Cognitive Impairment in People with Epilepsy
Abstract
1. Introduction
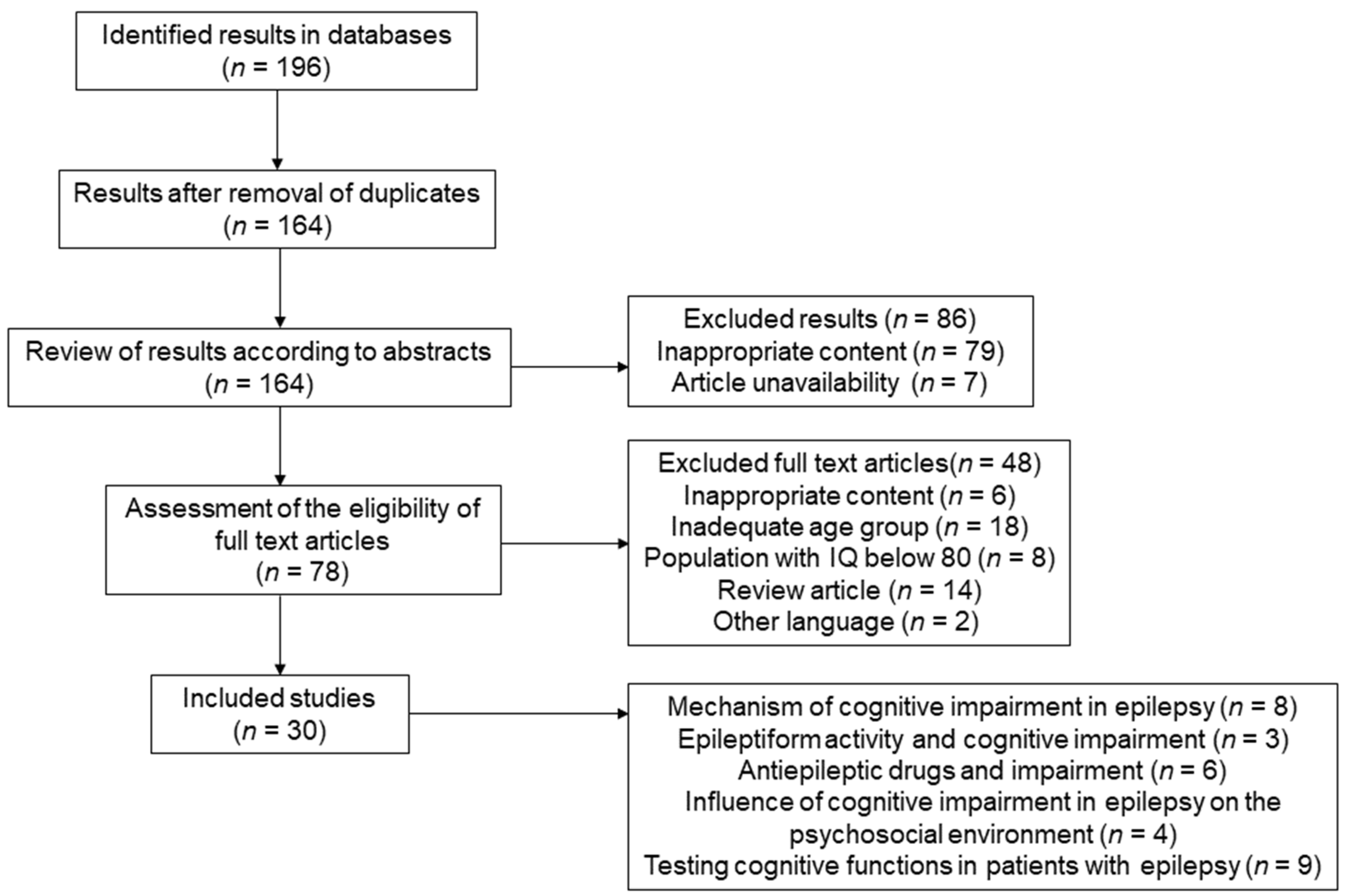
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Selection Process
3. Results
4. Discussion
4.1. Mechanisms of Cognitive Impairment in Epilepsy
4.2. Epileptiform Activity and Cognitive Impairment
4.3. Cognitive Impairment in Epilepsy and Social Connection
4.4. Effect of Antiepileptic Drugs on Cognitive Impairment
4.5. Effect of Epilepsy on Cognitive Abilities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.-S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and incidence of epilepsy. Neurology 2016, 88, 296–303. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshe, S.; Peltola, J.; Perez, E.R.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Panayiotopoulos, C.P. The Epilepsies: Seizures, Syndromes and Management; Bladon Medical Publishing: Oxfordshire, UK, 2005; pp. 1–75. [Google Scholar]
- Kuks, J.B.M.; Snoek, J.W. Textbook of Clinical Neurology, 1st ed.; Bohn Stafleu van Loghum: Houten, The Netherlands, 2018; pp. 225–227. [Google Scholar]
- Van Rijckevorsel, K. Cognitive problems related to epilepsy syndromes, especially malignant epilepsies. Seizure 2006, 15, 227–234. [Google Scholar] [CrossRef]
- Holmes, G.L. Cognitive impairment in epilepsy: The role of network abnormalities. Epileptic Disord. 2015, 17, 101–116. [Google Scholar] [CrossRef]
- Landi, S.; Petrucco, L.; Sicca, F.; Ratto, G.M. Transient Cognitive Impairment in Epilepsy. Front. Mol. Neurosci. 2019, 11, 458. [Google Scholar] [CrossRef]
- Helmstaedter, C.; Witt, J.-A. Epilepsy and cognition—A bidirectional relationship? Seizure 2017, 49, 83–89. [Google Scholar] [CrossRef]
- Hermann, B.; Seidenberg, M. Epilepsy and Cognition. Epilepsy Curr. 2007, 7, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Liu, C.; Lin, W.; Huang, H. Factors for cognitive impairment in adult epileptic patients. Brain Behav. 2020, 10, e01475. [Google Scholar] [CrossRef]
- Helmstaedter, C.; Aldenkamp, A.P.; Baker, G.A.; Mazarati, A.; Ryvlin, P.; Sankar, R. Disentangling the relationship between epilepsy and its behavioral comorbidities—The need for prospective studies in new-onset epilepsies. Epilepsy Behav. 2014, 31, 43–47. [Google Scholar] [CrossRef]
- Vrinda, M.; Arun, S.; Srikumar, B.N.; Kutty, B.M.; Shankaranarayana Rao, B.S. Temporal lobe epilepsy-induced neurodegeneration and cognitive deficits: Implications for aging. J. Chem. Neuroanat. 2019, 95, 146–153. [Google Scholar] [CrossRef]
- Titiz, A.S.; Mahoney, J.M.; Testorf, M.E.; Holmes, G.L.; Scott, R.C. Cognitive impairment in temporal lobe epilepsy: Role of online and offline processing of single cell information. Hippocampus 2014, 24, 1129–1145. [Google Scholar] [CrossRef]
