Basal Septal Hypertrophy as the Early Imaging Biomarker for Adaptive Phase of Remodeling Prior to Heart Failure
Abstract
1. Background
2. Basal Septal Hypertrophy and Clinic Observations
3. BSH and Animal Validation Studies
Striking Difference between Animal and Human BSH Data
4. Heart Failure with Preserved Ejection Fraction
5. Effective Medical Approach in Hypertensive Disease
6. Cardiovascular Outcomes of Patients with HFPEP Who Are on Effective Antihypertensive Treatment
Author Contributions
Funding
Conflicts of Interest
References
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Berenji, K.; Drazner, M.H.; Rothermel, B.A.; Hill, J.A. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H8–H16. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The transition from hypertension to heart failure: Contemporary update. JACC Heart Fail. 2017, 5, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Klingbeil, A.U.; Schneider, M.; Martus, P.; Messerli, F.H.; Schmieder, R.E. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am. J. Med. 2003, 115, 41–46. [Google Scholar] [CrossRef]
- Wachtell, K.; Gerdts, E.; Palmieri, V.; Olsen, M.H.; Nieminen, M.S.; Papademetriou, V.; Boman, K.; Dahlöf, B.; Aurigemma, G.P.; Rokkedal, J.E.; et al. In-treatment midwall and endocardial fractional shortening predict cardiovascular outcome in hypertensive patients with preserved baseline systolic ventricular function: The Losartan Intervention for Endpoint reduction study. J. Hypertens. 2010, 28, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Shiota, T.; Odabashian, J. Comparison by real-time three-dimensional echocardiography of left ventricular geometry in hypertrophic cardiomyopathy versus secondary left ventricular hypertrophy. Am. J. Cardiol. 2000, 85, 1035–1038. [Google Scholar] [CrossRef]
- Caselli, S.; Pelliccia, A.; Maron, M.; Santini, D.; Puccio, D.; Marcantonio, A.; Pandian, N.G.; De Castro, S. Differentiation of hypertrophic cardiomyopathy from other forms of left ventricular hypertrophy by means of three-dimensional echocardiography. Am. J. Cardiol. 2008, 102, 616–620. [Google Scholar] [CrossRef]
