Brain Natriuretic Peptide Biomarkers in Current Clinical and Therapeutic Scenarios of Heart Failure
Abstract
:1. Introduction
2. Variability of BNP and NT-proBNP Measurements
2.1. Measurement Variability
2.2. NT-proBNP and BNP: Serum Levels and Clinical Correlates
3. Natriuretic Peptides Defining Heart Failure Risk
4. Natriuretic Peptides in the Definition and Diagnosis of Heart Failure
5. Natriuretic Peptides for Stratifying Chronic Heart Failure Prognosis
6. Use of Natriuretic Peptides to Guide CHF Therapy
7. BNP and NT-proBNP in Acute Decompensated Heart Failure: Inpatient and Outpatient Utility
7.1. NPs during Admission for Acute Decompensated Heart Failure (ADHF)
7.2. NPs and Patient Monitoring after Discharge
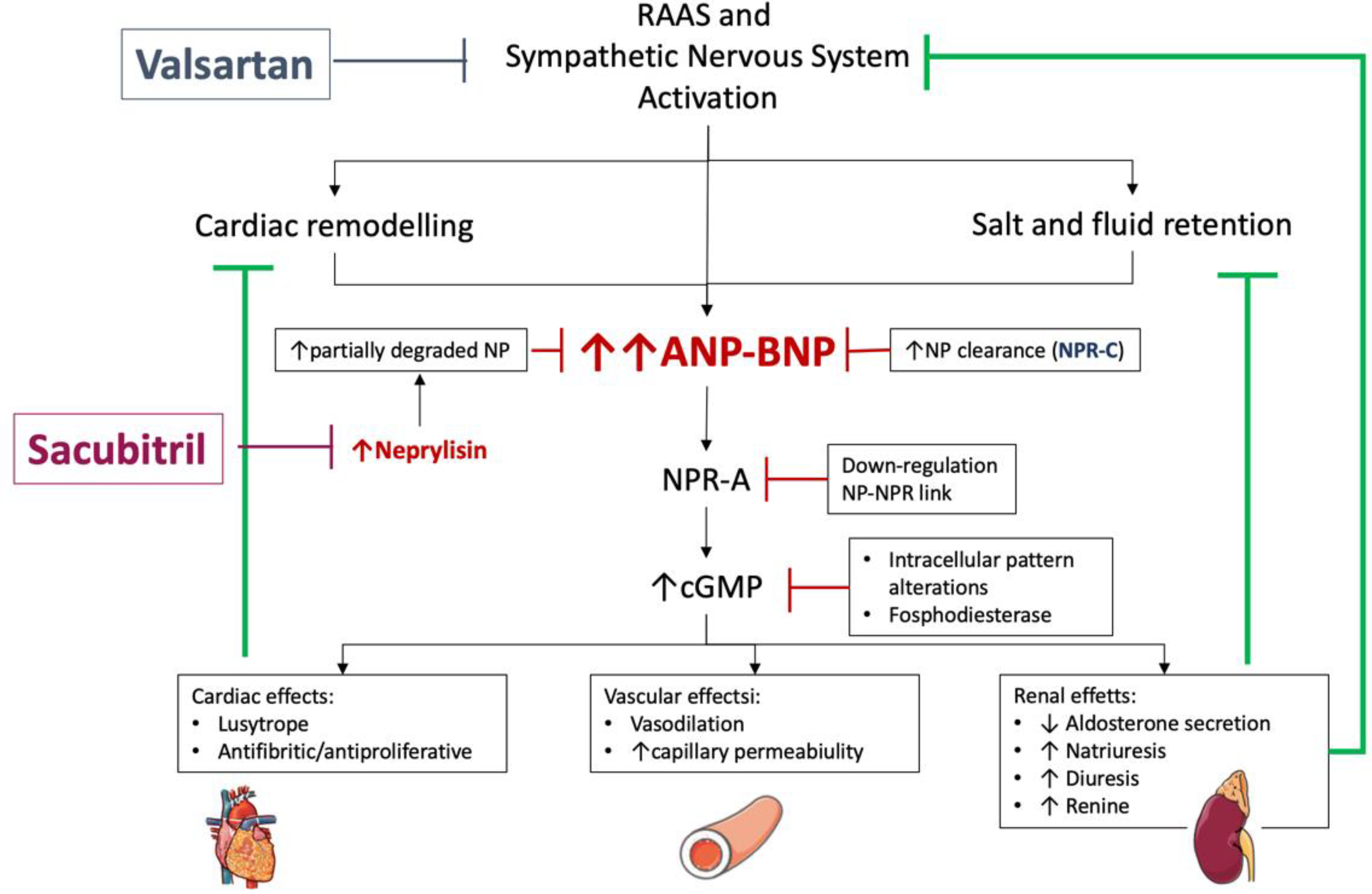
8. Natriuretic Peptides and New Modifier Drugs
8.1. Recent Randomized Trials and NPs
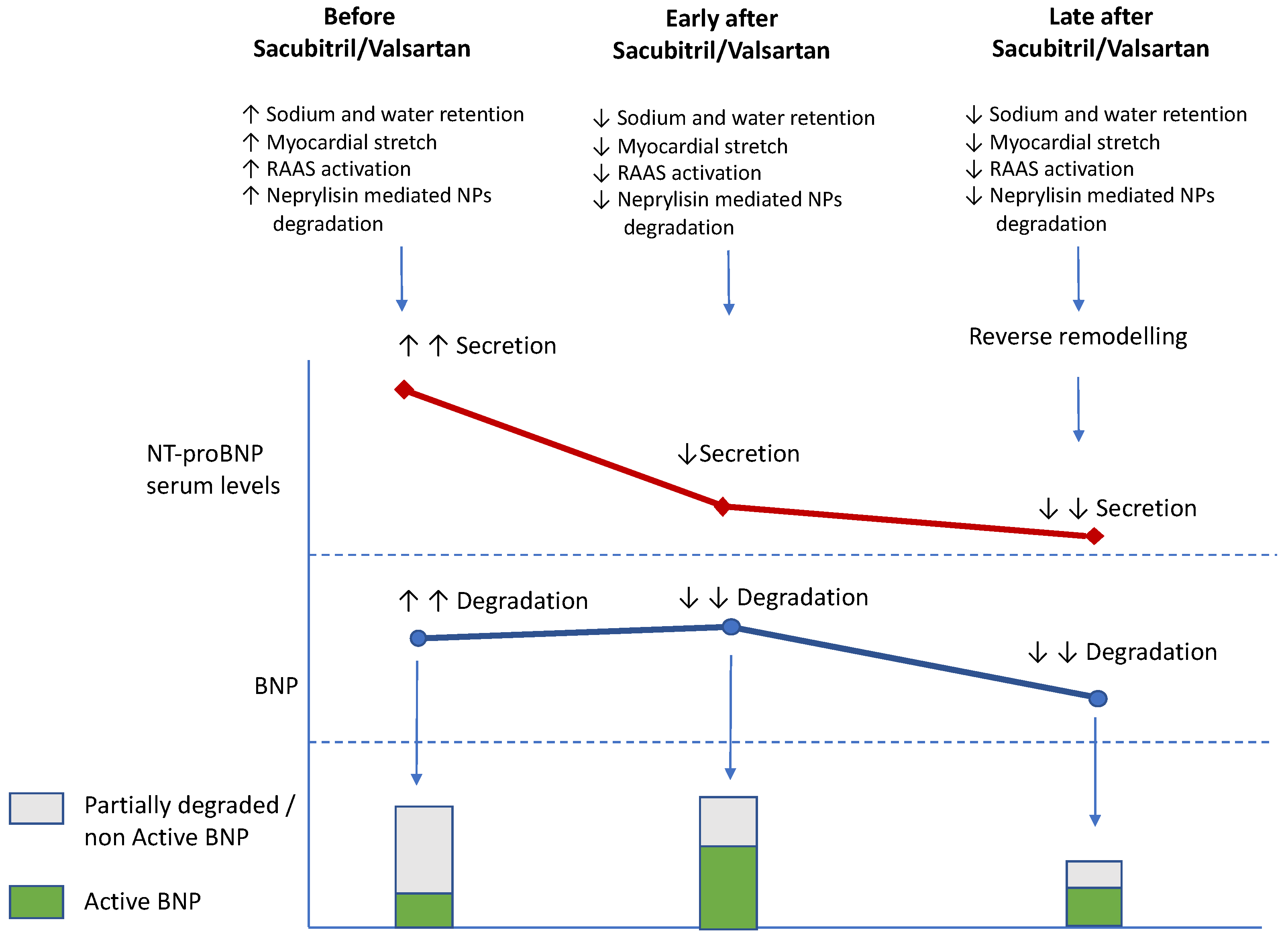
8.2. Sacubitril/Valsartan and NT-proBNP/BNP Changes
8.3. SGLT2i and NP Changes
9. Current Guideline Recommendations Regarding the Use of BNP and NT-proBNP
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Byrne, J.; McDonagh, T.; Morton, J.J.; McMurray, J.J. Measurement of brain natriuretic peptide. Lancet 1996, 348, 1589. [Google Scholar] [CrossRef]
- Aspromonte, N.; Gulizia, M.M.; Clerico, A.; Di Tano, G.; Emdin, M.; Feola, M.; Iacoviello, M.; Latini, R.; Mortara, A.; Valle, R.; et al. ANMCO/ELAS/SIBioC Consensus Document: Biomarkers in heart failure. Eur. Heart J. Suppl. 2017, 19 (Suppl. D), D102–D112. [Google Scholar] [CrossRef]
- Clerico, A.; Giannoni, A.; Vittorini, S.; Passino, C. Thirty years of the heart as an endocrine organ: Physiological role and clinical utility of cardiac natriuretic hormones. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H12–H20. [Google Scholar] [CrossRef] [Green Version]
- Diez, J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: Implications for therapy. Eur. J. Heart Fail. 2017, 19, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, S.S.; Stebbins, A.; Voors, A.A.; Hasselblad, V.; Ezekowitz, J.A.; Califf, R.M.; O’Connor, C.M.; Starling, R.C.; Hernandez, A.F. Effects of nesiritide and predictors of urine output in acute decompensated heart failure: Results from ASCEND-HF (acute study of clinical effectiveness of nesiritide and decompensated heart failure). J. Am. Coll. Cardiol. 2013, 62, 1177–1183. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; Redfield, M.M.; et al. PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bohm, M.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Packer, M.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Haass, M.; Miller, A.; et al. Effect of Empaglifozin on Worsening Heart Failure Events in Patients with heart failure and preserved ejection fraction: EMPEROR-Preserved Trial. Circulation 2021, 144, 1284–1494. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2020, 384, 117–128. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Diaz, R.; Felker, G.M. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N. Engl. J. Med. 2021, 384, 105–116. [Google Scholar] [CrossRef]
