Rapid Campimetry—A Novel Screening Method for Glaucoma Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
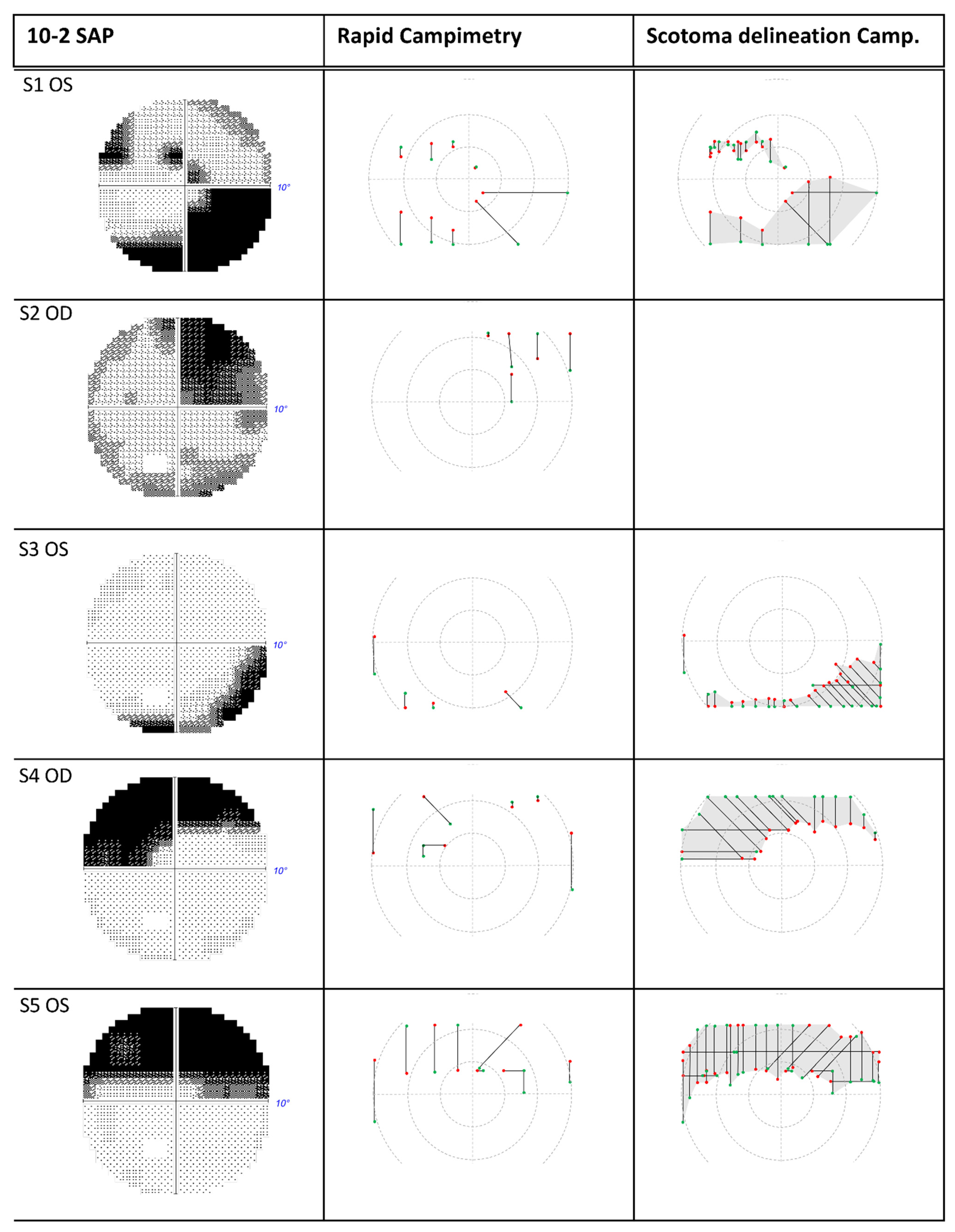
2.2. Standard Automated Perimetry Check (SAP)
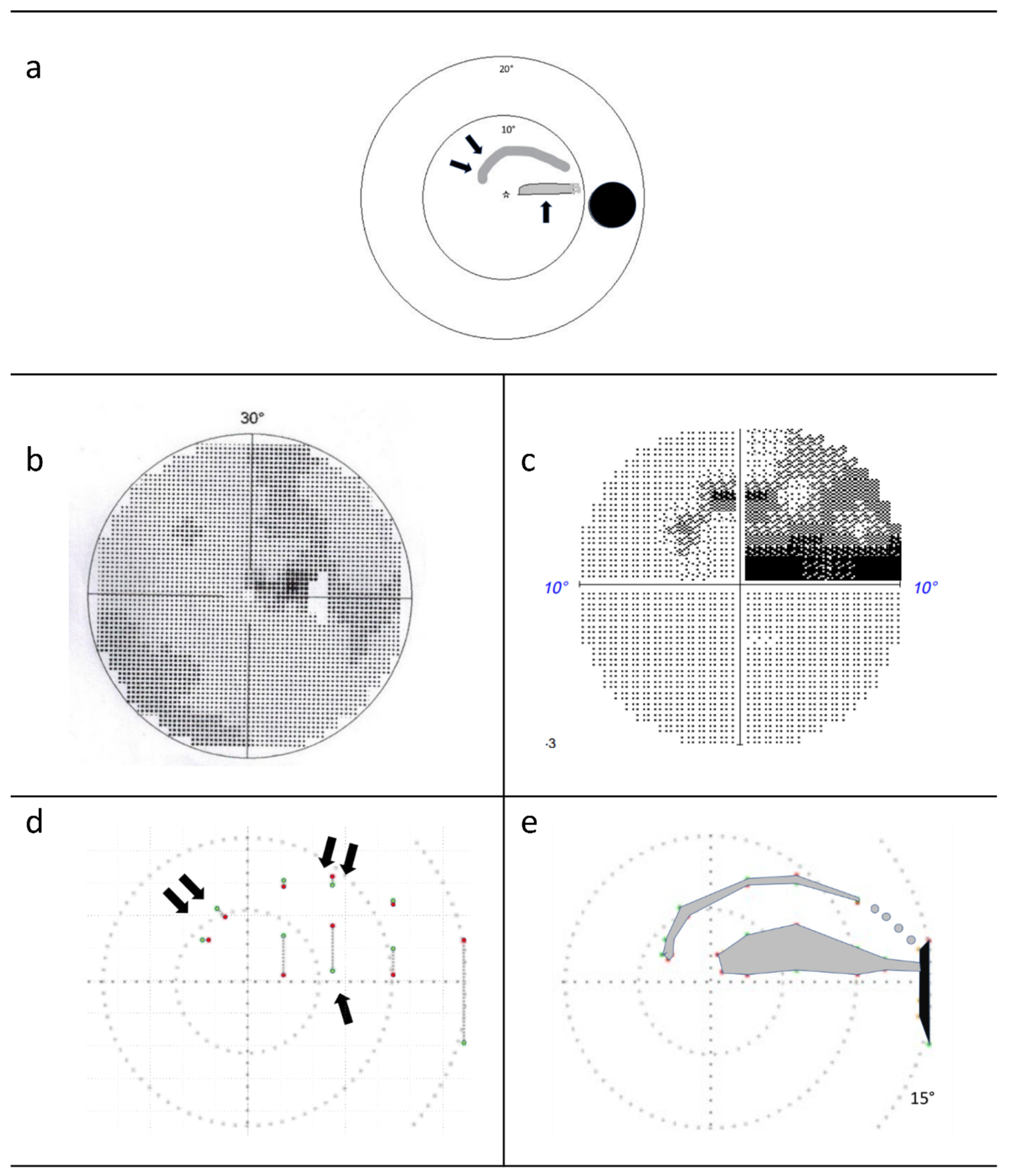
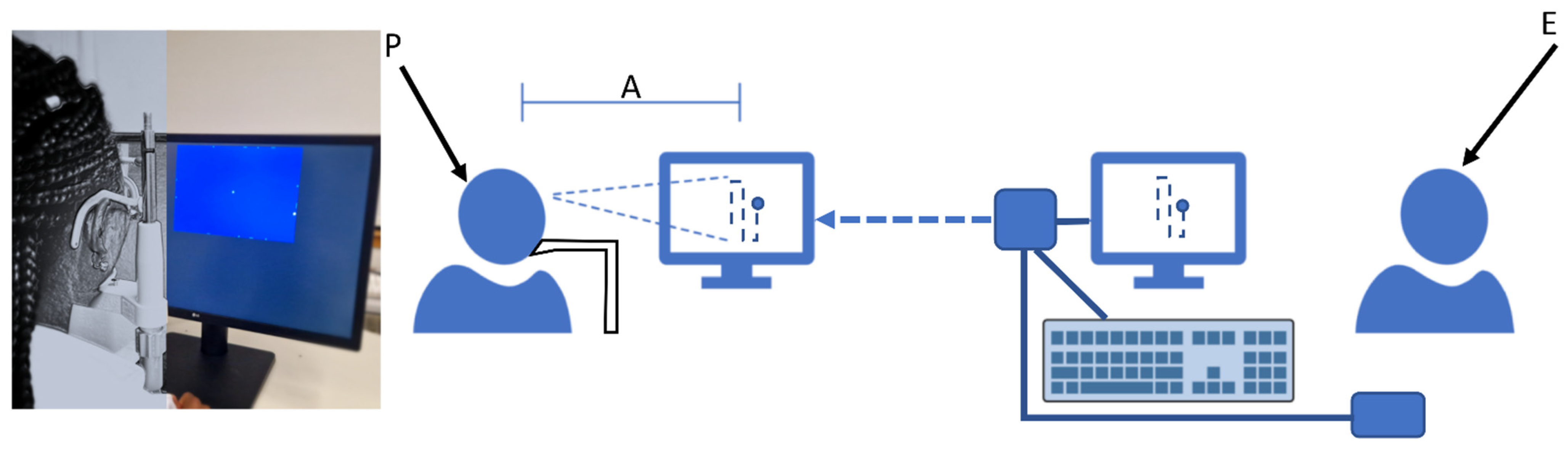
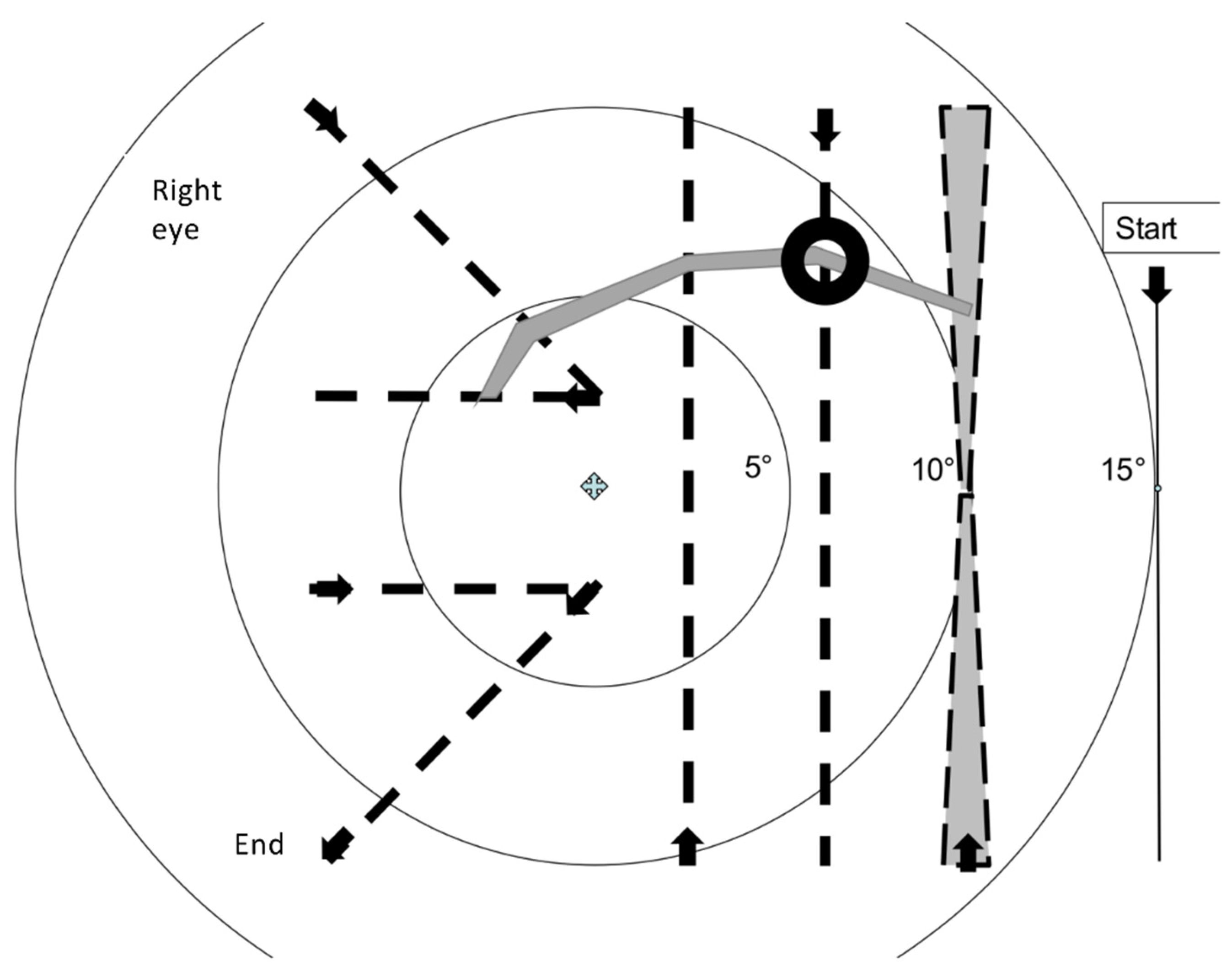
2.3. Rapid Campimetry
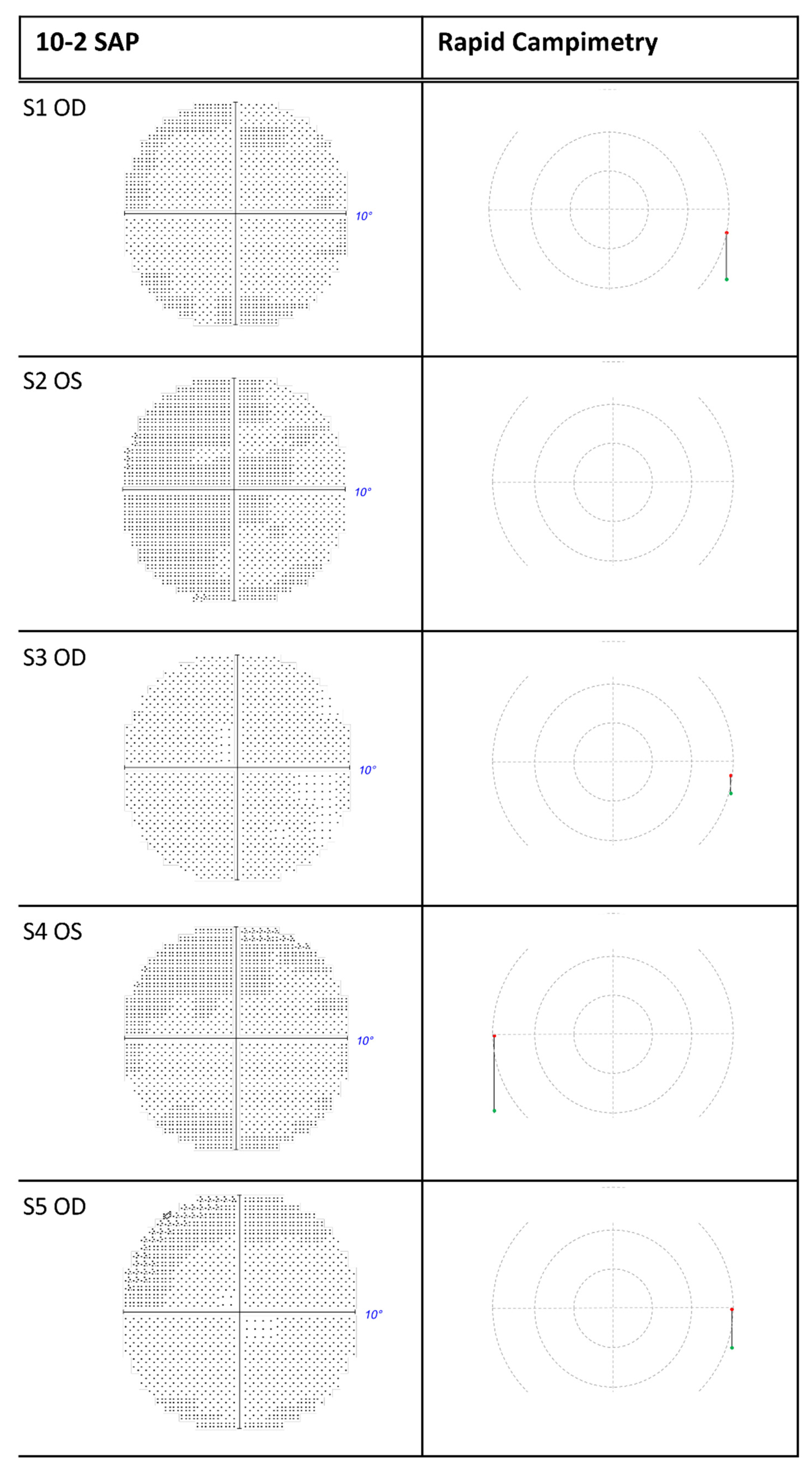
3. Results
4. Discussion
4.1. Increasing Attention via Fast Stimulus Movement
4.2. Proportion of the Examined Visual Field Area in the Paracentral Visual Field
4.3. Accuracy of Rapid Campimetry
4.4. Detection of Arc Scotomas
4.5. Automation of Test Point Movement in the Paramacular Visual Field
4.6. Limitations of the Study
4.7. Outlook
4.8. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Al-Nosairy, K.O.; Hoffmann, M.B.; Bach, M. Non-invasive Electrophysiology in Glaucoma, Structure and Function—A Review. Eye 2021, 35, 2374–2385. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.C.; Garway-Heath, D.F.; Goñi, F.J.; Rossetti, L.; Bengtsson, B.; Viswanathan, A.C.; Heijl, A. Practical Recommendations for Measuring Rates of Visual Field Change in Glaucoma. Br. J. Ophthalmol. 