- Plante, G.E. Predisease biological markers: Early diagnosis and prevention of arterial hypertension. Metabolism 2008, 57, S36–S39. [Google Scholar] [CrossRef] [PubMed]
- Julius, S.; Nesbitt, S.D.; Egan, B.M.; Weber, M.A.; Michelson, E.L.; Kaciroti, N.; Black, H.R.; Grimm, R.H.; Messerli, F.H.; Oparil, S.; et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N. Engl. J. Med. 2006, 354, 1685–1697. [Google Scholar] [CrossRef]
- Maron, B.J.; Edwards, J.E.; Epstein, S.E. Disproportionate ventricular septal thickening in patients with systemic hypertension. Chest 1978, 73, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Benessiano, J.R.; Hornych, A.L. Asymmetrical septal hypertrophy and borderline hypertension. Int. J. Cardiol. 1982, 2, 103–108. [Google Scholar] [CrossRef]
- Verdecchia, P.; Porcellati, C.; Zampi, I.; Schillaci, G.; Gatteschi, C.; Battistelli, M.; Bartoccini, C.; Borgioni, C.; Ciucci, A. Asymmetric left ventricular remodelling due to isolated septal thickening in patients with systemic hypertension and normal left ventricular masses. Am. J. Cardiol. 1994, 73, 247–252. [Google Scholar] [CrossRef]
- Lutas, E.M.; Devereux, R.B.; Reis, G.; Alderman, M.H.; Pickering, T.G.; Borer, J.S.; Laragh, J.H. Increased cardiac performance in mild essential hypertension. Left ventricular mechanics. Hypertension 1985, 7, 979–988. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Di Lorenzo, L.; Costantino, G.; Moccia, D.; Buonissimo, S.; de Divitiis, O. Supernormal contractility in primary hypertension without left ventricular hypertrophy. Hypertension 1988, 11, 457–463. [Google Scholar] [CrossRef]
- Yalçin, F.; Yigit, F.; Erol, T.; Baltali, M.; Korkmaz, M.E.; Müderrisoglu, H. Effect of dobutamine stress on basal septal tissue dynamics in hypertensive patients with basal septal hypertrophy. J. Hum. Hypertens. 2006, 20, 628–630. [Google Scholar] [CrossRef]