- Ponikowski, P.; Kirwan, B.A.; Anker, S.D.; Dorobantu, M.; Filippatos, G.; Martinez, F.A.; Metra, M.; Milicic, D.; Ohlsson, M.; Skouri, H.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 2020, 396, 1895–1904. [Google Scholar] [CrossRef]
- Clerico, A.; Del Ry, S.; Giannessi, D. Measurement of natriuretic cardiac hormones (ANP, BNP and related peptides) in clinical practice: The need for a new generation of immunoassay methods. Clin. Chem. 2000, 46, 1529–1534. [Google Scholar] [CrossRef] [Green Version]
- Clerico, A.; Recchia, F.A.; Passino, C.; Emdin, M. Cardiac endocrine function is an essential component of the homeostatic regulation network: Physiological and clinical implications. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H17–H29. [Google Scholar] [CrossRef] [Green Version]
- McCullough, P.A.; Omland, T.; Maisel, A.S. B-type natriuretic peptides: A diagnostic breakthrough for clinicians. Rev. Cardiovasc. Med. 2003, 4, 72–80. [Google Scholar]
- Noubiap, J.J.; Sanders, P.; Nattel, S.; Lau, D.H. Biomarkers in Atrial Fibrillation: Pathogenesis and Clinical Implications. Card. Electrophysiol. Clin. 2021, 13, 221–233. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Bohm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar]
- Betti, I.; Castelli, G.; Barchielli, A. The role of N-terminal PRO-brain natriuretic peptide and echocardiography for screening asymptomatic left ventricular dysfunction in a population at high risk for heart failure. The PROBE-HF study. J. Card. Fail. 2009, 15, 377–384. [Google Scholar] [CrossRef]
- Huelsmann, M.; Neuhold, S.; Strunk, G.; Moertl, D.; Berger, R.; Prager, R.; Abrahamian, H.; Riedl, M.; Pacher, R.; Clodi, M. NTproBNP has a high negative predictive value to rule-out short-term cardiovascular events in patients with diabetes with diabetes mellitus. Eur. Heart J. 2008, 29, 2259–2264. [Google Scholar] [CrossRef] [Green Version]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Inzucchi, E.E.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [Green Version]
- Neal, B.; Perkovic, V.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, G.; Desai, M.; Matthews, D.R. CANVAS program Collaborative Group. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. DECLARE-TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Xu, J.; Li, J.; Shaw, W.; Oh, R.; Pfeifer, M.; Butler, J.; Sattar, N.; Mahaffey, K.W.; Neal, B.; et al. Effects of Canagliflozin on Amino-Terminal Pro-B-Type Natriuretic Peptide: Implications for Cardiovascular Risk Reduction. J. Am. Coll. Cardiol. 2020, 76, 2076–2085. [Google Scholar] [CrossRef]
- McDonagh, T.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Piepoli, F.M.; Price, S.; Rosano, G.M.C.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42836, 3599–3726. [Google Scholar] [CrossRef]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, K.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Clopton, P.; Westheim, A.; et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Eng. J. Med. 2002, 347, 161. [Google Scholar] [CrossRef]
