Recovery Profiles of Sevoflurane and Desflurane with or without M-Entropy Guidance in Obese Patients: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection Criteria
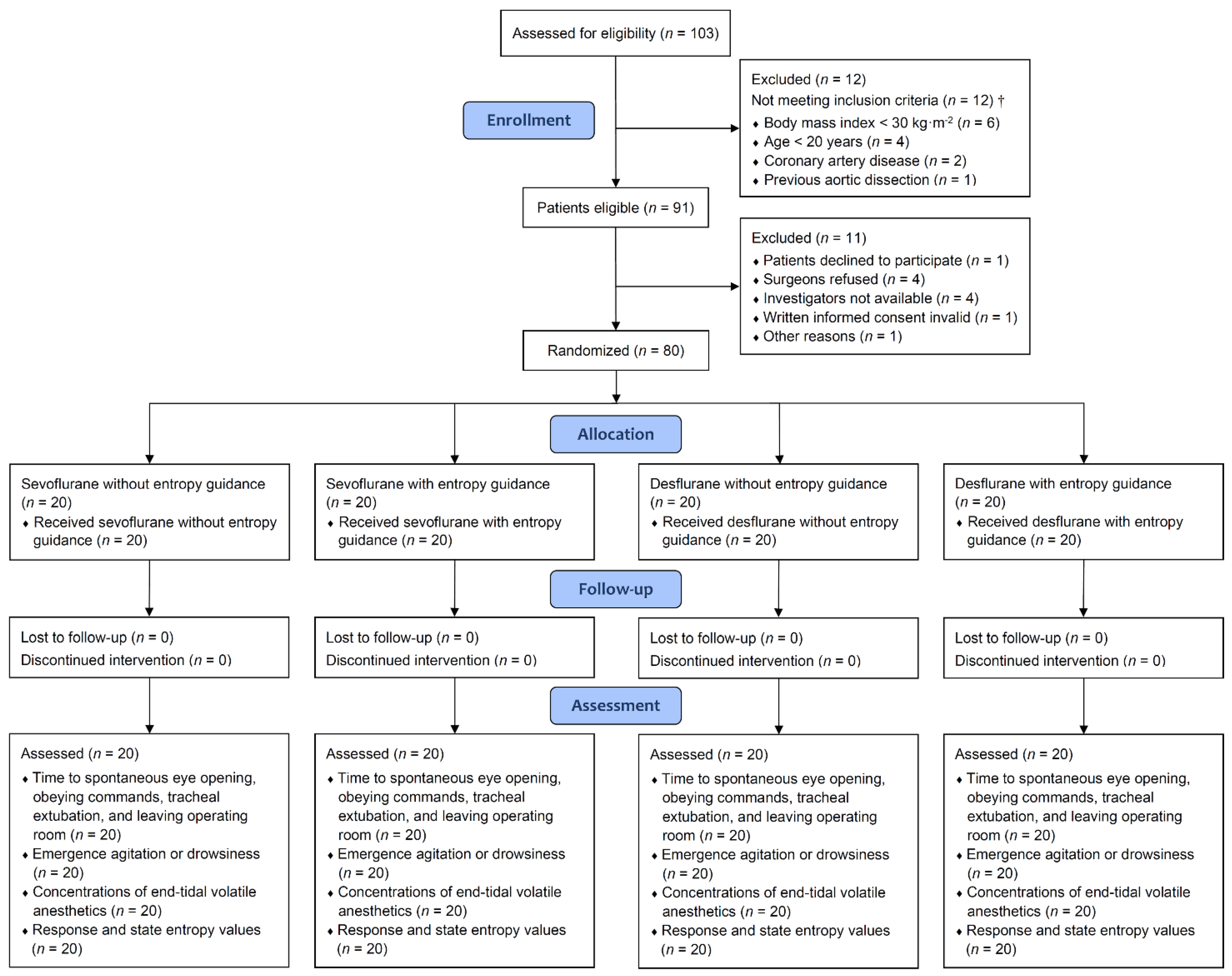
2.2. Randomization Methods
2.3. Anesthesia Management and M-Entropy Guidance
2.4. Outcome Measurement
2.5. Sample Size Estimation
2.6. Statistical Analysis
3. Results
3.1. Baseline Patient and Clinical Characteristics
3.2. Intraoperative Hemodynamic Changes
3.3. Study Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Fact Sheets: Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 October 2021).