- Gorantla, R.S.; Ahmed, S.; Voruganti, D.; Menzies, D.J. Hyperdynamic left ventricle on radionuclide myocardial perfusion imaging (RNMPI): A marker of diastolic dysfunction in patients presenting with dyspnea on exertion. Int. J. Cardiol. Heart Vasc. 2015, 9, 43–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yalçin, F.; Schindler, T.; Abraham, T.P. Hypertension should be ruled out in hyperdynamic left ventricle in radionuclide myocardial perfusion imaging, diastolic dysfunction, dyspnea on exertion. Int. J. Cardiol. Heart Vasc. 2015, 7, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Muderrisoglu, H.; Korkmaz, M.E.; Ozin, B.; Baltali, M.; Yigit, F. The effect of dobutamine stress on left ventricular outflow tract gradients in hypertensive patients with basal septal hypertrophy. Angiology 2004, 55, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Yalçin, H.; SeyfeliEAkgul, F. Stress-induced hypercontractility in patients with hypertension: An interesting imaging finding. Int. J. Cardiol. 2010, 143, E1–E3. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Abraham, T.P. Stress-induced regional features of left ventricle is related to pathogenesis of clinical conditions with both acute and chronic stress. Int. J. Cardiol. 2010, 145, 367–368. [Google Scholar] [CrossRef]
- Yalçin, F.; Kucukler, N.; Cingolani, O.; Mbiyangandu, B.; Sorensen, L.; Pinherio, A.; Abraham, M.R.; Abraham, T.P. Evolution of ventricular hypertrophy and myocardial mechanics in physiologic and pathologic hypertrophy. J. Appl. Physiol. 2019, 126, 354–362. [Google Scholar] [CrossRef]
- Yalçin, F.; Kucukler, N.; Cingolani, O.; Mbiyangandu, B.; Sorensen, L.; Pinherio, A.; Abraham, M.R.; Abraham, T.P. Intracavitary gradient in mice with early regional remodeling at the compensatory hyperactive stage prior to left ventricular tissue dysfunction. J. Am. Coll. Cardiol. 2020, 75, 1585. [Google Scholar] [CrossRef]
- Lee, P.T.; Dweck, M.R.; Prasher, S.; Shah, A.; Humphries, S.E.; Pennell, D.J.; Montgomery, H.E.; Payne, J.R. Left ventricular wall thickness and the presence of asymmetric hypertrophy in healthy young army recruits: Data from the LARGE heart study. Circ. Cardiovasc. Imaging 2013, 6, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Abraham, T.P.; Gottdiener, J.S. Letter by Yalcin et al. regarding article “Left ventricular wall thickness and the presence of asymmetric hypertrophy in healthy young army recruits: Data from the LARGE heart study”. Circ. Cardiovasc. Imaging 2013, 6, e28. [Google Scholar] [CrossRef] [PubMed]