- Jannuzzi, J.L.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Tung, R.; Foran-Melanson, S.; Lewandrowski, K.B. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am. J. Cardiol. 2005, 95, 948. [Google Scholar] [CrossRef]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Iwaz, J.A.; Maisal, A.L. Recent advances in point of care testing for natriuretic peptides: Potential impact on heart failure diagnosis and management. Expert Rev. Mol. Diagn. 2016, 16, 641–650. [Google Scholar] [CrossRef]
- Ro, R.; Thode, H.; Taylor, M.; Gulla, J.; Tetrault, E.; Singer, A. Comparison of the diagnostic characteristics of two B-Type natriuretiuc peptide point of care devices. J. Emerg. Med. 2011, 41, 661–667. [Google Scholar] [CrossRef]
- Peacock, W.F.; Braunwald, E.; Abtaham, W. National heart, Lung and Blood Institute Working Group on Emergence Department Management of Acute Heart Failure: Research challenges and opportunities. J. Am. Coll. Cardiol. 2010, 56, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Doust, J.A.; Pietrzak, E.; Dobson, A.; Glasziou, P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: Systematic review. BMJ 2005, 330, 625. [Google Scholar] [CrossRef] [Green Version]
- Latini, R.; Masson, S.; Anand, I.; Judd, D.; Maggioni, A.P.; Chiang, Y.T.; Bevilacqua, M.; Salio, M.; Cardano, P.; Dunselman, P.H.; et al. Valsartan Heart Failure Trial Investigators. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: The Valsartan Heart Failure Trial (Val-HeFT). Circulation 2002, 106, 2454–2458. [Google Scholar]
- Packer, M.; Coats, A.J.; Krum, H.; Rouleau, J.L.; DeMets, D.L. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: A substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation 2002, 110, 1780–1786. [Google Scholar]
- Grande, D.; Leone, M.; Rizzo, C.; Terlizzese, P.; Parisi, G.; Gioia, M.I.; Leopizzi, T.; Segreto, A.; Guida, P.; Romito, R.; et al. A Multiparametric Approach Based on NT-proBNP, ST2, and Galectin3 for Stratifying One Year Prognosis of Chronic Heart Failure Outpatients. J. Cardiovasc. Dev. Dis. 2017, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Savarese, G.; Musella, F.; D’Amore, C.; Vassallo, E.; Losco, T.; Gambardella, F.; Cecere, M.; Petraglia, L.; Pagano, G.; Fimiani, L.; et al. Changes of natriuretic peptides predict hospital admissions in patients with chronic heart failure: A meta-analysis. JACC Heart Fail. 2014, 2, 148–158. [Google Scholar] [CrossRef]
- Masson, S.; Latini, R.; Anand, I.S.; Barlera, S.; Angelici, L.; Vago, T.; Tognoni, G.; Cohn, J.N. Val-HeFT Investigators. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial). J. Am. Coll. Cardiol. 2008, 52, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Januzzi, J.L.; Troughton, R. Are serial BNP measurements useful in heart failure management? Serial natriuretic peptide measurements are useful in heart failure management. Circulation 2013, 127, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Jourdain, P.; Jondeau, G.; Funck, F.; Gueffet, P.; Le Helloco, A.; Donal, E.; Aupetit, J.F.; Aumont, M.C.; Galinier, M.; Eicher, J.C.; et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: The STARS-BNP Multicenter Study. J. Am. Coll. Cardiol. 2007, 49, 1733–1739. [Google Scholar] [CrossRef]