- Knierim, J.J. The hippocampus. Curr. Biol. 2015, 25, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M. The Human Frontal Lobes and Frontal Network Systems: An Evolutionary, Clinical, and Treatment Perspective. ISRN Neurol. 2013, 2013, 892459. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Hirsch, E.; Schmitz, B.; Carreño, M. Epilepsy, antiepileptic drugs (AEDs) and cognition. Acta Neurol. Scand. 2003, 108, 23–32. [Google Scholar] [CrossRef]
- Lenck-Santini, P.-P.; Scott, R.C. Mechanisms Responsible for Cognitive Impairment in Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022772. [Google Scholar] [CrossRef]
- Saniya, K.; Patil, B.G.; Chavan, M.D.; Prakash, K.G.; Sailesh, K.S.; Archana, R.; Johny, M. Neuroanatomical changes in brain structures related to cognition in epilepsy: An update. J. Nat. Sci. Biol. Med. 2017, 8, 139–143. [Google Scholar] [CrossRef]
- Bell, B.; Lin, J.J.; Seidenberg, M.; Hermann, B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat. Rev. Neurol. 2011, 7, 154–164. [Google Scholar] [CrossRef]
- Lin, H.; Holmes, G.L.; Kubie, J.L.; Muller, R.U. Recurrent seizures induce a reversible impairment in a spatial hidden goal task. Hippocampus 2009, 19, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Nickels, K.C.; Wirrell, E.C. Cognitive and Social Outcomes of Epileptic Encephalopathies. Semin. Pediatr. Neurol. 2017, 24, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Frank, H.Y.; Massimo, M.; Ruth, E.W.; Carol, A.R.; Franck, K.; Kimberly, A.B.; William, J.S.; Stanley, M.G.; Todd, S.; William, A.C. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 2006, 9, 1142–1149. [Google Scholar]
- Macdonald, R.L.; Kang, J.Q.; Gallagher, M.J. Mutations in GABAA receptor subunits associated with genetic epilepsies. J. Physiol. 2010, 588, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Bateup, H.S.; Takasaki, K.T.; Saulnier, J.L.; Denefrio, C.L.; Sabatini, B.L. Loss of Tsc1 in vivo impairs hippocampal mGluR-LTD and increases excitatory synaptic function. J. Neurosci. 2011, 31, 8862–8869. [Google Scholar] [CrossRef]
- Imran, H.Q.; Shani, S.; Kile, P.M.; Yalan, Z.; Syed, R.A.; Michael, R.M.; Maria, C.M.; Michael, J.M.; Eugenia, M.J.; Fred, H.G.; et al. An Epilepsy-Associated KCNT1 Mutation Enhances Excitability of Human iPSC-Derived Neurons by Increasing Slack KNa Currents. J. Neurosci. 2019, 39, 7438–7449. [Google Scholar]
- Staley, K.J. Molecular mechanisms of epilepsy. Nat. Neurosci. 2015, 18, 367–372. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Shatskikh, T.N.; Liu, X.; Holmes, G.L. Impaired single cell firing and long-term potentiation parallels memory impairment following recurrent seizures. Eur. J. Neurosci. 2007, 25, 3667–3677. [Google Scholar] [CrossRef]
- Han, T.; Qin, Y.; Mou, C.; Wang, M.; Jiang, M.; Liu, B. Seizure induced synaptic plasticity alteration in hippocampus is mediated by IL-1β receptor through PI3K/Akt pathway. Am. J. Transl. Res. 2016, 8, 4499–4509. [Google Scholar]