- Małek, Ł.A.; Czajkowska, A.; Mróz, A.; Witek, K.; Barczuk-Falecka, M.; Nowicki, D.; Postuła, M.; Werys, K. Left ventricular hypertrophy in middle-aged endurance athletes: Is it blood pressure related? Blood Press. Monit. 2019, 24, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Yalçin, H.; Abraham, T.P. Exercise hypertension should be recalled in basal septal hypertrophy as the early imaging biomarker in patients with stressed heart morphology. Blood Press. Monit. 2020, 25, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Muderrisoglu, H. Takotsubo cardiomyopathy may be associated with cardiac geometric features as observed in hypertensive heart disease. Int. J. Cardiol. 2009, 135, 251–252. [Google Scholar] [CrossRef]
- Yalçin, F.; Abraham, M.R.; Abraham, T.P. Myocardial aspects in aortic stenosis and functional increased afterload conditions in patients with stressed heart morphology. Ann. Thorac. Cardiovasc. Surg. 2021, 27, 332–334. [Google Scholar] [CrossRef]
- Marciniak, M.; Gilbert, A.; Loncaric, F.; Fernandes, J.F.; Bijnens, B.; Sitges, M.; King, A.; Crispi, F.; Lamata, P. Septal curvature as a robust and reproducible marker for basal septal hypertrophy. J. Hypertens. 2021, 39, 1421–1428. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Abraham, M.R.; Abraham, T.P. Basal septal hypertrophy: Extremely sensitive region to variety of stress stimuli and stressed heart morphology. J. Hypertens. 2021, in press. [Google Scholar]
- Maurer, M.S.; King, D.L.; El-Khoury Rumbarger, L.; Packer, M.; Burkhoff, D. Left heart failure with a normal ejection fraction: Identification of different pathophysiologic mechanisms. J. Card. Fail. 2005, 11, 177–187. [Google Scholar] [CrossRef]