2008, 92, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Wang, Y.-T.; Jung, T.-P.; Zao, J.K.; Chien, Y.-Y.; Diniz-Filho, A.; Daga, F.B.; Lin, Y.-P.; Wang, Y.; Medeiros, F.A. Detecting Glaucoma with a Portable Brain-Computer Interface for Objective Assessment of Visual Function Loss. JAMA Ophthalmol. 2017, 135, 550–557. [Google Scholar] [CrossRef]
- Wu, Z.; Medeiros, F.A. Recent Developments in Visual Field Testing for Glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 141–146. [Google Scholar] [CrossRef]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.V.; Liebmann, J.M.; Ritch, R. Glaucomatous Damage of the Macula. Prog. Retin Eye Res. 2013, 32, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Bach, M.; Sulimma, F.; Gerling, J. Little Correlation of the Pattern Electroretinogram (PERG) and Visual Field Measures in Early Glaucoma. Doc. Ophthalmol. 1997, 94, 253–263. [Google Scholar] [CrossRef]
- Blumberg, D.M.; De Moraes, C.G.; Prager, A.J.; Yu, Q.; Al-Aswad, L.; Cioffi, G.A.; Liebmann, J.M.; Hood, D.C. Association between Undetected 10-2 Visual Field Damage and Vision-Related Quality of Life in Patients with Glaucoma. JAMA Ophthalmol. 2017, 135, 742–747. [Google Scholar] [CrossRef]