- Troughton, R.W.; Frampton, C.M.; Yandle, T.G.; Espiner, E.A.; Nicholls, M.G.; Richards, A.M. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000, 355, 1126–1130. [Google Scholar] [CrossRef]
- Lainchbury, J.G.; Troughton, R.W.; Strangman, K.M.; Frampton, C.M.; Pilbrow, A.; Yandle, T.G.; Hamid, A.K.; Nicholls, M.G.; Richards, A.M. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: Results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J. Am. Coll. Cardiol. 2009, 55, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Davarzani, N.; Sanders-van Wijk, S.; Karel, J.; Maeder, M.T.; Leibundgut, G.; Gutmann, M.; Pfisterer, M.E.; Rickenbacher, P.; Peeters, R.; Brunner-la Rocca, H.P. N-Terminal Pro-B-Type Natriuretic Peptide-Guided Therapy in Chronic Heart Failure Reduces Repeated Hospitalizations-Results From TIME-CHF. J. Card. Fail. 2017, 23, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Schou, M.; Gustafsson, F.; Videbaek, L.; Andersen, H.; Toft, J.; Nyvad, O.; Ryde, H.; Fog, L.; Jensen, J.C.; Nielsen, O.W.; et al. Adding serial N-terminal pro brain natriuretic peptide measurements to optimal clinical management in outpatients with systolic heart failure: A multicentre randomized clinical trial (NorthStar monitoring study). Eur. J. Heart Fail. 2013, 15, 818–827. [Google Scholar] [CrossRef]
- Eurlings, L.W.; van Pol, P.E.; Kok, W.E.; van Wijk, S.; Lodewijks-van der Bolt, C.; Ah, B.; Lok, D.J.; Crijns, H.J.; van Kraaij, D.J.; de Jonge, N.; et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: Results of the PRIMA (can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J. Am. Coll. Cardiol. 2010, 56, 2090–2100. [Google Scholar]
- Januzzi, J.J.; Rehman, S.U.; Mohammed, A.A.; Bhardwaj, A.; Barajas, L.; Kim, N.A.; Baggish, A.L. Use of amino-terminal pro B type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J. Am. Coll. Cardiol. 2011, 58, 1881–1889. [Google Scholar] [CrossRef] [Green Version]
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.J.; Mark, D.B.; Passmore, G.; Whellan, D.J. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients with Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017, 318, 713–720. [Google Scholar] [CrossRef]
- Fiuzat, M.; Ezekowitz, J.; Alemayehu, W.; Westerhout, C.M.; Sbolli, M.; Cani, D.; Whellan, D.J.; Ahmad, T.; Adams, K.; Patel, C.B.; et al. Assessment of Limitations to Optimization of Guideline-Directed Medical Therapy in Heart Failure From the GUIDE-IT Trial: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2020, 5, 757–764. [Google Scholar] [CrossRef]
- Fonarow, G.C.; ADHERE Scientific Advosory Committee. The Acute decompensated heart failure national registry (ADHERE): Opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev. Cardiovasc. Med. 2003, 4, 21–30. [Google Scholar]