- Tan, Y.T.; Wenzelburger, F.; Lee, E.; Heatlie, G.; Leyva, F.; Patel, K.; Frenneaux, M.; Sanderson, J. The pathophysiology of heart failure with normal ejection fraction: Exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J. Am. Coll. Cardiol. 2009, 54, 36–46. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Küçükler, N.; Abraham, T.P. Quantitative left ventricular contractility analysis under stress: A new practical approach in follow-up of hypertensive patients. J. Hum. Hypertens. 2011, 25, 578–584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diez, J.; Frohlich, E.D. A translational approach to hypertensive heart disease. Hypertension 2010, 55, 1–8. [Google Scholar] [CrossRef]
- Rudolph, A.; Abdel-Aty, H.; Bohl, S.; Boyé, P.; Zagrosek, A.; Dietz, R.; Schulz-Menger, J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J. Am. Coll. Cardiol. 2009, 53, 284–291. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Devereux, R.B.; Koren, M.J.; Mensah, G.A.; Casale, P.N.; Laragh, J.H. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation 1996, 93, 259–265. [Google Scholar] [CrossRef]
- Bigi, R.; Bestetti, A.; Strinchini, A.; Conte, A.; Gregori, D.; Brusoni, B.; Fiorentini, C. Combined assessment of left ventricular perfusion and function by gated single-photon emission computed tomography for the risk stratification of high-risk hypertensive patients. J. Hypertens. 2006, 24, 767. [Google Scholar] [CrossRef] [PubMed]
- González, A.; López, B.; Ravassa, S.; Querejeta, R.; Larman, M.; Díez, J.; Fortuño, M.A. Stimulation of cardiac apoptosis in essential hypertension: Potential role of angiotensin II. Hypertension 2002, 39, 75–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- López, B.; Querejeta, R.; Varo, N.; González, A.; Larman, M.; Martínez Ubago, J.L.; Díez, J. Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation 2001, 104, 286–291. [Google Scholar] [CrossRef]