- le Roux, C.W.; Chubb, B.; Nørtoft, E.; Borglykke, A. Obesity and healthcare resource utilization: Results from Clinical Practice Research Database (CPRD). Obes. Sci. Pract. 2018, 4, 409–416. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Welbourn, R.; Hollyman, M.; Kinsman, R.; Dixon, J.; Liem, R.; Ottosson, J.; Ramos, A.; Våge, V.; Al-Sabah, S.; Brown, W. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes. Surg. 2019, 29, 782–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, E.; Martin, F.; Pollard, B. Delayed recovery of consciousness after general anaesthesia. BJA Educ. 2020, 20, 173–179. [Google Scholar] [CrossRef]
- Tait, A.R.; Voepel-Lewis, T.; Burke, C.; Kostrzewa, A.; Lewis, I. Incidence and risk factors for perioperative adverse respiratory events in children who are obese. Anesthesiology 2008, 108, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorell, A.; MacCormick, A.D.; Awad, S.; Reynolds, N.; Roulin, D.; Demartines, N.; Vignaud, M.; Alvarez, A.; Singh, P.M.; Lobo, D.N. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery after Surgery (ERAS) Society Recommendations. World J. Surg. 2016, 40, 2065–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juvin, P.; Vadam, C.; Malek, L.; Dupont, H.; Marmuse, J.P.; Desmonts, J.M. Postoperative recovery after desflurane, propofol, or isoflurane anesthesia among morbidly obese patients: A prospective, randomized study. Anesth. Analg. 2000, 91, 714–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Baerdemaeker, L.E.C.; Struys, M.M.R.F.; Jacobs, S.T.E.F.A.N.; Den Blauwen, N.M.M.; Bossuyt, G.R.P.J.; Pattyn, P.; Mortier, E.P. Optimization of desflurane administration in morbidly obese patients: A comparison with sevoflurane using an “inhalation bolus” technique. Br. J. Anaesth. 2003, 91, 638–650. [Google Scholar] [CrossRef] [Green Version]
- Strum, E.M.; Szenohradszki, J.; Kaufman, W.A.; Anthone, G.J.; Manz, I.L.; Lumb, P.D. Emergence and recovery characteristics of desflurane versus sevoflurane in morbidly obese adult surgical patients: A prospective, randomized study. Anesth. Analg. 2004, 99, 1848–1853. [Google Scholar] [CrossRef]
- La Colla, L.; Albertin, A.; La Colla, G.; Mangano, A. Faster wash-out and recovery for desflurane vs sevoflurane in morbidly obese patients when no premedication is used. Br. J. Anaesth. 2007, 99, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, R.E.; Malhotra, A.; Cakmakkaya, O.S.; Hall, K.T.; McKay, W.R.; Apfel, C.C. Effect of increased body mass index and anaesthetic duration on recovery of protective airway reflexes after sevoflurane vs desflurane. Br. J. Anaesth. 2010, 104, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, A.; Jain, A.K.; Sehgal, R.; Sood, J. Hemodynamics and early recovery characteristics of desflurane versus sevoflurane in bariatric surgery. J. Anaesth. Clin. Pharmacol. 2013, 29, 36–40. [Google Scholar] [CrossRef]