- Cheng, V.; Kazanagra, R.; Garcia, A.; Gardetto, N.; Clopton, P.; Maisel, A. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: A pilot study. J. Am. Coll. Cardiol. 2001, 37, 386. [Google Scholar] [CrossRef] [Green Version]
- Kazanegra, R.; Cheng, V.; Garcia, A.; Krishnaswamy, P.; Gardetto, N.; Clopton, P.; Maisel, A. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: A pilot study. J. Card. Fail. 2001, 7, 21–29. [Google Scholar] [CrossRef]
- Valle, R.; Aspromonte, N.; Giovinazzo, P.; Carbonieri, E.; Chiatto, M.; di Tano, G.; Feola, M.; Milli, M.; Fontebasso, A.; Barro, S.; et al. B-type natriuretic Peptide-guided treatment for predicting outcome in patients hospitalized in sub-intensive care unit with acute heart failure. J. Card. Fail. 2008, 14, 219–224. [Google Scholar] [CrossRef]
- McQuade, C.N.; Mizus, M.; Wald, J.W.; Goldberg, L.; Jessup, M.; Umscheid, C.A. Brain-Type Natriuretic Peptide and Amino-Terminal Pro-Brain-Type Natriuretic Peptide Discharge Thresholds for Acute Decompensated Heart Failure: A Systematic Review. Ann. Intern. Med. 2017, 166, 180–190. [Google Scholar] [CrossRef]
- Carubelli, V.; Lombardi, C.; Lazzarini, V.; Bonadei, I.; Gorga, E.; Metra, M. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J. Cardiovasc. Med. 2016, 17, 828–839. [Google Scholar] [CrossRef]
- Bettencourt, P.; Azevedo, A.; Pimenta, J.; Friões, F.; Ferreira, S.; Ferreira, A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 2004, 110, 2168–2174. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.S.; Stevenson, L.W. Rehospitalization for heart failure: Predict or prevent? Circulation 2012, 126, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Chioncel, O.; Mebazaa, A.; Harjola, V.P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. ESC Heart Failure Long-Term Registry Investigators. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef] [Green Version]
- Massari, F.; Iacoviello, M.; Scicchitano, P.; Mastropasqua, F.; Guida, P.; Riccioni, G.; Speziale, G.; Caldarola, P.; Ciccone, M.M.; Di Somma, S. Accuracy of bioimpedance vector analysis and brain natriuretic peptide in detection of peripheral edema in acute and chronic heart failure. Heart Lung. 2016, 45, 319–326. [Google Scholar]
- Massari, F.; Scicchitano, P.; Iacoviello, M.; Passantino, A.; Guida, P.; Sanasi, M.; Piscopo, A.; Romito, R.; Valle, R.; Caldarola, P.; et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J. Cardiol. 2020, 75, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Lainscak, M.; Anderson, L.; Gayat, E.; Grapsa, J.; Harjola, V.P.; Manka, R.; Nihoyannopoulos, P.; Filardi, P.P.; Vrettou, R.; Anker, S.D.; et al. Imaging in patients with suspected acute heart failure: Timeline approach position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 181–195. [Google Scholar]
- Kamran, H.; Tang, W.H.W. Medical management of acute heart failure. Fac. Rev. 2021, 10, 82. [Google Scholar] [CrossRef]