- Núñez, J.; Zamorano, J.L.; Pérez De Isla, L.; Palomeque, C.; Almería, C.; Rodrigo, J.L.; Corteza, J.; Banchs, J.; Macaya, C. Differences in regional systolic and diastolic function by Doppler tissue imaging in patients with hypertrophic cardiomyopathy and hypertrophy caused by hypertension. J. Am. Soc. Echocardiogr. 2004, 17, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Shiota, M.; Greenberg, N.; Thomas, J.D.; Shiota, T. Real time three-dimensional echocardiography evaluation of mitral annular characteristics in patients with myocardial hypertrophy. Echocardiography 2008, 25, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Niizuma, S.; Inuzuka, Y.; Kawashima, T.; Okuda, J.; Tamaki, Y.; Iwanaga, Y.; Narazaki, M.; Matsuda, T.; Soga, T.; et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ. Heart Fail. 2010, 3, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Topaloglu, C.; Kucukler, N.; Ofgeli, M.; Abraham, T.P. Could early septal involvement in the remodeling process be related to the advance hypertensive heart disease? Int. J. Cardiol. Heart Vasc. 2015, 7, 241–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paulus, W.J.; Tschöpe, C.; Sanderson, J.E.; Rusconi, C.; Flachskampf, F.A.; Rademakers, F.E.; Marino, P.; Smiseth, O.A.; De Keulenaer, G.; Leite-Moreira, A.F.; et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007, 28, 2539–2550. [Google Scholar] [CrossRef]
- Yu, C.M.; Lin, H.; Yang, H.; Kong, S.L.; Zhang, Q.; Lee, S.W. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation 2002, 105, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Borlaug, B.A.; Rosen, B.; Hay, I.; Ferruci, L.; Morell, C.H.; Lakatta, E.G.; Najjar, S.S.; Kass, D.A. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community. J. Am. Coll. Cardiol. 2007, 49, 198–207. [Google Scholar] [CrossRef]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Anderson, S.; Donovan, M.; Iverson, E.; Staiger, C.; et al. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Kijima, Y.; Nishibe, A. Myocardial fibrosis is associated with diastolic heart failure in hypertensive patients—Noninvasive assessment by cardiac magnetic resonance. Eur. Heart J. 2010, 31, S-457. [Google Scholar]