- De Moraes, C.G.; Hood, D.C.; Thenappan, A.; Girkin, C.A.; Medeiros, F.A.; Weinreb, R.N.; Zangwill, L.M.; Liebmann, J.M. 24-2 Visual Fields Miss Central Defects Shown on 10-2 Tests in Glaucoma Suspects, Ocular Hypertensives, and Early Glaucoma. Ophthalmology 2017, 124, 1449–1456. [Google Scholar] [CrossRef]
- Park, H.-Y.L.; Hwang, B.-E.; Shin, H.-Y.; Park, C.K. Clinical Clues to Predict the Presence of Parafoveal Scotoma on Humphrey 10-2 Visual Field Using a Humphrey 24-2 Visual Field. Am. J. Ophthalmol. 2016, 161, 150–159. [Google Scholar] [CrossRef]
- Quigley, H.A.; Dunkelberger, G.R.; Green, W.R. Retinal Ganglion Cell Atrophy Correlated with Automated Perimetry in Human Eyes with Glaucoma. Am. J. Ophthalmol. 1989, 107, 453–464. [Google Scholar] [CrossRef]
- Bullimore, M.A.; Wood, J.M.; Swenson, K. Motion Perception in Glaucoma. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3526–3533. [Google Scholar]
- Wall, M.; Ketoff, K.M. Random Dot Motion Perimetry in Patients with Glaucoma and in Normal Subjects. Am. J. Ophthalmol. 1995, 120, 587–596. [Google Scholar] [CrossRef]