- Arain, S.R.; Barth, C.D.; Shankar, H.; Ebert, T.J. Choice of volatile anesthetic for the morbidly obese patient: Sevoflurane or desflurane. J. Clin. Anesth. 2005, 17, 413–419. [Google Scholar] [CrossRef]
- De Baerdemaeker, L.E.; Jacobs, S.; Den Blauwen, N.M.; Pattyn, P.; Herregods, L.L.; Mortier, E.P.; Struys, M.M. Postoperative results after desflurane or sevoflurane combined with remifentanil in morbidly obese patients. Obes. Surg. 2006, 16, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.C.; Sah, N.; Phelps, A.L.; O’Donnell, J.; Romeo, R.C. Desflurane versus sevoflurane for laparoscopic gastroplasty in morbidly obese patients. J. Clin. Anesth. 2007, 19, 3–8. [Google Scholar] [CrossRef]
- Lewis, S.R.; Pritchard, M.W.; Fawcett, L.J.; Punjasawadwong, Y. Bispectral index for improving intraoperative awareness and early postoperative recovery in adults. Cochrane Database Syst. Rev. 2019, 9, CD003843. [Google Scholar] [PubMed]
- Pandazi, A.; Bourlioti, A.; Kostopanagiotou, G. Bispectral Index (BIS) monitoring in morbidly obese patients undergoing gastric bypass surgery: Experience in 23 patients. Obes. Surg. 2005, 15, 58–62. [Google Scholar] [CrossRef]
- Ibraheim, O.; Alshaer, A.; Mazen, K.; El-Dawlatly, A.; Turkistani, A.; Alkathery, K.; Al-Zahrani, T.; Al-Dohayan, A.; Bukhari, A. Effect of bispectral index (BIS) monitoring on postoperative recovery and sevoflurane consumption among morbidly obese patients undergoing laparoscopic gastric banding. Middle East J. Anesthesiol. 2008, 19, 819–830. [Google Scholar]
- Van Lancker, P.; Dillemans, B.; Bogaert, T.; Mulier, J.P.; De Kock, M.; Haspeslagh, M. Ideal versus corrected body weight for dosage of sugammadex in morbidly obese patients. Anaesthesia 2011, 66, 721–725. [Google Scholar] [CrossRef]
- Ely, E.W.; Truman, B.; Shintani, A.; Thomason, J.W.W.; Wheeler, A.P.; Gordon, S.; Francis, J.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003, 289, 2983–2991. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.L.; Cherng, Y.G.; Chen, S.Y.; Su, Y.H.; Huang, S.Y.; Lo, P.H.; Lee, Y.-Y.; Tam, K.-W. Postoperative recovery after anesthesia in morbidly obese patients: A systematic review and meta-analysis of randomized controlled trials. Can. J. Anaesth. 2015, 62, 907–917. [Google Scholar] [CrossRef] [Green Version]
- Sample-Size Determination. In Statistical Methods in Medical Research; Armitage, P.; Berry, G.; Matthews, J.N.S. (Eds.) Blackwell Science: Malden, MA, USA, 2002; pp. 137–146. [Google Scholar]