- Tan, Y.T.; Wenzelburger, F.; Lee, E.; Heatlie, G.; Frenneaux, M.; Sanderson, J.E. Abnormal left ventricular function occurs on exercise in well-treated hypertensive subjects with normal resting echocardiography. Heart 2010, 96, 948–955. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Lam, C.S.; Olson, T.P. Cardiovascular reserve function in heart failure with preserved ejection fraction: Systolic versus diastolic determinants. Circulation 2008, 118, S1022. [Google Scholar]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Bunting, B.A.; Smith, B.H.; Sutherland, S.E. The Asheville Project: Clinical and economic outcomes of a community-based long-term medication therapy management program for hypertension and dyslipidemia. J. Am. Pharm. Assoc. 2008, 48, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Weston, S.A.; Redfield, M.M.; Jacobsen, S.J.; Pakhomov, S.; Nkomo, V.T.; Meverden, R.A.; Roger, V.L. Systolic and diastolic heart failure in the community. JAMA 2006, 296, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Tajer, C.; Mariani, J.; Abreu, M. Clinical profile and in-hospital outcomes in patients admitted for heart failure with preserved or reduced ejection fraction: The Epi-Cardio prospective survey. Eur. Heart J. 2010, 31, S457. [Google Scholar]
- Fung, J.W.; Sanderson, J.E.; Yip, G.W.; Zhang, Q.; Yu, C.M. Impact of atrial fibrillation in heart failure with normal ejection fraction: A clinical and echocardiographic study. J. Card. Fail. 2007, 13, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Somaratne, J.B.; Berry, C.; McMurray, J.J.; Poppe, K.K.; Doughty, R.N.; Whalley, G.A. The prognostic significance of heart failure with preserved left ventricular ejection fraction: A literature-based meta-analysis. Eur. J. Heart Fail. 2009, 11, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Badheka, A.O.; Rathod, A.; Kizilbash, M.A.; Bhardwaj, A.; Ali, O.; Afonso, L.; Jacob, S. Comparison of mortality and morbidity in patients with atrial fibrillation and heart failure with preserved versus decreased left ventricular ejection fraction. Am. J. Cardiol. 2011, 108, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Varela-Roman, A.; Gonzalez-Juanatey, J.R.; Basante, P.; Trillo, R.; Garcia-Seara, J.; Martinez-Sande, J.L.; Gude, F. Clinical characteristics and prognosis of hospitalisedinpatients with heart failure and preserved or reduced left ventricular ejection fraction. Heart 2002, 88, 249–254. [Google Scholar] [CrossRef]
