time-metabolic efficient strategy to improve physical fitness of obese women. Eur. J. Appl. Physiol. 2020, 120, 1051–1061. [Google Scholar] [CrossRef]
- Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Brazo-Sayavera, J.; Burtscher, M.; Timón, R.; Olcina, G. Effects of High-Intensity Interval Training Under Normobaric Hypoxia on Cardiometabolic Risk Markers in Overweight/Obese Women. High Alt. Med. Biol. 2018, 19, 356–366. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, F.L.; Milsom, W.K.; Mitchell, G.S. Time domains of the hypoxic ventilatory response. Respir. Physiol. 1998, 112, 123–134. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 2017, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Petrassi, F.A.; Hodkinson, P.D.; Walters, P.L.; Gaydos, S.J. Hypoxic hypoxia at moderate altitudes: Review of the state of the science. Aviat. Space Environ. Med. 2012, 83, 975–984. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Lambourne, K.; Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef]
- Rąglewska, P. Dancing as Non-Pharmacological Treatment for Healthy Aging in the COVID-19 Era; a Gerontological Perspective. TSS 2021, 28, 173–177. [Google Scholar] [CrossRef]
- Alves, C.R.R.; Tessaro, V.H.; Teixeira, L.A.C.; Murakava, K.; Roschel, H.; Gualano, B.; Takito, M.Y. Influence of Acute High-Intensity Aerobic Interval Exercise Bout on Selective Attention and Short-Term Memory Tasks. Percept. Mot. Ski. 2014, 118, 63–72. [Google Scholar] [CrossRef]
- Winter, B.; Breitenstein, C.; Mooren, F.C.; Voelker, K.; Fobker, M.; Lechtermann, A.; Krueger, K.; Fromme, A.; Korsukewitz, C.; Floel, A.; et al. High impact running improves learning. Neurobiol. Learn. Mem. 2007, 87, 597–609. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Suga, T.; Takenaka, S.; Tanaka, D.; Takeuchi, T.; Hamaoka, T.; Isaka, T.; Hashimoto, T. Greater impact of acute high-intensity interval exercise on post-exercise executive function compared to moderate-intensity continuous exercise. Physiol. Behav. 2016, 155, 224–230. [Google Scholar] [CrossRef]
- Ando, S.; Hatamoto, Y.; Sudo, M.; Kiyonaga, A.; Tanaka, H.; Higaki, Y. The Effects of Exercise Under Hypoxia on Cognitive Function. PLoS ONE 2013, 8, e63630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Aquino-Lemos, V.; Santos, R.V.T.; Antunes, H.K.M.; Lira, F.S.; Bittar, I.G.L.; Caris, A.V.; Tufik, S.; de Mello, M.T. Acute physical exercise under hypoxia improves sleep, mood and reaction time. Physiol. Behav. 2016, 154, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, T.; Sudo, M.; Higaki, Y.; Kiyonaga, A.; Tanaka, H.; Ando, S. Does moderate hypoxia alter working memory and executive function during prolonged exercise? Physiol. Behav. 2015, 139, 290–296. [Google Scholar] [CrossRef]
- Komiyama, T.; Katayama, K.; Sudo, M.; Ishida, K.; Higaki, Y.; Ando, S. Cognitive function during exercise under severe hypoxia. Sci. Rep. 2017, 7, 10000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, O.-K.; Kong, Z.; Loprinzi, P.D.; Shi, Q.; Sun, S.; Zou, L.; Hu, Y.; Nie, J. Severe Hypoxia Does Not Offset the Benefits of Exercise on Cognitive Function in Sedentary Young Women. Int. J. Environ. Res. Public Health 2019, 16, 1003. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.; Burns, K.; Fennell, C.; Kim, J.-H.; Gunstad, J.; Glickman, E.; McDaniel, J. The Influence of Exercise on Cognitive Performance in Normobaric Hypoxia. High Alt. Med. Biol. 2015, 16, 298–305. [Google Scholar] [CrossRef]