- Elbakry, A.E.; Sultan, W.E.; Ibrahim, E. A comparison between inhalational (desflurane) and total intravenous anaesthesia (propofol and dexmedetomidine) in improving postoperative recovery for morbidly obese patients undergoing laparoscopic sleeve gastrectomy: A double-blinded randomised controlled trial. J. Clin. Anesth. 2018, 45, 6–11. [Google Scholar] [PubMed]
- Nimmo, A.F.; Absalom, A.R.; Bagshaw, O.; Biswas, A.; Cook, T.M.; Costello, A.; Grimes, S.; Mulvey, D.; Shinde, S.; Wiles, M.D. Guidelines for the safe practice of total intravenous anaesthesia (TIVA): Joint Guidelines from the Association of Anaesthetists and the Society for Intravenous Anaesthesia. Anaesthesia 2019, 74, 211–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, A.; Huang, J.; Schroeder, D.; Sprung, J.; Weingarten, T. Agitation in adults in the post-anaesthesia care unit after general anaesthesia. Br. J. Anaesth. 2018, 121, 1052–1058. [Google Scholar] [CrossRef] [Green Version]
- Munk, L.; Andersen, G.; Møller, A.M. Post-anaesthetic emergence delirium in adults: Incidence, predictors and consequences. Acta Anaesthesiol. Scand. 2016, 60, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Sung, T.Y. Emergence agitation: Current knowledge and unresolved questions. Korean J. Anaesthesiol. 2020, 73, 471–485. [Google Scholar] [CrossRef]
- Faulk, D.J.; Twite, M.D.; Zuk, J.; Pan, Z.; Wallen, B.; Friesen, R.H. Hypnotic depth and the incidence of emergence agitation and negative postoperative behavioral changes. Paediatr. Anaesth. 2010, 20, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Oh, A.Y.; Seo, K.S.; Kim, S.D.; Kim, C.S.; Kim, H.S. Delayed emergence process does not result in a lower incidence of emergence agitation after sevoflurane anesthesia in children. Acta Anaesthesiol. Scand. 2005, 49, 297–299. [Google Scholar] [CrossRef]
- Choi, G.J.; Baek, C.W.; Kang, H.; Park, Y.H.; Yang, S.Y.; Shin, H.Y.; Jung, Y.H.; Woo, Y.C.; Lee, U.L. Emergence agitation after orthognathic surgery: A randomised controlled comparison between sevoflurane and desflurane. Acta Anaesthesiol. Scand. 2015, 59, 224–231. [Google Scholar] [CrossRef]
- Kim, M.S.; Moon, B.E.; Kim, H.; Lee, J.R. Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br. J. Anaesth. 2013, 110, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Isik, B.; Arslan, M.; Tunga, A.D.; Kurtipek, O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr. Anaesth. 2006, 16, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Varughese, S.; Ahmed, R. Environmental and occupational considerations of anesthesia: A narrative review and update. Anesth. Analg. 2021, 133, 826–835. [Google Scholar] [CrossRef] [PubMed]