- Goonewardena, S.N.; Gemignani, A.; Ronan, A.; Vasaiwala, S.; Blair, J.; Brennan, J.M.; Shah, D.P.; Spencer, K.T. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc. Imaging 2008, 1, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Maisel, A.S. Practical approaches to treating patients with acute decompensated heart failure. J. Clin. Fail. 2001, 7 (Suppl. S1), 13–17. [Google Scholar] [CrossRef]
- Maisel, A.; Barnard, D.; Jaski, B.; Frivold, G.; Marais, J.; Azer, M.; Taub, P.R.; Kupfer, K.; Clopton, P.; Greenberg, B. Primary results of the HABIT trial. JACC 2013, 61, 1726–1735. [Google Scholar] [CrossRef]
- Simioniuc, A.; Carluccio, E.; Ghio, S.; Rossi, A.; Biagioli, P.; Reboldi, G.; Galeotti, G.G.; Lu, F.; Zara, C.; Whalley, G.; et al. Investigators of the Network Labs Ultrasound (NEBULA) in Heart Failure Study Group. Echo and natriuretic peptide guided therapy improves outcome and reduces worsening renal function in systolic heart failure: An observational study of 1137 outpatients. Int. J. Cardiol. 2016, 224, 416–423. [Google Scholar] [CrossRef]
- Pellicori, P.; Urbinati, A.; Shah, P.; MacNamara, A.; Kazmi, S.; Dierckx, R.; Zhang, J.; Cleland, J.G.F.; Clark, A.L. What proportion of patients with chronic heart failure are eligible for sacubitril-valsartan? Eur. J. Heart Fail. 2017, 19, 768–778. [Google Scholar] [CrossRef]
- Angelini, G.; Albanese, M.; Ursi, R.; Lisi, F.; Bellino, M.C.; Amato, L.; Parisi, G.; Brunetti, N.D.; Piazzola, G.; Ciccone, M.M.; et al. Elegibility of outpatients with chronic heart failure for sodium-glucose co-transporter-2 inhibitors. ESC Heart Fail. 2021, 8, 2951–2958. [Google Scholar] [CrossRef]
- Buggey, J.; Mentz, R.J.; DeVore, A.D.; Velazquez, E.J. Angiotensin receptor neprilysin inhibition in heart failure: Mechanistic action and clinical impact. J. Card. Fail. 2015, 21, 741–750. [Google Scholar] [CrossRef]
- Miller, W.L.; Phelps, M.A.; Wood, C.M. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ. Heart Fail. 2011, 4, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayes-Genis, A.; Barallat, J.; de Antonio, M.; Domingo, M.; Zamora, E.; Urrutia, A.J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J. Am. Coll. Cardiol. 2015, 65, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Pezel, T.; Picard, F.; Sadoune, M.; Arrigo, M.; Beauvais, F.; Launay, J.M.; Cohen-Solal, A.; Vodovar, N.; Logeart, D. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: A mechanistic clinical study. Eur. J. Heart Fail. 2019, 21, 598–605. [Google Scholar]
- Packer, M.; McMurray, J.J.V.; Desai, A.S.; Gog, J.; Lefkowitz, M.P.; Starling, R.C.; Teerlink, J.R.; Vanhaecke, J.; Vinereanu, D.; Wong, R.C.C. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Zile, M.R.; Claggett, B.L.; Desai, A.S.; Shi, V.C.; Solomon, S.D. Prognostic implications of changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients With Heart Failure. J. Am. Coll. Cardiol. 2016, 68, 2425–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, A.S.; Solomon, S.D.; Shah, A.M. Effect of Sacubitril-Valsartan vs. Enalapril on Aortic Stiffness in Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2019, 322, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Prescott, M.F.; Butler, J. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment with Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA 2019, 322, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Khan, M.S.; Marx, N.; Lam, C.S.; Schnaidt, S.; Ofstad, A.P.; Brueckmann, M.; Jamal, W.; et al. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients With Heart Failure by Baseline Diabetes Status: Results From the EMPEROR-Reduced Trial. Circulation 2021, 143, 337–349. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef] [Green Version]
- Fragasso, G.; Petrie, M. Sodium-glucose co-transporter 2 inhibitors in heart failure: Beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1495–1503. [Google Scholar]
- Gronda, E.; Jessup, M.; Iacoviello, M.; Palazzuoli, A.; Napoli, C. Glucose Metabolism in the Kidney: Neurohormonal Activation and Heart Failure Development. J. Am. Heart Assoc. 2020, 9, e018889. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; Pitt, B.; Austin, B.; Malone, M.; Margulies, K.; et al. Dapaglifozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with heart failure with reduced Ejection Fraction: The DEFINE HF Trial. Circulation 2019, 140, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; DeMets, D.; Inzucchi, S.E.; Kober, L.; Martinez, F.A.; Sabatine, M.S.; Sjostrand, M.; Solomon, S.D. DAPA-HF Committees and Investigators. A trial to evaluate the effect of the SGLT2 inhibitor dapaglifozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur. J. Heart Fail. 2019, 21, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Januzzi, J.L., Jr.; Zannad, F.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Ferreira, J.P.; Sattar, N.; Verma, S.; Vedin, O.; et al. EMPEROR-Reduced Trial Committees and Investigators. Prognostic Importance of NT-proBNP and Effect of Empagliflozin in the EMPEROR-Reduced Trial. J. Am. Coll. Cardiol. 2021, 78, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [PubMed]
- National Guideline Centre (UK). Chronic Heart Failure in Adults: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2018. [Google Scholar]
| Study | Patients (Age) | Therapeutic Target | NP Target | At Target (%) | Lower NP than Control | Results |
|---|---|---|---|---|---|---|
| BNP-guided | ||||||
| STARS-BNP [42] | 220 (65) | <100 pg/mL | Low | 33 | Yes | Reduction in HF death and hospital stay |
| Troughton [43] | 69 (70) | <200 pmol/L | Low | N.a. | Reduction in total cardiovascular events | |
| NT-proBNP-guided | ||||||
| BATTLESCARED [44] | 364 (76) | NT-proBNP increase 150 HF score > 2.0 | No | Minority | Yes | Improved one-year survival; improved 3-year survival in < 75 yrs |
| TIME-CHF [45] | 499 (77) | NT-proBNP <400 (<75 yrs) <800 (>75 yrs) NYHA class II | No | 49 | Yes | No effect on HF hospitalization, only a positive trend |
| NorthStarAdherence [46] | 921 | NT-proBNP 1000 for shift from primary care to HF clinic | - | - | - | No improvement in adherence |