- Jung, M.; Zou, L.; Yu, J.J.; Ryu, S.; Kong, Z.; Yang, L.; Kang, M.; Lin, J.; Li, H.; Smith, L.; et al. Does exercise have a protective effect on cognitive function under hypoxia? A systematic review with meta-analysis. J. Sport Health Sci. 2020, 9, 562–577. [Google Scholar] [CrossRef]
- Bouak, F.; Vartanian, O.; Hofer, K.; Cheung, B. Acute Mild Hypoxic Hypoxia Effects on Cognitive and Simulated Aircraft Pilot Performance. Aerosp. Med. Hum. Perform. 2018, 89, 526–535. [Google Scholar] [CrossRef]
- Dobashi, S.; Horiuchi, M.; Endo, J.; Kiuchi, M.; Koyama, K. Cognitive Function and Cerebral Oxygenation During Prolonged Exercise Under Hypoxia in Healthy Young Males. High Alt. Med. Biol. 2016, 17, 214–221. [Google Scholar] [CrossRef]
- Kim, C.-H.; Ryan, E.J.; Seo, Y.; Peacock, C.; Gunstad, J.; Muller, M.D.; Ridgel, A.L.; Glickman, E.L. Low intensity exercise does not impact cognitive function during exposure to normobaric hypoxia. Physiol. Behav. 2015, 151, 24–28. [Google Scholar] [CrossRef]
- Lefferts, W.K.; Babcock, M.; Tiss, M.J.; Ives, S.J.; White, C.N.; Brutsaert, T.D.; Heffernan, K.S. Effect of hypoxia on cerebrovascular and cognitive function during moderate intensity exercise. Physiol. Behav. 2016, 165, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Ochi, G.; Kanazawa, Y.; Hyodo, K.; Suwabe, K.; Shimizu, T.; Fukuie, T.; Byun, K.; Soya, H. Hypoxia-induced lowered executive function depends on arterial oxygen desaturation. J. Physiol. Sci. 2018, 68, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Duckworth, L.; Barlow, M.J.; Deighton, K.; Matu, J.; Williams, E.L.; Woods, D.; Xie, L.; Stephan, B.C.M.; Siervo, M.; et al. Effects of Dietary Nitrate Supplementation on Physiological Responses, Cognitive Function, and Exercise Performance at Moderate and Very-High Simulated Altitude. Front. Physiol. 2017, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Loprinzi, P.D.; Guan, H.; Zou, L.; Kong, Z.; Hu, Y.; Shi, Q.; Nie, J. The Effects of High-Intensity Interval Exercise and Hypoxia on Cognition in Sedentary Young Adults. Medicina 2019, 55, 43. [Google Scholar] [CrossRef] [Green Version]
- Subudhi, A.W.; Dimmen, A.C.; Roach, R.C. Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J. Appl. Physiol. 2007, 103, 177–183. [Google Scholar] [CrossRef]
- Goodall, S.; Twomey, R.; Amann, M. Acute and chronic hypoxia: Implications for cerebral function and exercise tolerance. Fatigue 2014, 2, 73–92. [Google Scholar] [CrossRef] [Green Version]
- Cockcroft, E.J.; Williams, C.A.; Weaver, H.; O’Connor, A.; Jackman, S.R.; Armstrong, N.; Barker, A.R. Acute Exercise and Insulin Sensitivity in Boys: A Time-Course Study. Int. J. Sports Med. 2017, 38, 967–974. [Google Scholar] [CrossRef]
- Kong, Z.; Shi, Q.; Sun, S.; Tong, T.K.; Zhang, H.; Nie, J. High-intensity interval exercise lowers postprandial glucose concentrations more in obese adults than lean adults. Prim. Care Diabetes 2019, 13, 568–573. [Google Scholar] [CrossRef]