| SEVO without M-Entropy n = 20 | SEVO with M-Entropy n = 20 | DES without M-Entropy n = 20 | DES with M-Entropy n = 20 | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age, years | 38.0 | 7.3 | 37.9 | 10.7 | 37.0 | 10.5 | 33.9 | 7.5 | 0.4471 |
| Sex, male | 12 | 60.0 | 7 | 35.0 | 11 | 55.0 | 8 | 40.0 | 0.3328 |
| Body mass index, linear, kg·m−2 | 41.4 | 37.5, 46.4 (32.3, 56.3) | 38.3 | 34.4, 41.8 (31.9, 52.5) | 41.2 | 37.2, 47.5 (32.7, 59.2) | 39.3 | 34.4, 42.2 (30.9, 47.9) | 0.2512 |
| Body mass index, binary, kg·m−2 | 0.4660 | ||||||||
| <40 | 8 | 40.0 | 12 | 60.0 | 8 | 40.0 | 11 | 55.0 | |
| ≥40 | 12 | 60.0 | 8 | 40.0 | 12 | 60.0 | 9 | 45.0 | |
| Waist circumference, cm | 126.0 | 12.6 | 125.4 | 14.1 | 127.0 | 17.5 | 122.6 | 12.9 | 0.7919 |
| ASA physical status | 0.4660 | ||||||||
| II | 8 | 40.0 | 12 | 60.0 | 8 | 40.0 | 11 | 55.0 | |
| III | 12 | 60.0 | 8 | 40.0 | 12 | 60.0 | 9 | 45.0 | |
| Current cigarette smoking | 7 | 35.0 | 6 | 30.0 | 11 | 55.0 | 10 | 50.0 | 0.3236 |
| Current alcohol drinking | 4 | 20.0 | 4 | 20.0 | 4 | 20.0 | 2 | 10.0 | 0.8177 |
| Coexisting disease | |||||||||
| Hypertension | 11 | 55.0 | 5 | 25.0 | 7 | 35.0 | 4 | 20.0 | 0.0925 |
| Diabetes mellitus | 3 | 15.0 | 3 | 15.0 | 4 | 20.0 | 4 | 20.0 | >0.9999 |
| Obstructive sleep apnea | 8 | 40.0 | 9 | 45.0 | 9 | 45.0 | 6 | 30.0 | 0.7410 |
| Fatty liver | 17 | 85.0 | 15 | 75.0 | 15 | 75.0 | 17 | 85.0 | 0.7949 |
| Preoperative blood test | |||||||||
| Hemoglobin, g·dL−1 | 14.7 | 13.9, 15.5 (12.3, 17.8) | 14.3 | 13.2, 15.1 (8.7, 16.8) | 14.8 | 14.0, 15.8 (12.5, 17.7) | 14.5 | 13.9, 15.1 (10.8, 16.6) | 0.4802 |
| eGFR, mL·min·1.73 m−2 | 93.5 | 84.4, 116.5 (53.9, 166.7) | 119.9 | 103.1, 129.3 (82.8, 153.9) | 99.2 | 87.0, 126.8 (70.4, 166.5) | 121.9 | 99.8, 133.2 (80.5, 189.9) | 0.0105 |
| Sodium, mmol·L−1 | 139 | 137, 140 (136, 144) | 139 | 137, 141 (130, 145) | 139 | 137, 140 (135, 144) | 138 | 137, 139 (134, 143) | 0.8009 |
| Potassium, mmol·L−1 | 3.9 | 3.8, 4.0 (3.3, 4.4) | 3.9 | 3.6, 4.1 (3.4, 4.4) | 3.8 | 3.7, 4.0 (3.5, 4.4) | 4.1 | 3.8, 4.1 (3.3, 4.2) | 0.4356 |
| Alanine aminotransferase, U·L−1 | 30 | 27, 40 (18, 84) | 34 | 25, 44 (12, 159) | 28 | 24, 35 (15, 80) | 36 | 22, 62 (15, 242) | 0.5160 |
| Aspartate aminotransferase, U·L−1 | 34 | 21, 44 (17, 77) | 37 | 23, 58 (16, 142) | 33 | 22, 38 (18, 67) | 40 | 23, 69 (12, 305) | 0.4865 |
| SEVO without M-Entropy n = 20 | SEVO with M-Entropy n = 20 | DES without M-Entropy n = 20 | DES with M-Entropy n = 20 | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| RE value before induction | 98 | 97, 99 (93, 100) | 98 | 97, 98 (91, 98) | 98 | 97, 99 (95, 100) | 98 | 97, 99 (89, 100) | 0.6215 |