- Postnikova, T.Y.; Zubareva, O.E.; Kovalenko, A.A.; Kim, K.K.; Magazanik, L.G.; Zaitsev, A.V. Status epilepticus impairs synaptic plasticity in rat hippocampus and is followed by changes in expression of NMDA receptors. Biochemistry 2017, 82, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.F.; Bliss, T.V.P. Plasticity in the human central nervous system. Brain 2006, 129, 1659–1673. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Carmant, L. Seizures and Epilepsy: An Overview for Neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426. [Google Scholar] [CrossRef]
- Kropotov, J.D. Functional Neuromarkers for Psychiatry: Applications for Diagnosis and Treatment; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–242. [Google Scholar]
- Kleen, J.K.; Scott, R.C.; Holmes, G.L.; Lenck-Santini, P.P. Hippocampal interictal spikes disrupt cognition in rats. Ann. Neurol. 2010, 67, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.K.; Scott, R.C.; Holmes, G.L.; Roberts, D.W.; Rundle, M.M.; Testorf, M.; Lenck-Santini, P.-P.; Jobst, B.C. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology 2013, 81, 18–24. [Google Scholar] [CrossRef]
- Ung, H.; Cazares, C.; Nanivadekar, A.; Kini, L.; Wagenaar, J.; Becker, D.; Krieger, A.; Lucas, T.; Litt, B.; Davis, K.A. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain 2017, 140, 2157–2168. [Google Scholar] [CrossRef]
- International League Against Epilepsy. Coping with Epilepsy. Epilepsia 2003, 44, 43–44. [Google Scholar] [CrossRef]
- Adolphs, R. The Social Brain: Neural Basis of Social Knowledge. Annu. Rev. Psychol. 2009, 60, 693–716. [Google Scholar] [CrossRef]
- Steiger, B.K.; Jokeit, H. Why epilepsy challenges social life. Seizure 2017, 44, 194–198. [Google Scholar] [CrossRef]
- Amlerova, J.; Cavanna, A.E.; Bradac, O.; Javurkova, A.; Raudenska, J.; Marusic, P. Emotion recognition and social cognition in temporal lobe epilepsy and the effect of epilepsy surgery. Epilepsy Behav. 2014, 36, 86–89. [Google Scholar] [CrossRef]
- Bujarski, K.A.; Flashman, L.L.; Li, Z.; Tosteson, T.D.; Jobst, B.C.; Thadani, V.M.; Kobylarz, E.J.; Roberts, D.W.; Roth, R.M. Investigating social cognition in epilepsy using a naturalistic task. Epilepsia 2016, 57, 1515–1520. [Google Scholar] [CrossRef]
- Kobau, R.; Zahran, H.; Thurman, D.J.; Zack, M.M.; Henry, T.R.; Schachter, S.C.; Price, P.H. Epilepsy surveillance among adults--19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill. Summ. 2008, 57, 1–20. [Google Scholar]
- Rai, D.; Kerr, M.P.; McManus, S.; Jordanova, V.; Lewis, G.; Brugha, T.S. Epilepsy and psychiatric comorbidity: A nationally representative population-based study. Epilepsia 2012, 53, 1095–1103. [Google Scholar] [CrossRef]
- International League Against Epilepsy. Available online: https://www.ilae.org/ (accessed on 17 December 2021).