- Silverman, S.E.; Trick, G.L.; Hart, W.M., Jr. Motion Perception Is Abnormal in Primary Open-Angle Glaucoma and Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 1990, 31, 722–729. [Google Scholar]
- Wall, M.; Jennisch, C.S.; Munden, P.M. Motion Perimetry Identifies Nerve Fiber Bundlelike Defects in Ocular Hypertension. Arch. Ophthalmol. 1997, 115, 26–33. [Google Scholar] [CrossRef]
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 2021, 105, 1–169. [CrossRef]
- Aulhorn, E. Subjective Examination Methods in Glaucoma Diagnosis. Buch Augenarzt 1976, 69, 128–139. [Google Scholar]
- Lachenmayr, B.; Vivell, P.M.O. Perimetrie; Thieme: Stuttgart, Germany, 1992. [Google Scholar]
- Pembury Smith, M.Q.R.; Ruxton, G.D. Camouflage in Predators. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1325–1340. [Google Scholar] [CrossRef]
- Rönne, H. Über das Gesichtsfeld beim Glaukom. Klin Mbl Augenheilkunde 1909, 47, 12–33. [Google Scholar]
- Goldmann, H. Demonstration unseres neuen Projektionskugelperimeters samt theoretischen und klinischen Bemerkungen über Perimetrie. OPH 1946, 111, 187–192. [Google Scholar] [CrossRef]