| SE value before induction | 88 | 87, 90 (86, 94) | 88 | 86, 89 (82, 90) | 89 | 88, 89 (82, 90) | 87 | 86, 89 (72, 91) | 0.0896 |
| Intravenous anesthetics | |||||||||
| Lidocaine, mg | 100 | 80, 100 (60, 100) | 80 | 80, 100 (60, 100) | 95 | 80, 100 (80, 100) | 80 | 80, 100 (60, 100) | 0.3515 |
| Fentanyl, μg | 200 | 150, 200 (150, 250) | 175 | 150, 200 (100, 200) | 200 | 150, 200 (150, 250) | 200 | 188, 200 (150, 250) | 0.1270 |
| Propofol, mg | 200 | 155, 200 (150, 200) | 160 | 145, 200 (130, 200) | 200 | 150, 200 (120, 200) | 175 | 150, 200 (120, 200) | 0.1486 |
| Dexamethasone, mg | 5 | 5, 5 (5, 5) | 5 | 5, 5 (5, 5) | 5 | 5, 5 (5, 5) | 5 | 5, 5 (5, 5) | >0.9999 |
| Glycopyrrolate, mg | 0.2 | 0.2, 0.2 (0.2, 0.2) | 0.2 | 0.2, 0.2 (0.2, 0.2) | 0.2 | 0.2, 0.2 (0.2, 0.2) | 0.2 | 0.2, 0.2 (0.2, 0.2) | >0.9999 |
| Rocuronium, mg | 100 | 75, 115 (60, 140) | 95 | 80, 100 (60, 160) | 100 | 88, 123 (70, 140) | 95 | 85, 135 (70, 200) | 0.2216 |
| Sugammadex, mg | 185 | 150, 200 (135, 220) | 165 | 155, 193 (150, 200) | 200 | 170, 200 (130, 210) | 170 | 150, 200 (130, 220) | 0.2877 |
| Duration of anesthesia, min | 120 | 110, 148 (75, 280) | 116 | 100, 135 (90, 210) | 123 | 100, 150 (90, 170) | 128 | 91, 146 (65, 200) | 0.8408 |
| Amount of intravenous fluids, mL | 800 | 625, 1000 (350, 1350) | 750 | 650, 900 (350, 1200) | 700 | 600, 850 (500, 1000) | 725 | 600, 800 (400, 1000) | 0.5477 |
| Sevoflurane Anesthesia n = 40 | Desflurane Anesthesia n = 40 | p | No M-Entropy Guidance n = 40 | M-Entropy Guidance n = 40 | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time to spontaneous eye opening, s | 454 | 257, 569 (149, 982) | 315 | 182, 392 (121, 778) | 0.0034 | 372 | 249, 569 (125, 982) | 333 | 193, 475 (121, 814) | 0.0922 |
| Time to obeying commands, s | 494 | 391, 609 (210, 1158) | 361 | 233, 434 (130, 825) | <0.0001 | 422 | 330, 609 (180, 1158) | 384 | 273, 520 (130, 884) | 0.1060 |
| Time to tracheal extubation, s | 571 | 450, 697 (232, 1066) | 385 | 254, 490 (160, 1144) | 0.0001 | 504 | 388, 664 (205, 1144) | 412 | 281, 590 (160, 1047) | 0.0675 |
| Time to leaving operating room, s | 849 | 677, 911 (443, 1400) | 683 | 538, 800 (410, 1255) | 0.0008 | 765 | 665, 902 (443, 1372) | 707 | 549, 855 (410, 1400) | 0.1658 |
| Emergence agitation | 13 | 32.5 | 10 | 25.0 | 0.4586 | 17 | 42.5 | 6 | 15.0 | 0.0066 |
| Drowsiness after tracheal extubation | 3 | 7.5 | 2 | 5.0 | >0.9999 | 2 | 5.0 | 3 | 7.5 | >0.9999 |
| Intraoperative awareness or recall | 0 | 0 | 0 | 0 | NA | 0 | 0 | 0 | 0 | NA |
| Time percentage of RE > 60 | 24.4 | 13.3, 45.1 (1.9, 77.3) | 12.0 | 7.2, 18.8 (2.5, 51.5) | 0.0004 | 15.5 | 8.3, 31.1 (1.9, 77.3) | 18.3 | 11.0, 28.9 (2.5, 66.7) | 0.5475 |
