An Evaluation of the Physicochemical Properties of Preservative-Free 0.005% (w/v) Latanoprost Ophthalmic Solutions, and the Impact on In Vitro Human Conjunctival Goblet Cell Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Physicochemical Characterization
2.3. Human Conjunctival Goblet Cell Cultivation
2.4. Cell Survival Analysis
2.5. Immunohistochemical Staining
2.6. Statistics
3. Results
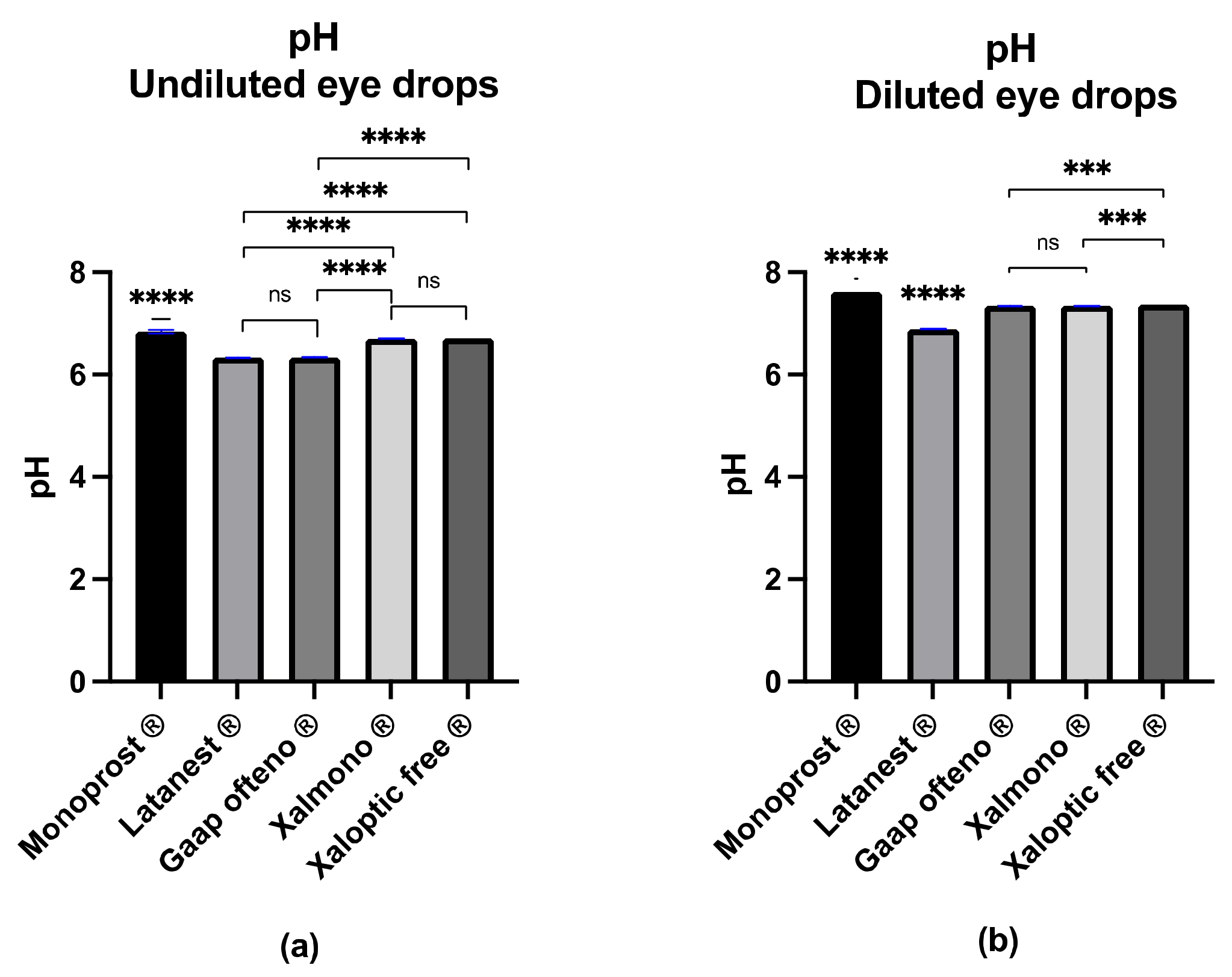
3.1. pH Value
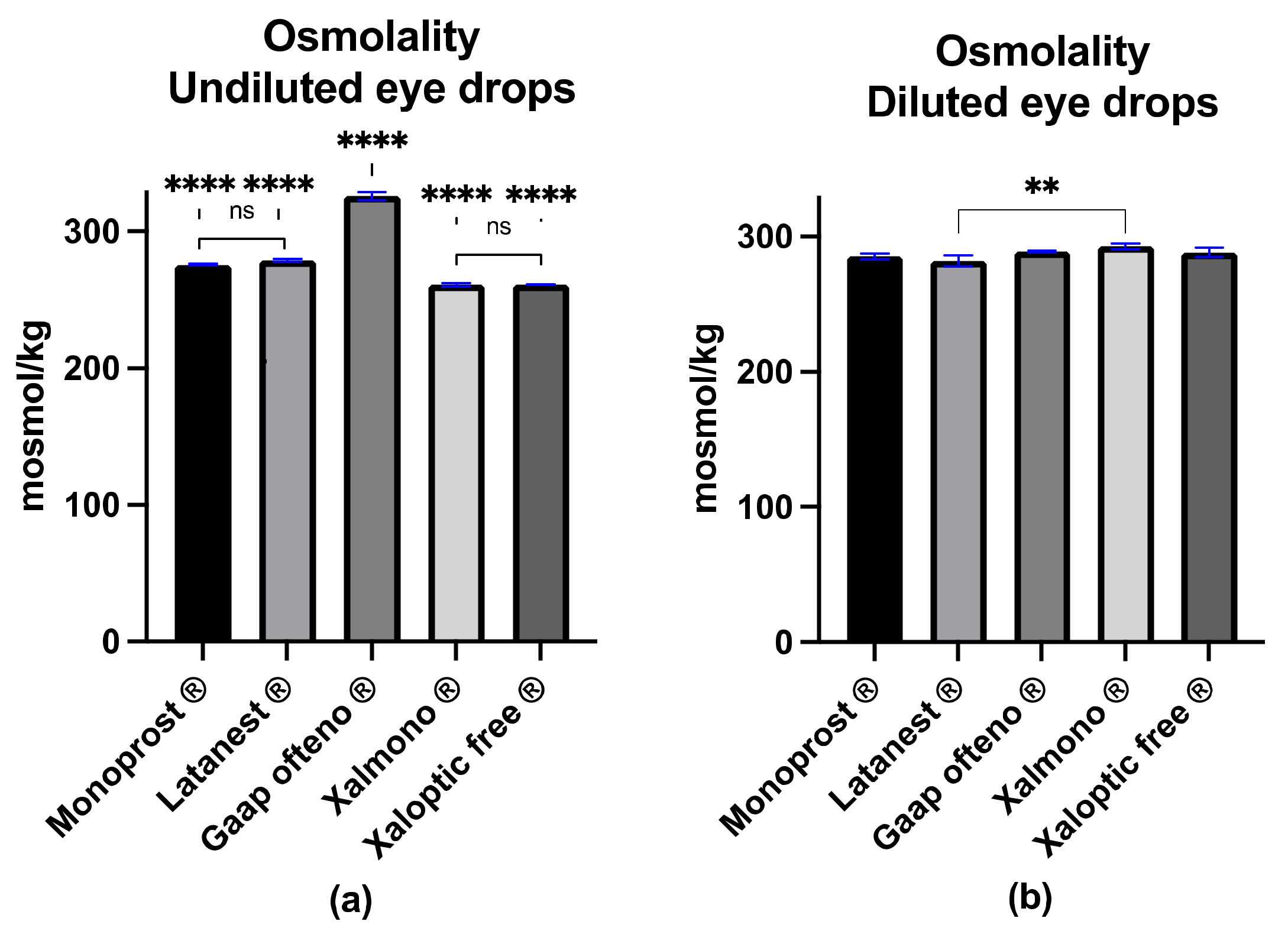
3.2. Osmolality
3.3. Surface Tension
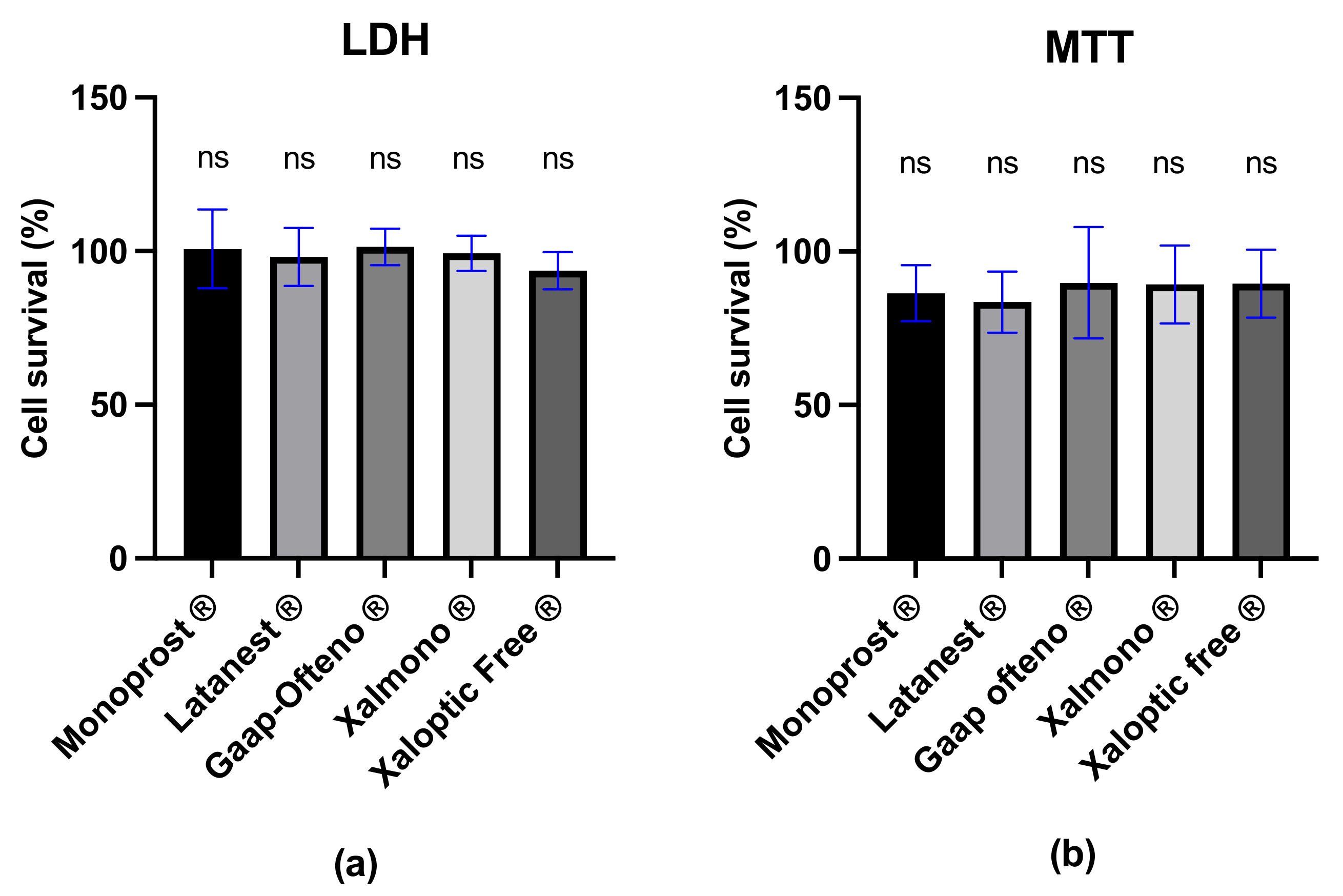
3.4. Cell Survival
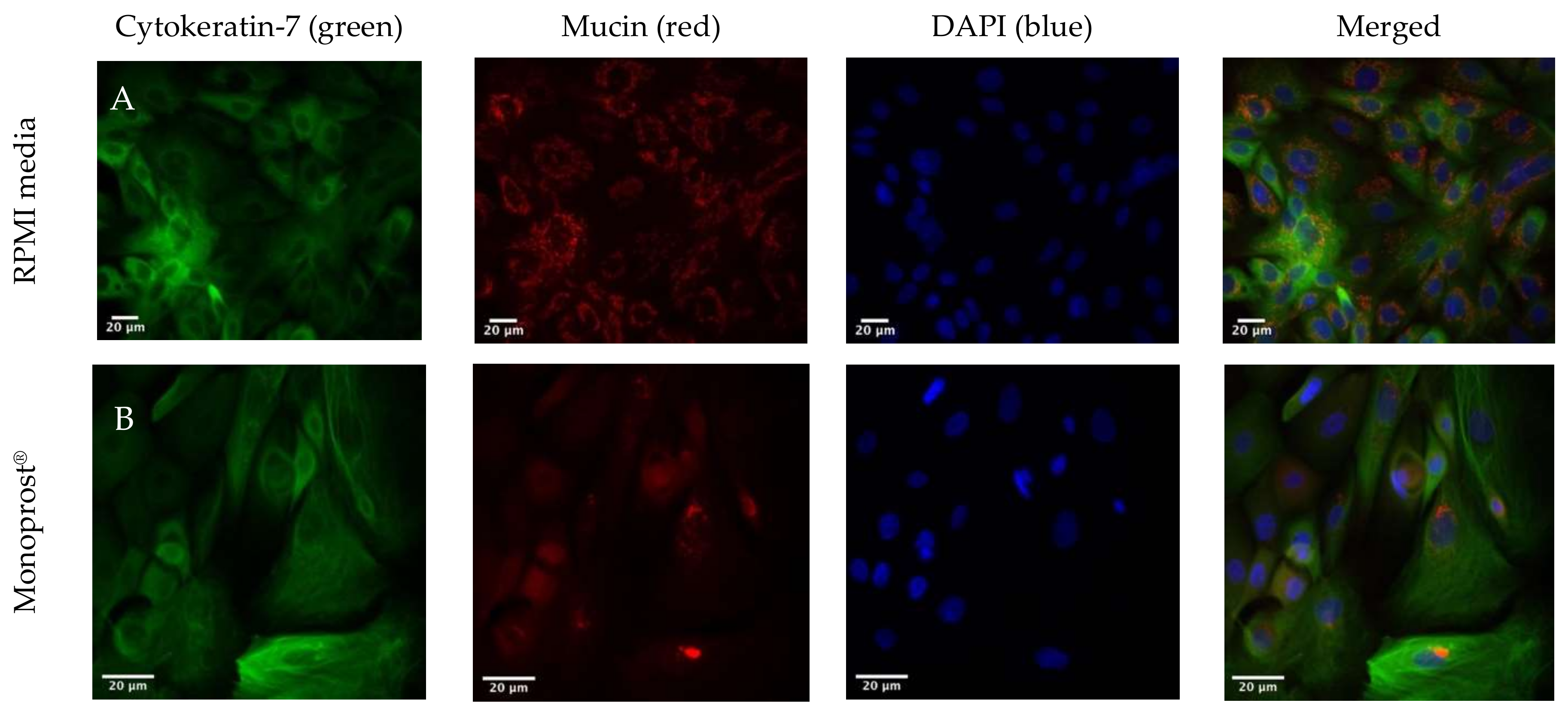
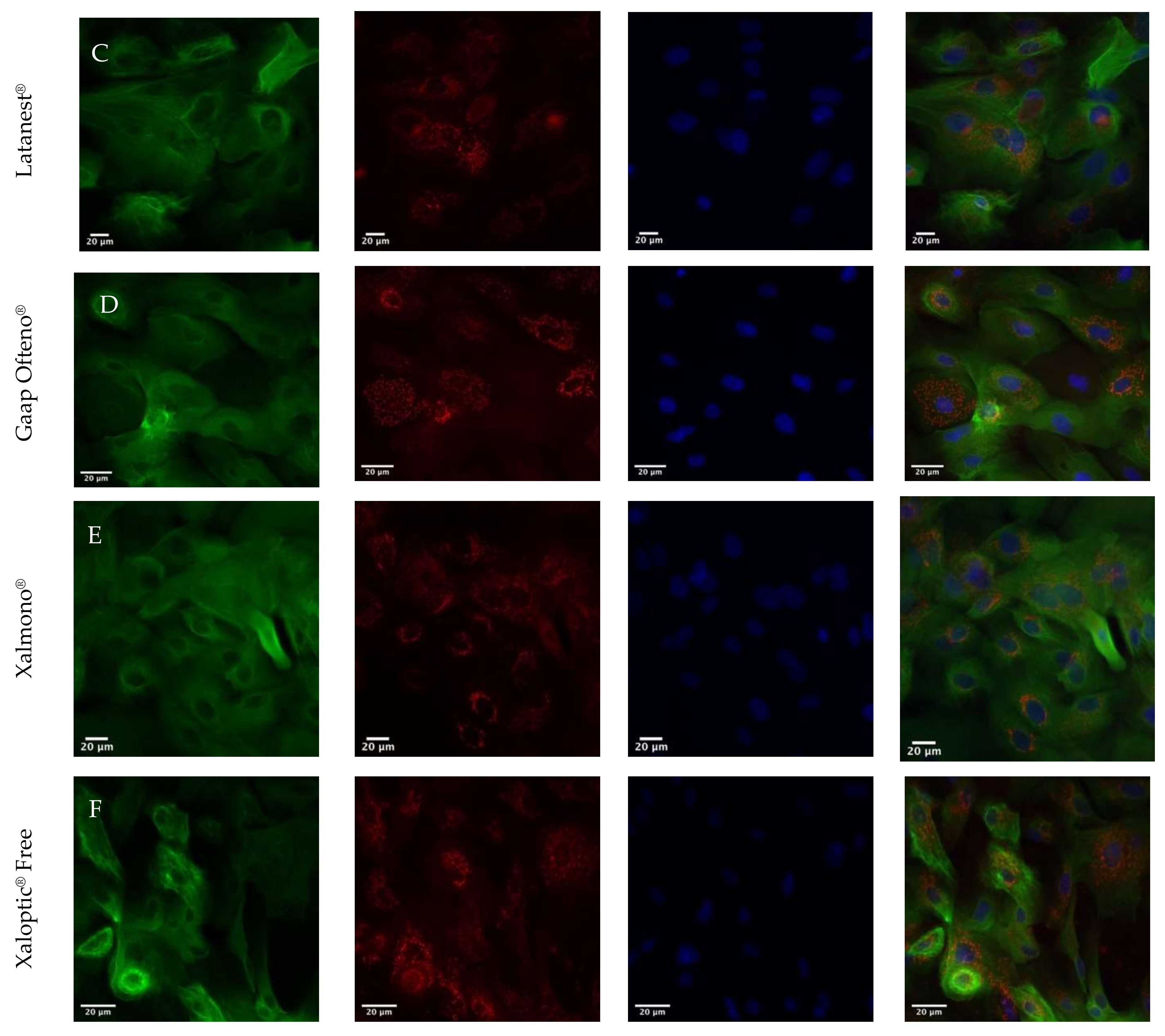