- Schiefer, U.; Papageorgiou, E.; Sample, P.A.; Pascual, J.P.; Selig, B.; Krapp, E.; Paetzold, J. Spatial Pattern of Glaucomatous Visual Field Loss Obtained with Regionally Condensed Stimulus Arrangements. Invest. Ophthalmol. Vis. Sci. 2010, 51, 5685–5689. [Google Scholar] [CrossRef]
- Traynis, I.; De Moraes, C.G.; Raza, A.S.; Liebmann, J.M.; Ritch, R.; Hood, D.C. Prevalence and Nature of Early Glaucomatous Defects in the Central 10° of the Visual Field. JAMA Ophthalmol. 2014, 132, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.V.; Odel, J.G.; Greenstein, V.C.; Liebmann, J.M.; Ritch, R. Initial Arcuate Defects within the Central 10 Degrees in Glaucoma. Invest. Ophthalmol. Vis. Sci. 2011, 52, 940–946. [Google Scholar] [CrossRef]
- Phu, J.; Kalloniatis, M. Comparison of 10-2 and 24-2C Test Grids for Identifying Central Visual Field Defects in Glaucoma and Suspect Patients. Ophthalmology 2021, 128, 1405–1416. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cursiefen, C.; Wisse, M.; Cursiefen, S.; Jünemann, A.; Martus, P.; Korth, M. Migraine and Tension Headache in High-Pressure and Normal-Pressure Glaucoma. Am. J. Ophthalmol. 2000, 129, 102–104. [Google Scholar] [CrossRef]