- Dries, D.L.; Exner, D.V.; Gersh, B.J.; Domanski, M.J.; Waclawiw, M.A.; Stevenson, L.W. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: A retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 1998, 32, 695–703. [Google Scholar] [PubMed]
- Senni, M.; Tribouilloy, C.M.; Rodeheffer, R.J.; Jacobsen, S.J.; Evans, J.M.; Bailey, K.R.; Redfield, M.M. Congestive heart failure in the community: A study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 1998, 98, 2282–2289. [Google Scholar] [CrossRef]
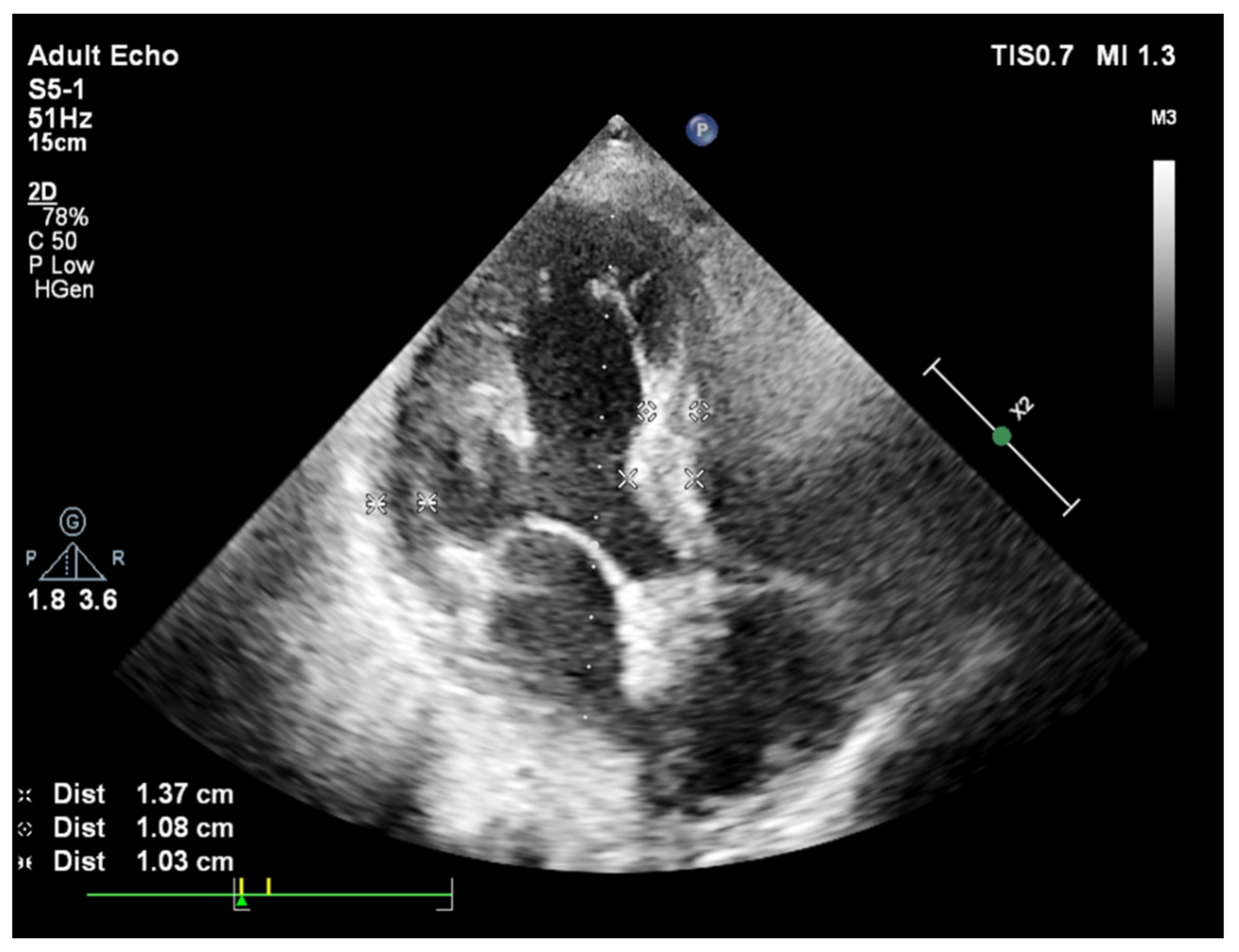
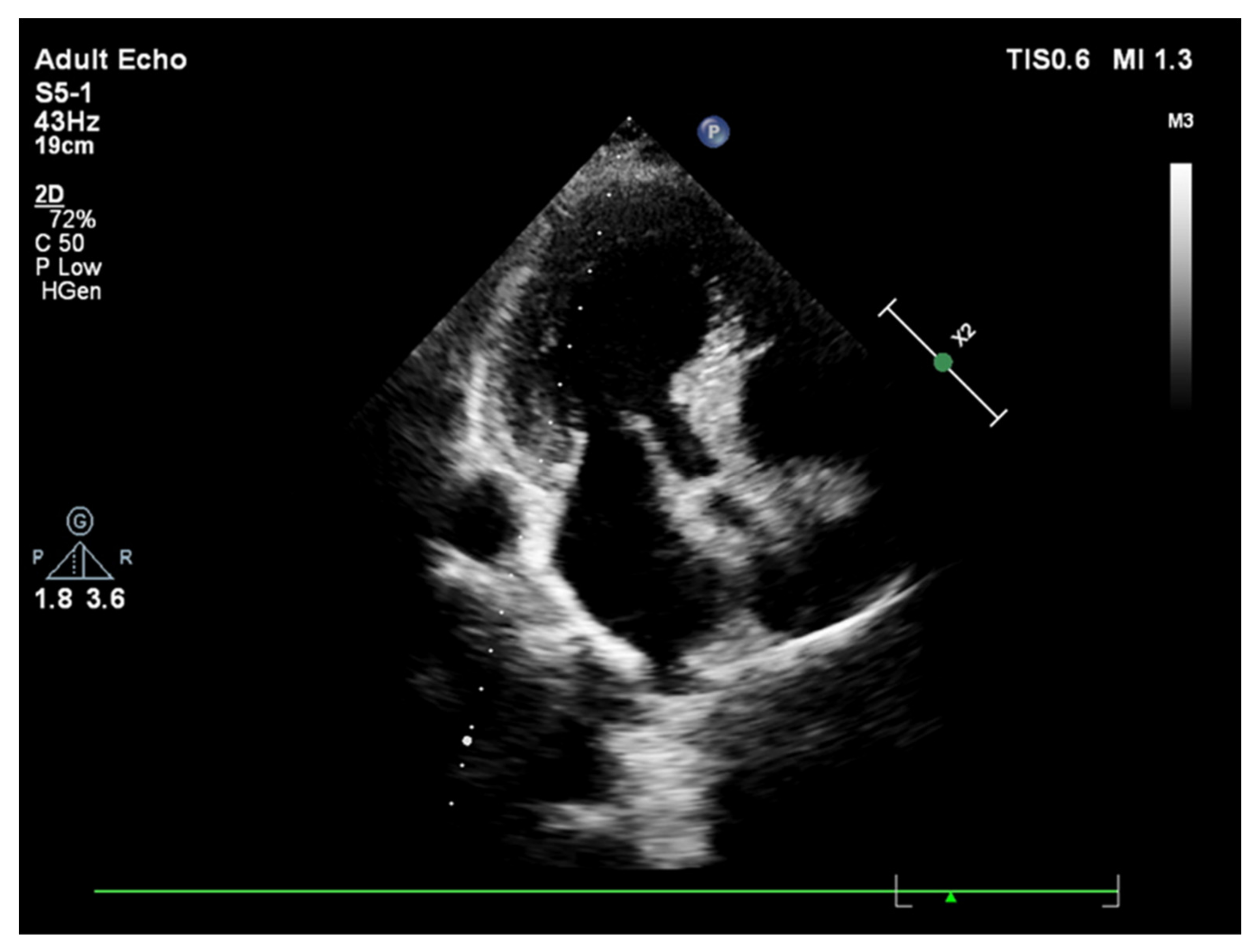
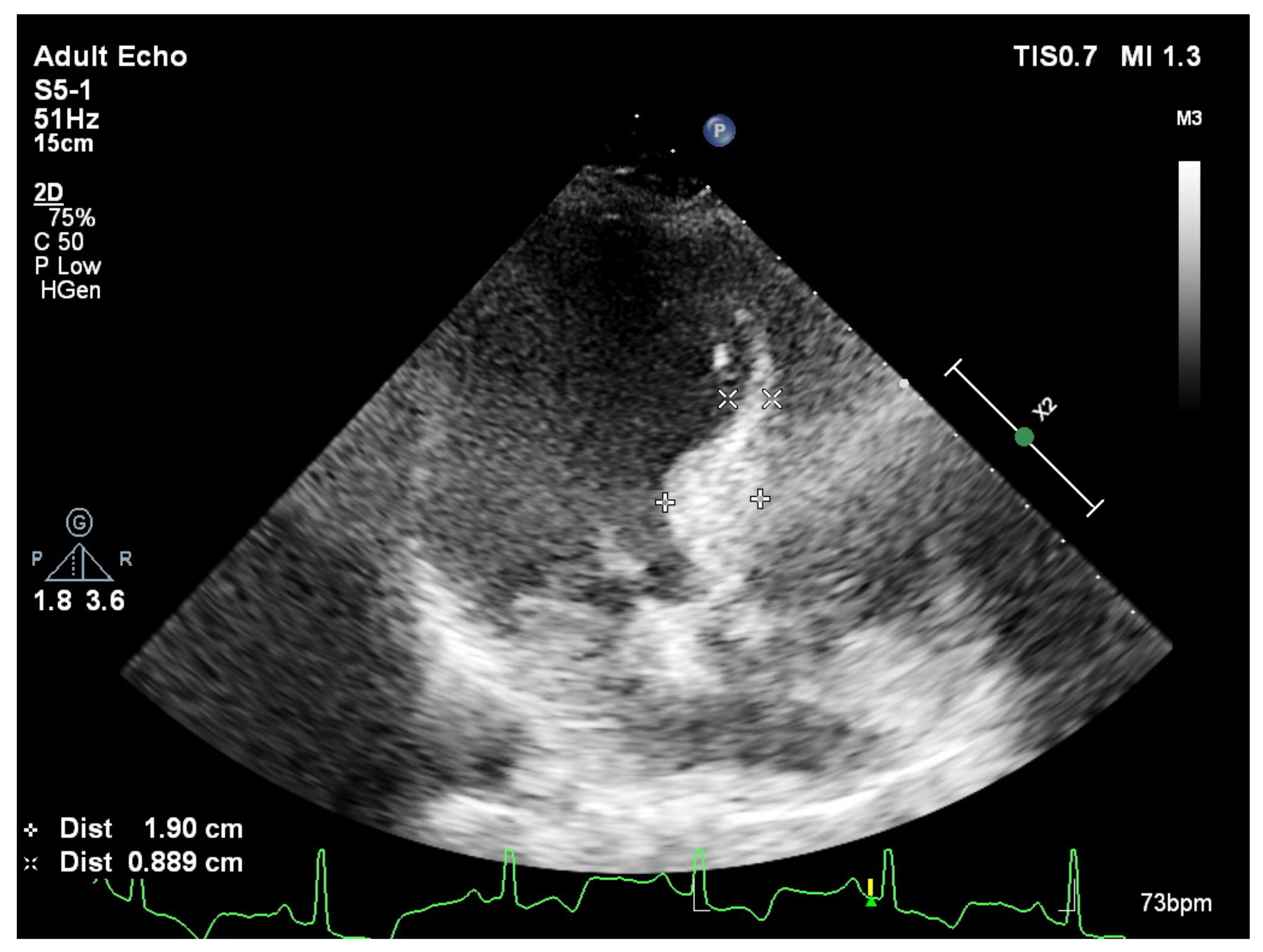
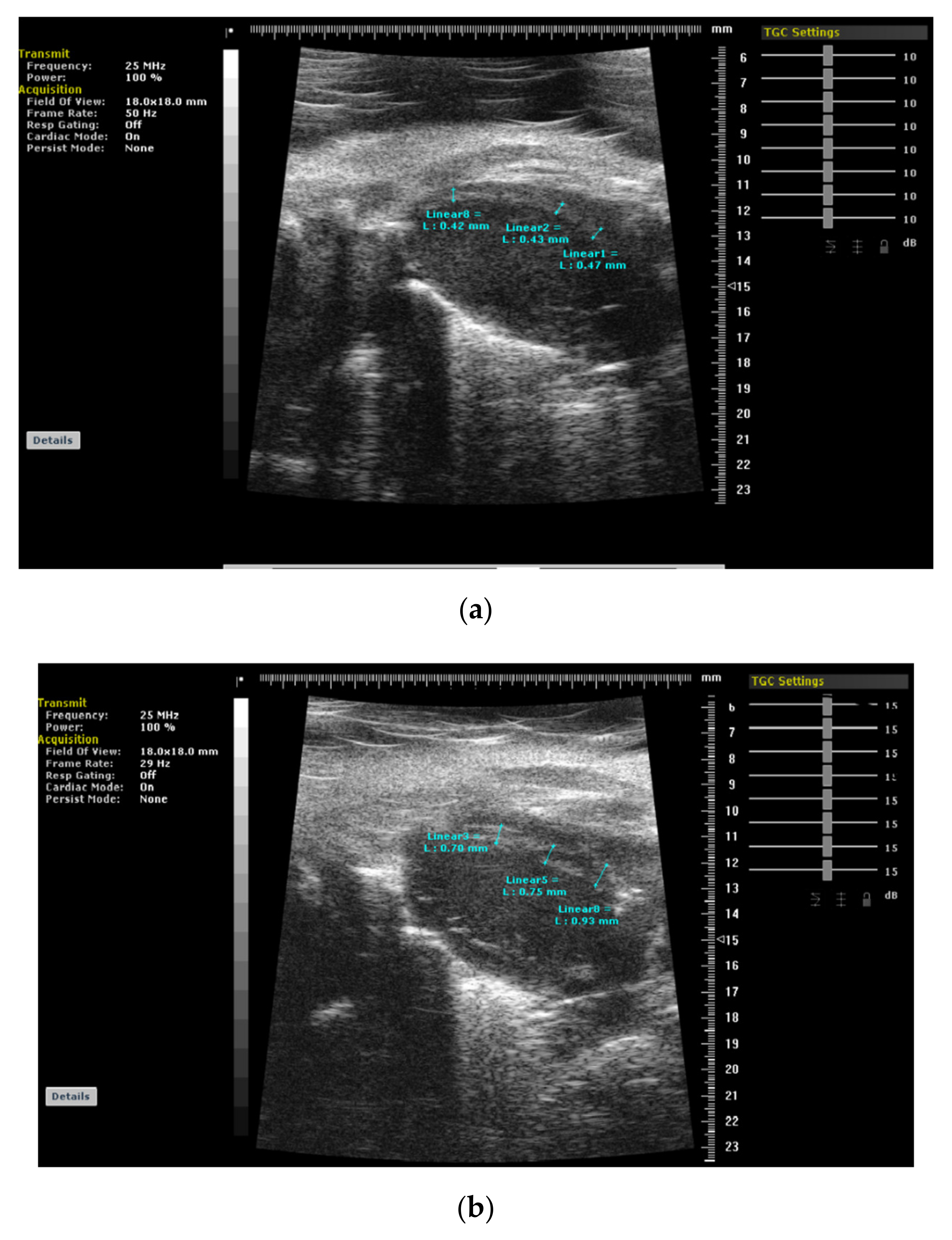
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalçin, F.; Yalçin, H.; Küçükler, N.; Arslan, S.; Akkuş, O.; Kurtul, A.; Abraham, M.R. Basal Septal Hypertrophy as the Early Imaging Biomarker for Adaptive Phase of Remodeling Prior to Heart Failure. J. Clin. Med. 2022, 11, 75. https://doi.org/10.3390/jcm11010075
Yalçin F, Yalçin H, Küçükler N, Arslan S, Akkuş O, Kurtul A, Abraham MR. Basal Septal Hypertrophy as the Early Imaging Biomarker for Adaptive Phase of Remodeling Prior to Heart Failure. Journal of Clinical Medicine. 2022; 11(1):75. https://doi.org/10.3390/jcm11010075
Chicago/Turabian StyleYalçin, Fatih, Hulya Yalçin, Nagehan Küçükler, Serbay Arslan, Oguz Akkuş, Alparslan Kurtul, and Maria Roselle Abraham. 2022. "Basal Septal Hypertrophy as the Early Imaging Biomarker for Adaptive Phase of Remodeling Prior to Heart Failure" Journal of Clinical Medicine 11, no. 1: 75. https://doi.org/10.3390/jcm11010075
APA StyleYalçin, F., Yalçin, H., Küçükler, N., Arslan, S., Akkuş, O., Kurtul, A., & Abraham, M. R. (2022). Basal Septal Hypertrophy as the Early Imaging Biomarker for Adaptive Phase of Remodeling Prior to Heart Failure. Journal of Clinical Medicine, 11(1), 75. https://doi.org/10.3390/jcm11010075