3.5. Immunohistochemical Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, J.; Varma, R. Intraocular Pressure: Modulation as Treatment for Glaucoma. Am. J. Ophthalmol. 2011, 152, 340–344.e2. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lindsley, K.; Rouse, B.; Hong, H.; Shi, Q.; Friedman, D.S.; Friedman, D.S.; Wormald, R.; Dickersin, K. Comparative Effectiveness of First-Line Medications for Primary Open-Angle Glaucoma: A Systematic Review and Network Meta-analysis. Ophthalmology 2016, 123, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudouin, C. Allergic reaction to topical eyedrops. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 459–463. [Google Scholar] [CrossRef]
- Manni, G.; Centofanti, M.; Oddone, F.; Parravano, M.; Bucci, M.G. Interleukin-1β tear concentration in glaucomatous and ocular hypertensive patients treated with preservative-free nonselective beta-blockers. Am. J. Ophthalmol. 2005, 139, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Baudouin, C.; Hamard, P.; Liang, H.; Creuzot-Garcher, C.; Bensoussan, L.; Brignole, F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology 2004, 111, 2186–2192. [Google Scholar] [CrossRef]
- Stahl, U.; Willcox, M.; Stapleton, F. Osmolality and tear film dynamics. Clin. Exp. Optom. 2012, 95, 3–11. [Google Scholar] [CrossRef]
- Portal, C.; Gouyer, V.; Gottrand, F.; Desseyn, J.L. Ocular mucins in dry eye disease. Exp. Eye Res. 2019, 186, 107724. [Google Scholar] [CrossRef]
- Gipson, I.K. Goblet cells of the conjunctiva: A review of recent findings. Prog. Retin. Eye Res. 2016, 54, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Jun, R.M.; Cho, M.S.; Choi, K.R. Comparison