| Gender | Age [Years] | BCVA [logMAR] OD | BCVA [logMAR] OS | MD 10-2 OD [dB] | MD 10-2 OS [dB] | |
|---|---|---|---|---|---|---|
| S1 | m | 81 | 0.0 | 0.2 | 0.29 | −15.48 |
| S2 | f | 80 | 0.80 | 0.4 | −14.52 * | −1.73 * |
| S3 | m | 55 | −0.1 | −0.1 | 1.20 | −3.64 |
| S4 | m | 70 | 0.0 | 0.1 | −10.77 | −1.03 |
| S5 | f | 62 | 0.0 | 0.0 | −0.40 | −13.30 |
| Distance [°] From Fixation Point | Diameter [mm] of the Test Point | Angle Diameter [°] of the Test Point |
|---|---|---|
| 0.0° | 1.05 | 0.16° |
| 1.0° | 1.16 | 0.17° |
| 2.5° | 1.33 | 0.19° |
| 5.0° | 1.61 | 0.23° |
| 7.5° | 1.88 | 0.27° |
| 10.0° | 2.17 | 0.31° |
| 12.5° | 2.45 | 0.35° |
| 15.0° | 2.72 | 0.39° |
| Greatest Test Point Thickness [cm] | Smallest Test Point Thickness [cm] | Sum of Both Test Point Thicknesses [cm] | Half the Running Distance of the Test Point [cm] | Area [cm2] | |
|---|---|---|---|---|---|
| Vertical 1 | 0.26 | 0.22 | 0.48 | 7 | 3.35 |
| Vertical 2 | 0.24 | 0.18 | 0.42 | 7 | 2.93 |
| Vertical 3 | 0.22 | 0.13 | 0.35 | 7 | 2.47 |
| Diagonal 1 + 2 | 0.24 | 0.14 | 0.38 | 7.44 | 2.81 |
| Horizontal 1 + 2 | 0.19 | 0.14 | 0.33 | 5.6 | 1.85 |