- Martin, R.C.; Griffith, H.R.; Faught, E.; Gilliam, F.; Mackey, M.; Vogtle, L. Cognitive Functioning in Community Dwelling Older Adults with Chronic Partial Epilepsy. Epilepsia 2005, 46, 298–303. [Google Scholar] [CrossRef]
- Griffith, H.R.; Martin, R.C.; Bambara, J.K.; Marson, D.C.; Faught, E. Older adults with epilepsy demonstrate cognitive impairments compared with patients with amnestic mild cognitive impairment. Epilepsy Behav. 2006, 8, 161–168. [Google Scholar] [CrossRef]
- Piazzini, A.; Canevini, M.P.; Turner, K.; Chifari, R.; Canger, R. Elderly People and Epilepsy: Cognitive Function. Epilepsia 2006, 47, 82–84. [Google Scholar] [CrossRef]
- Miller, L.A.; Galioto, R.; Tremont, G.; Davis, J.; Bryant, K.; Roth, J.; LaFrance, J.W.C.; Blum, A.S. Cognitive impairment in older adults with epilepsy: Characterization and risk factor analysis. Epilepsy Behav. 2016, 56, 113–117. [Google Scholar] [CrossRef]
- Hermann, B.; Meador, K.J.; Gaillard, W.D.; Cramer, J.A. Cognition across the lifespan: Antiepileptic drugs, epilepsy, or both? Comorbidities at Baseline. Epilepsy Behav. 2010, 17, 1–5. [Google Scholar] [CrossRef]
- Witt, J.-A.; Elger, C.E.; Helmstaedter, C. Adverse cognitive effects of antiepileptic pharmacotherapy: Each additional drug matters. Eur. Neuropsychopharmacol. 2015, 25, 1954–1959. [Google Scholar] [CrossRef]
- Eddy, C.M.; Rickards, H.E.; Cavanna, A.E. The cognitive impact of antiepileptic drugs. Ther. Adv. Neurol. Disord. 2011, 4, 385–407. [Google Scholar] [CrossRef]
- Vossler, D.G.; Weingarten, M.; Gidal, B.E.; The American Epilepsy Society Treatments Committee. Summary of Antiepileptic Drugs Available in the United States of America. Epilepsy Curr. 2018, 18, 1–26. [Google Scholar] [CrossRef]
- Blum, D.; Meador, K.; Biton, V.; Fakhoury, T.; Shneker, B.; Chung, S.; Mills, K.; Hammer, A.; Isojarvi, J. Cognitive effects of lamotrigine compared with topiramate in patients with epilepsy. Neurology 2006, 67, 400–406. [Google Scholar] [CrossRef]
- Lee, S.-A.; Lee, H.-W.; Heo, K.; Shin, D.-J.; Song, H.-K.; Kim, O.-J.; Lee, S.-M.; Kim, S.-O.; Lee, B.-I. Cognitive and behavioral effects of lamotrigine and carbamazepine monotherapy in patients with newly diagnosed or untreated partial epilepsy. Seizure 2011, 20, 49–54. [Google Scholar] [CrossRef][Green Version]
- Gomer, B.; Wagner, K.; Frings, L.; Saar, J.; Carius, A.; Härle, M.; Steinhoff, B.J.; Schulze-Bonhage, A. The influence of antiepileptic drugs on cognition: A comparison of levetiracetam with topiramate. Epilepsy Behav. 2007, 10, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, A.; Chifari, R.; Canevini, M.P.; Turner, K.; Fontana, S.P.; Canger, R. Levetiracetam: An improvement of attention and of oral fluency in patients with partial epilepsy. Epilepsy Res. 2006, 68, 181–188. [Google Scholar] [CrossRef]
- Witt, J.-A.; Elger, C.E.; Helmstaedter, C. Impaired verbal fluency under topiramate—Evidence for synergistic negative effects of epilepsy, topiramate, and polytherapy. Eur. J. Neurol. 2012, 20, 130–137. [Google Scholar] [CrossRef]