of the ocular surface changes following the use of two different prostaglandin F2? analogues containing benzalkonium chloride or polyquad in rabbit eyes. Cutan. Ocul. Toxicol. 2015, 34, 195–202. [Google Scholar] [CrossRef]
- Anwar, Z.; Wellik, S.R.; Galor, A. Glaucoma therapy and ocular surface disease: Current literature and recommendations. Curr. Opin. Ophthalmol. 2013, 24, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C. Detrimental effect of preservatives in eyedrops: Implications for the treatment of glaucoma. Acta Ophthalmol. 2008, 86, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Ayaki, M.; Iwasawa, A. Cytotoxicity of prostaglandin analog eye drops preserved with benzalkonium chloride in multiple corneoconjunctival cell lines. Clin. Ophthalmol. (Auckl. NZ) 2010, 4, 919–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Aller, M.; Guinchard, S.; Guillarme, D.; Pupier, M.; Jeannerat, D.; Rivara-Minten, E.; Veuthey, J.-L.; Gurny, R. New prostaglandin analog formulation for glaucoma treatment containing cyclodextrins for improved stability, solubility and ocular tolerance. Eur. J. Pharm. Biopharm. 2015, 95, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Fabrice Mercier, C.-F.F. inventor; Laboratoires Thea, Clermont Ferrand (FR), assignee. Polymeric Delivery System for A Nonviscous Prostaglandin-Based Solution without Preservatives. U.S. Patent 8637054-B2, 20 December 2012. [Google Scholar]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
- Hsu, K.H.; Gupta, K.; Nayaka, H.; Donthi, A.; Kaul, S.; Chauhan, A. Multidose Preservative Free Eyedrops by Selective Removal of Benzalkonium Chloride from Ocular Formulations. Pharm. Res. 2017, 34, 2862–2872. [Google Scholar] [CrossRef]
- SAIDANE, L.P.B. How to deliver preservative-free eye drops in a multidose system with a safer alternative to filters? Investig. Ophthalmol. Vis. Sci. 2017, 58, 4460. [Google Scholar]
- EMA. Questions and Answers on Generic Medicines. Available online: https://www.ema.europa.eu/en/documents/medicine-qa/questions-answers-generic-medicines_en.pdf (accessed on 3 August 2021).
- EMA. Generic and Hybrid Medicines. Available online: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/generic-hybrid-medicines (accessed on 3 August 2021).