| PRISMA [47] | 345 (72) | Increase after discharge > 10% NT-proBNP > 850 | High | 80 | No | No effects on days of admission and HF hospitalization |
| PROTECT [48] | 151 (63) | NT-proBNP < 1000 | Low | 44 | No | Decrease in events |
| GUIDE-IT [49,50] | 1100 (75) | NT-proBNP < 1000 | No | N.a. | No | Decrease in events |
| Trial (Treatment) | Main Inclusion Criteria | Main Exclusion Criteria | NT-proBNP/BNP Inclusion Criteria | Enrolled/Screened Patients (Excluded for NPs) | |||
|---|---|---|---|---|---|---|---|
| PARADIGM-HF (Sacubitril/Valsartan vs. Enalapril) |
|
| hHF (last 12 months) | 8442/10,513 (n.a.) | |||
| Yes | No | ||||||
| BNP | ≥100 | ≥150 | |||||
| NT-proBNP | ≥400 | ≥600 | |||||
| PARAGON-HF (Sacubitril/Valsartan vs. Valsartan) |
|
| NT-proBNP | hHF (last 12 months) | 4822/10,359 (n.a.) | ||
| Yes | No | ||||||
| SR | 200 | 300 | |||||
| AF | 600 | 900 | |||||
| DAPA-HF (Dapagliflozin vs. Placebo) |
|
| NT-proBNP hHF (last 12 months) | If AF | 4744/8134 (n.a.) | ||
| Yes | No | ||||||
| ≥400 | ≥600 | ≥900 | |||||
| EMPEROR-reduced |
|
| NT-proBNP | 3730/7220 (2603, 75%) | |||
| LVEF | hHF (12 months) | ||||||
| Rhythm | Yes | No | |||||
| 36–40% | SR AF | ≥600 ≥1200 | ≥2500 ≥5000 | ||||
| 31–35% | SR AF | ≥ 600 ≥1200 | ≥1000 ≥2000 | ||||
| ≤30% | SR AF | ≥600 ≥1200 | ≥600 ≥1200 | ||||
| EMPEROR-preserved |
|
| SR | AF | 5988/11,583 (4353, 78%) | ||
| NT-proBNP | >300 | >900 | |||||
| SOLOIST (Sotaglifozin vs. Placebo) |
|
| BNP | SR AF | ≥150 ≥450 | 1222/1549 (85, 26%) | |
| NT-proBNP | SR AF | ≥600 ≥1800 | |||||
| VICTORIA-HF Vericiguat vs. Placebo |
|
| BNP | SR AF | ≥300 ≥500 | 5050/6857 (1078, 70%) | |
| NT-proBNP | SR AF | ≥1000 ≥1600 | |||||
| GALACTIC-HF Omecamtiv Mecarbil vs. Placebo |
|
| BNP | SR AF | ≥125 ≥375 | 8256/11,421 (1467, 46%) | |
| NT-proBNP | SR AF | ≥400 ≥1200 | |||||
| AFFIRM-HF Ferric Carboxymaltose vs. Placebo |
| BNP | SR AF | ≥400 ≥600 | 1132/1525 (n.a.) | ||
| NT-proBNP | SR AF | ≥1200 >2400 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcidi, G.; Goffredo, G.; Correale, M.; Brunetti, N.D.; Iacoviello, M. Brain Natriuretic Peptide Biomarkers in Current Clinical and Therapeutic Scenarios of Heart Failure. J. Clin. Med. 2022, 11, 3192. https://doi.org/10.3390/jcm11113192
Alcidi G, Goffredo G, Correale M, Brunetti ND, Iacoviello M. Brain Natriuretic Peptide Biomarkers in Current Clinical and Therapeutic Scenarios of Heart Failure. Journal of Clinical Medicine. 2022; 11(11):3192. https://doi.org/10.3390/jcm11113192
Chicago/Turabian StyleAlcidi, Gianmarco, Giovanni Goffredo, Michele Correale, Natale Daniele Brunetti, and Massimo Iacoviello. 2022. "Brain Natriuretic Peptide Biomarkers in Current Clinical and Therapeutic Scenarios of Heart Failure" Journal of Clinical Medicine 11, no. 11: 3192. https://doi.org/10.3390/jcm11113192
APA StyleAlcidi, G., Goffredo, G., Correale, M., Brunetti, N. D., & Iacoviello, M. (2022). Brain Natriuretic Peptide Biomarkers in Current Clinical and Therapeutic Scenarios of Heart Failure. Journal of Clinical Medicine, 11(11), 3192. https://doi.org/10.3390/jcm11113192