- Taylor, J.; Kolamunnage-Dona, R.; Marson, A.G.; Smith, P.E.M.; Aldenkamp, A.P.; Baker, G.A.; on behalf of the SANAD study group. Patients with epilepsy: Cognitively compromised before the start of antiepileptic drug treatment? Epilepsia 2010, 51, 48–56. [Google Scholar] [CrossRef]
- Loring, D.W.; Marino, S.; Meador, K.J. Neuropsychological and Behavioral Effects of Antiepilepsy Drugs. Neuropsychol. Rev. 2007, 17, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Leeman-Markowski, B.A.; Schachter, S.C. Treatment of Cognitive Deficits in Epilepsy. Neurol. Clin. 2016, 34, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Black, L.C.; Schefft, B.K.; Howe, S.R.; Szaflarski, J.P.; Yeh, H.-S.; Privitera, M.D. The effect of seizures on working memory and executive functioning performance. Epilepsy Behav. 2010, 17, 412–419. [Google Scholar] [CrossRef]
- Ozer, C.A.; Kurt, P.; Yener, G.; Alkin, T.; Oztura, I.; Baklan, B. Comparison of Cognitive Impairment between Patients having Epilepsy and Psychogenic Nonepileptic Seizures. Nöro Psikiyatri Arşivi 2015, 52, 163–168. [Google Scholar] [CrossRef]
- Anzellotti, F.; Dono, F.; Evangelista, G.; Di Pietro, M.; Carrarini, C.; Russo, M.; Ferrante, C.; Sensi, S.L.; Onofrj, M. Psychogenic Non-epileptic Seizures and Pseudo-Refractory Epilepsy, a Management Challenge. Front. Neurol. 2020, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Ahmad, H. Thought suppression predicts task switching deficits in patients with frontal lobe epilepsy. Neurosciences 2015, 20, 153–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Author (by Year) | Sample Size (n) | Main Findings | Limitations |
|---|---|---|---|
| Martin et al. (2005) | n(PWE) = 25 n(control) = 27 |
|
|
| Griffith et al. (2006) | n(PWE) = 26 n(control) = 26 |
|
|
| Piazzini et al. (2006) | n(PWE) = 40 n(control) = 40 |
|
|
| Black et al. (2010) | n(PWE) = 207 n(control) = 216 |
|
|
| Taylor et al. (2010) | n(PWE) = 155 n(control) = 87 |
|
|
| Gul et al. (2015) | n(PWE) = 30 n(control) = 30 |
|
|
| Ozer et al. (2015) | n(PWE) = 20 n(PNES) = 11n(control) = 20 |
|
|
| Miller et al. (2016) | n(PWE) = 38 n(control) = 29 |
|
|
| Wang et al. (2020) | n(PWE) = 257 * compared with the normal reference ranges |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novak, A.; Vizjak, K.; Rakusa, M. Cognitive Impairment in People with Epilepsy. J. Clin. Med. 2022, 11, 267. https://doi.org/10.3390/jcm11010267
Novak A, Vizjak K, Rakusa M. Cognitive Impairment in People with Epilepsy. Journal of Clinical Medicine. 2022; 11(1):267. https://doi.org/10.3390/jcm11010267
Chicago/Turabian StyleNovak, Ajda, Karmen Vizjak, and Martin Rakusa. 2022. "Cognitive Impairment in People with Epilepsy" Journal of Clinical Medicine 11, no. 1: 267. https://doi.org/10.3390/jcm11010267
APA StyleNovak, A., Vizjak, K., & Rakusa, M. (2022). Cognitive Impairment in People with Epilepsy. Journal of Clinical Medicine, 11(1), 267. https://doi.org/10.3390/jcm11010267