- Kolko, M.; Koch Jensen, P. The physical properties of generic latanoprost ophthalmic solutions are not identical. Acta Ophthalmol. 2017, 95, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Angmo, D.; Wadhwani, M.; Velpandian, T.; Kotnala, A.; Sihota, R.; Dada, T. Evaluation of physical properties and dose equivalency of generic versus branded latanoprost formulations. Int. Ophthalmol. 2017, 37, 423–428. [Google Scholar] [CrossRef]
- Müllertz, O.; Hedengran, A.; Mouhammad, Z.A.; Freiberg, J.; Nagymihály, R.; Jacobsen, J.; Larsen, S.W.; Bair, J.; Utheim, T.P.; Dartt, D.A.; et al. Impact of benzalkonium chloride-preserved and preservative-free latanoprost eye drops on cultured human conjunctival goblet cells upon acute exposure and differences in physicochemical properties of the eye drops. BMJ Open Ophthalmol. 2021, 6, e000892. [Google Scholar] [CrossRef] [PubMed]
- Hedengran, A.; Begun, X.; Müllertz, O.; Mouhammad, Z.; Vohra, R.; Bair, J.; Dartt, D.A.; Cvenkel, B.; Heegaard, S.; Petrovski, G.; et al. Benzalkonium Chloride-Preserved Anti-Glaucomatous Eye Drops and Their Effect on Human Conjunctival Goblet Cells in vitro. Biomed. Hub 2021, 6, 69–75. [Google Scholar] [CrossRef]
- Skalicky, S.E.; Goldberg, I.; McCluskey, P. Ocular Surface Disease and Quality of Life in Patients With Glaucoma. Am. J. Ophthalmol. 2012, 153, 1–9.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Vadoothker, S.; Munir, W.M.; Saeedi, O. Ocular Surface Disease and Glaucoma Medications: A Clinical Approach. Eye Contact Lens 2019, 45, 11–18. [Google Scholar] [CrossRef]
- Evangelista, M.; Koverech, A.; Messano, M.; Pescosolido, N. Comparison of Three Lubricant Eye Drop Solutions in Dry Eye Patients. Optom. Vis. Sci. 2011, 88, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Foulks, G.N. The Correlation Between the Tear Film Lipid Layer and Dry Eye Disease. Surv. Ophthalmol. 2007, 52, 369–374. [Google Scholar] [CrossRef]
- Fischer, F.H.; Wiederholt, M. Human precorneal tear film pH measured by microelectrodes. Graefes Arch. Clin. Exp. Ophthalmol. 1982, 218, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Jitendra, S.P.; Banik, A.; Dixit, S. A new trend: Ocular drug delivery system. Pharm. Sci. 2011, 2, 720–744. [Google Scholar]
- Garcia-Valldecabres, M.; López-Alemany, A.; Refojo, M.F. pH Stability of ophthalmic solutions. Optom. J. Am. Optom. Assoc. 2004, 75, 161–168. [Google Scholar] [CrossRef]
- Nagyová, B.; Tiffany, J.M. Components responsible for the surface tension of human tears. Curr. Eye Res. 1999, 19, 4–11. [Google Scholar] [CrossRef]
- Hotujac Grgurević, M.; Juretić, M.; Hafner, A.; Lovrić, J.; Pepić, I. Tear fluid-eye drops compatibility assessment using surface tension. Drug Dev. Ind. Pharm. 2017, 43, 275–282. [Google Scholar] [CrossRef]
- Van Santvliet, L.; Ludwig, A. Determinants of eye drop size. Surv. Ophthalmol. 2004, 49, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Brasnu, E.; Brignole-Baudouin, F.; Riancho, L.; Guenoun, J.-M.; Warnet, J.-M.; Baudouin, C. In Vitro Effects of Preservative-Free Tafluprost and Preserved Latanoprost, Travoprost, and Bimatoprost in a Conjunctival Epithelial Cell Line. Curr. Eye Res. 2008, 33, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Agnifili, L.; Fasanella, V.; Curcio, C.; Ciabattoni, C.; Mastropasqua, R.; Toto, L.; Ciancaglini, M. Conjunctival goblet cells density and preservative-free tafluprost therapy for glaucoma: An in vivo confocal microscopy and impression cytology study. Acta Ophthalmol. 2013, 91, e397–e405. [Google Scholar] [CrossRef] [PubMed]