| Total | 13.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, F.; Al-Nosairy, K.O.; Kramer, F.H.; Meltendorf, C.; Djouoma, N.; Thieme, H.; Hoffmann, M.B.; Hoffmann, F. Rapid Campimetry—A Novel Screening Method for Glaucoma Diagnosis. J. Clin. Med. 2022, 11, 2156. https://doi.org/10.3390/jcm11082156
Müller F, Al-Nosairy KO, Kramer FH, Meltendorf C, Djouoma N, Thieme H, Hoffmann MB, Hoffmann F. Rapid Campimetry—A Novel Screening Method for Glaucoma Diagnosis. Journal of Clinical Medicine. 2022; 11(8):2156. https://doi.org/10.3390/jcm11082156
Chicago/Turabian StyleMüller, Fabian, Khaldoon O. Al-Nosairy, Francie H. Kramer, Christian Meltendorf, Nidele Djouoma, Hagen Thieme, Michael B. Hoffmann, and Friedrich Hoffmann. 2022. "Rapid Campimetry—A Novel Screening Method for Glaucoma Diagnosis" Journal of Clinical Medicine 11, no. 8: 2156. https://doi.org/10.3390/jcm11082156
APA StyleMüller, F., Al-Nosairy, K. O., Kramer, F. H., Meltendorf, C., Djouoma, N., Thieme, H., Hoffmann, M. B., & Hoffmann, F. (2022). Rapid Campimetry—A Novel Screening Method for Glaucoma Diagnosis. Journal of Clinical Medicine, 11(8), 2156. https://doi.org/10.3390/jcm11082156