- Nie, J.; Kong, Z.; Baker, J.S.; Tong, T.K.; Lei, S.H.; Shi, Q. Acute changes in glycemic homeostasis in response to brief high-intensity intermittent exercise in obese adults. J. Exerc. Sci. Fit. 2012, 10, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Gillen, J.B.; Little, J.P.; Punthakee, Z.; Tarnopolsky, M.A.; Riddell, M.C.; Gibala, M.J. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 575–577. [Google Scholar] [CrossRef]
- Mackenzie, R.; Maxwell, N.; Castle, P.; Brickley, G.; Watt, P. Acute hypoxia and exercise improve insulin sensitivity (SI2*) in individuals with type 2 diabetes. Diabetes/Metab. Res. Rev. 2011, 27, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.E. Role of glucose in regulating the brain and cognition. Am. J. Clin. Nutr. 1995, 61, 987S–995S. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Parker, P.Y.; Donohoe, R.T. The supply of glucose to the brain and cognitive functioning. J. Biosoc. Sci. 1996, 28, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Graveling, A.J.; Deary, I.J.; Frier, B.M. Acute Hypoglycemia Impairs Executive Cognitive Function in Adults With and Without Type 1 Diabetes. Diabetes Care 2013, 36, 3240–3246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- William, B. Gene Adams Exercise Physiology Laboratory Manual; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. NeuroImage 2010, 50, 1702–1710. [Google Scholar] [CrossRef]
- Levine, T.R.; Hullett, C.R. Eta Squared, Partial Eta Squared, and Misreporting of Effect Size in Communication Research. Hum. Commun. Res. 2002, 28, 612–625. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013; ISBN 978-1-134-74270-7. [Google Scholar]
- Carter, C.S.; Mintun, M.; Cohen, J.D. Interference and Facilitation Effects during Selective Attention: An H215O PET Study of Stroop Task Performance. NeuroImage 1995, 2, 264–272. [Google Scholar] [CrossRef]
- Ludyga, S.; Pühse, U.; Lucchi, S.; Marti, J.; Gerber, M. Immediate and sustained effects of intermittent exercise on inhibitory control and task-related heart rate variability in adolescents. J. Sci. Med. Sport 2018, 22, 96–100. [Google Scholar] [CrossRef]
- Phillips, J.B.; Hørning, D.; Funke, M.E. Cognitive and Perceptual Deficits of Normobaric Hypoxia and the Time Course to Performance Recovery. Aerosp. Med. Hum. Perform. 2015, 86, 357–365. [Google Scholar] [CrossRef]
- Ogoh, S.; Ainslie, P.N. Cerebral blood flow during exercise: Mechanisms of regulation. J. Appl. Physiol. 2009, 107, 1370–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMorris, T.; Collard, K.; Corbett, J.; Dicks, M.; Swain, J.P. A test of the catecholamines hypothesis for an acute exercise–cognition interaction. Pharmacol. Biochem. Behav. 2008, 89, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Chiew, A.E.; Rimke, A.N.; Chan, G.; Rampuri, Z.H.; Kozak, M.D.; Boulé, N.G.; Steinback, C.D.; Davenport, M.H.; Day, T.A. Blood glucose concentration is unchanged during exposure to acute normobaric hypoxia in healthy humans. Physiol. Rep. 2021, 9, e14932. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Goto, K.; Ishida, K.; Ogita, F. Substrate utilization during exercise and recovery at moderate altitude. Metabolism 2010, 59, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Wadley, G.D.; Lee-Young, R.S.; Canny, B.J.; Wasuntarawat, C.; Chen, Z.P.; Hargreaves, M.; Kemp, B.E.; McConell, G.K. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E694–E702. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.R.; Williamson, D.L.; Fealy, C.E.; Kriz, D.A.; Krishnan, R.K.; Huang, H.; Ahn, J.; Loomis, J.L.; Kirwan, J.P. Acute altitude-induced hypoxia suppresses plasma glucose and leptin in healthy humans. Metabolism 2010, 59, 200–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiasson, J.-L.; Shikama, H.; Chu, D.T.W.; Exton, J.H. Inhibitory Effect of Epinephrine on Insulin-stimulated Glucose Uptake by Rat Skeletal Muscle. J. Clin. Investig. 1981, 68, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Goto, K.; Morishima, T.; Kurobe, K.; Huang, Z.; Ogita, F. Augmented Carbohydrate Oxidation under Moderate Hypobaric Hypoxia Equivalent to Simulated Altitude of 2500 m. Tohoku J. Exp. Med. 2015, 236, 163–168. [Google Scholar] [CrossRef] [Green Version]

| N | M | S | |
|---|---|---|---|
| SpO2 (%) | |||
| Baseline | 98 ± 1 | 98 ± 1 | 98 ± 1 |
| Pre | 98 ± 3 | 89 ± 3 *,a | 78 ± 4 *,**,a,b |
| Post 0 | 96 ± 3 | 84 ± 5 *,a,b | 74 ± 58 *,**,a,b |
| Post 10 | 97 ± 1 | 89 ± 4 *,a | 79 ± 5 *,**,a |
| Post 30 | 97 ± 1 | 97 ± 2 b,c,d | 96 ± 3 b,c,d |
| Post 60 | 97 ± 1 | 97 ± 2 b,c,d | 97 ± 2 b,c,d |
| HR (bpm) | |||
| Baseline | 63 ± 7 | 62 ± 6 | 64 ± 5 |
| Pre | 65 ± 6 | 72 ± 8 *,a | 79 ± 10 *,**,a,b |
| Post 0 | 167 ± 9 a,b | 168 ± 11 a,b | 168 ± 7 a,b |
| Post 10 | 92 ± 11 a,b,c | 94 ± 16 a,b,c | 95 ± 12 a,b,c |
| Post 30 | 86 ± 11 a,b,c,d | 86 ± 13 a,b,c,d | 87 ± 11 a,b,c,d |
| Post 60 | 81 ± 10 a,b,c,d,e | 80 ± 11 a,b,c,d,e | 80 ±9 a,b,c,d,e |
| RPE20 | |||
| Baseline | 6 ± 0 | 6 ± 1 | 6 ± 0 |
| Pre | 7 ± 1 | 7 ± 1 | 7 ± 1 |
| Post 0 | 17 ± 2 a,b | 18 ± 2 a,b | 17 ± 2 a,b |
| Post 10 | 8 ± 2 c | 8 ± 2 c | 8 ± 2 c |
| Post 30 | 7 ± 2 c | 7 ± 1 c | 7 ± 2 c |
| Post 60 | 7 ± 1 c | 7 ± 1 c | 6 ± 1 c |
| N | M | S | |
|---|---|---|---|
| Peak power (W/kg) | 11.6 ± 1.9 | 11.4 ± 1.8 | 9.9 ± 2.8 *,# |
| Average power (W/kg) | 6.4 ± 0.8 | 6.1 ± 0.7 * | 5.9 ± 0.9 * |
| Fatigue index (%) | 45.0 ± 13.6 | 48.8 ± 14.9 | 51.0 ± 18.4 |
| Exercise HR (bpm) | 169 ± 9 | 169 ± 11 | 168 ± 9 |
| %HRmax (%) | 93.1 ± 4.3 | 93.2 ± 5.4 | 92.8 ± 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, O.-K.; Sun, S.; Nie, J.; Shi, Q.; Kong, Z. Sprint Interval Exercise Improves Cognitive Performance Unrelated to Postprandial Glucose Fluctuations at Different Levels of Normobaric Hypoxia. J. Clin. Med. 2022, 11, 3159. https://doi.org/10.3390/jcm11113159
Lei O-K, Sun S, Nie J, Shi Q, Kong Z. Sprint Interval Exercise Improves Cognitive Performance Unrelated to Postprandial Glucose Fluctuations at Different Levels of Normobaric Hypoxia. Journal of Clinical Medicine. 2022; 11(11):3159. https://doi.org/10.3390/jcm11113159
Chicago/Turabian StyleLei, On-Kei, Shengyan Sun, Jinlei Nie, Qingde Shi, and Zhaowei Kong. 2022. "Sprint Interval Exercise Improves Cognitive Performance Unrelated to Postprandial Glucose Fluctuations at Different Levels of Normobaric Hypoxia" Journal of Clinical Medicine 11, no. 11: 3159. https://doi.org/10.3390/jcm11113159
APA StyleLei, O.-K., Sun, S., Nie, J., Shi, Q., & Kong, Z. (2022). Sprint Interval Exercise Improves Cognitive Performance Unrelated to Postprandial Glucose Fluctuations at Different Levels of Normobaric Hypoxia. Journal of Clinical Medicine, 11(11), 3159. https://doi.org/10.3390/jcm11113159