- Susanna, R., Jr.; Medeiros, F.A. The pros and cons of different prostanoids in the medical management of glaucoma. Curr. Opin. Ophthalmol. 2001, 12, 149–156. [Google Scholar] [CrossRef]
- Honrubia, F.; Garcia-Sanchez, J.; Polo, V.; De La Casa, J.M.M.; Soto, J. Conjunctival hyperaemia with the use of latanoprost versus other prostaglandin analogues in patients with ocular hypertension or glaucoma: A meta-analysis of randomised clinical trials. Br. J. Ophthalmol. 2009, 93, 316–321. [Google Scholar] [CrossRef]
- Cucherat, M.; Stalmans, I.; Rouland, J.F. Relative efficacy and safety of preservative-free latanoprost (T2345) for the treatment of open-angle glaucoma and ocular hypertension: An adjusted Indirect comparison meta-analysis of randomized clinical trials. J. Glaucoma 2014, 23, e69–e75. [Google Scholar] [CrossRef]
- Rouland, J.-F.; Traverso, C.E.; Stalmans, I.; El Fekih, L.; Delval, L.; Renault, D.; Baudouin, C. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br. J. Ophthalmol. 2013, 97, 196–200. [Google Scholar] [CrossRef]
- Gonzalez, J.R.; Baiza-Duran, L.; Quintana-Hau, J.; Tornero-Montano, R.; Castaneda-Hernandez, G.; Ortiz, M.; Oceguera, F.A.; Loustaunau, M.B.; Gastelum, M.C.; Mejia, J.G.; et al. Comparison of the stability, efficacy, and adverse effect profile of the innovator 0.005% latanoprost ophthalmic solution and a novel cyclodextrin-containing formulation. (Clinical report). J. Clin. Pharmacol. 2007, 47, 121–126. [Google Scholar] [CrossRef]
- Ghate, D.; Edelhauser, H.F. Barriers to Glaucoma Drug Delivery. J. Glaucoma 2008, 17, 147–156. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freiberg, J.C.; Hedengran, A.; Heegaard, S.; Petrovski, G.; Jacobsen, J.; Cvenkel, B.; Kolko, M. An Evaluation of the Physicochemical Properties of Preservative-Free 0.005% (w/v) Latanoprost Ophthalmic Solutions, and the Impact on In Vitro Human Conjunctival Goblet Cell Survival. J. Clin. Med. 2022, 11, 3137. https://doi.org/10.3390/jcm11113137
Freiberg JC, Hedengran A, Heegaard S, Petrovski G, Jacobsen J, Cvenkel B, Kolko M. An Evaluation of the Physicochemical Properties of Preservative-Free 0.005% (w/v) Latanoprost Ophthalmic Solutions, and the Impact on In Vitro Human Conjunctival Goblet Cell Survival. Journal of Clinical Medicine. 2022; 11(11):3137. https://doi.org/10.3390/jcm11113137
Chicago/Turabian StyleFreiberg, Josefine C., Anne Hedengran, Steffen Heegaard, Goran Petrovski, Jette Jacobsen, Barbara Cvenkel, and Miriam Kolko. 2022. "An Evaluation of the Physicochemical Properties of Preservative-Free 0.005% (w/v) Latanoprost Ophthalmic Solutions, and the Impact on In Vitro Human Conjunctival Goblet Cell Survival" Journal of Clinical Medicine 11, no. 11: 3137. https://doi.org/10.3390/jcm11113137
APA StyleFreiberg, J. C., Hedengran, A., Heegaard, S., Petrovski, G., Jacobsen, J., Cvenkel, B., & Kolko, M. (2022). An Evaluation of the Physicochemical Properties of Preservative-Free 0.005% (w/v) Latanoprost Ophthalmic Solutions, and the Impact on In Vitro Human Conjunctival Goblet Cell Survival. Journal of Clinical Medicine, 11(11), 3137. https://doi.org/10.3390/jcm11113137