| Time percentage of RE ranged 40–60 | 61.6 | 48.8, 77.3 (22.2, 92.5) | 72.2 | 48.7, 83.3 (9.7, 95.0) | 0.2346 | 51.7 | 30.2, 67.8 (9.7, 90.7) | 78.9 | 68.4, 83.3 (27.8, 95.0) | <0.0001 |
| Time percentage of RE < 40 | 3.8 | 0, 13.2 (0, 55.6) | 8.3 | 0, 33.2 (0, 87.1) | 0.0589 | 17.4 | 1.4, 42.2 (0, 87.1) | 0 | 0, 5.9 (0, 19.2) | <0.0001 |
| Average RE value | 56 | 51, 63 (42, 70) | 52 | 46, 55 (36, 62) | 0.0002 | 50 | 44, 58 (36, 70) | 55 | 52, 59 (47, 68) | 0.0045 |
| Time percentage of SE > 60 | 21.1 | 13.0, 37.9 (1.9, 77.3) | 11.0 | 6.0, 16.7 (2.5, 51.5) | 0.0010 | 12.8 | 7.1, 31.1 (1.9, 77.3) | 16.0 | 11.0, 24.2 (2.5, 61.1) | 0.4883 |
| Time percentage of SE ranged 40–60 | 67.4 | 46.9, 80.0 (22.2, 92.5) | 71.0 | 51.3, 81.3 (9.7, 95.0) | 0.8663 | 50.0 | 34.1, 69.6 (9.7, 88.9) | 77.8 | 71.8, 84.0 (33.3, 95.0) | <0.0001 |
| Time percentage of SE < 40 | 3.8 | 0, 13.8 (0, 55.6) | 14.3 | 3.4, 34.9 (0, 87.1) | 0.0074 | 19.7 | 4.0, 49.1 (0, 87.1) | 3.4 | 0, 9.6 (0, 28.6) | <0.0001 |
| Average SE value | 55 | 49, 60 (41, 68) | 50 | 45, 53 (35, 60) | 0.0003 | 48 | 43, 56 (35, 68) | 54 | 50, 57 (45, 66) | 0.0063 |
| Average level of end-tidal SEVO, % | 1.63 | 1.49, 1.76 (1.21, 2.29) | NA | NA | NA | 1.66 | 1.57, 1.76 (1.21, 2.16) | 1.54 | 1.46, 1.83 (1.33, 2.29) | 0.4091 |
| Average level of end-tidal SEVO, aaMAC | 0.76 | 0.71, 0.83 (0.57, 1.12) | NA | NA | NA | 0.78 | 0.73, 0.83 (0.57, 0.99) | 0.73 | 0.69, 0.84 (0.63, 1.12) | 0.4165 |
| Average level of end-tidal DES, % | NA | NA | 4.69 | 4.26, 5.20 (2.83, 6.83) | NA | 4.85 | 4.60, 5.23 (3.65, 6.83) | 4.41 | 4.03, 5.09 (2.83, 5.95) | 0.0531 |
| Average level of end-tidal DES, aaMAC | NA | NA | 0.69 | 0.62, 0.78 (0.42, 0.95) | NA | 0.73 | 0.67, 0.80 (0.51, 0.95) | 0.64 | 0.59, 0.75 (0.42, 0.85) | 0.0358 |
| SEVO without M-Entropy n = 20 | SEVO with M-Entropy n = 20 | DES without M-Entropy n = 20 | DES with M-Entropy n = 20 | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Time to spontaneous eye opening, s | 409 | 239, 570 (185, 982) | 463 | 330, 564 (149, 814) | 371 | 254, 488 (125, 778) | 218 | 154, 333 (121, 495) | 0.0012 |
| Time to obeying commands, s | 532 | 353, 663 (235, 1158) | 488 | 442, 591 (210, 884) | 400 | 318, 537 (180, 825) | 310 | 203, 368 (130, 548) | 0.0001 |
| Time to tracheal extubation, s | 575 | 398, 712 (255, 1066) | 571 | 489, 671 (232, 1047) | 449 | 362, 604 (205, 1144) | 313 | 197, 385 (160, 617) | <0.0001 |
| Time to leaving operating room, s | 791 | 665, 936 (443, 1372) | 851 | 738, 908 (548, 1400) | 765 | 656, 849 (490, 1255) | 569 | 459, 702 (410, 865) | 0.0003 |
| Emergence agitation | 10 | 50.0 | 3 | 15.0 | 7 | 35.0 | 3 | 15.0 | 0.0370 |
| Drowsiness after tracheal extubation | 0 | 0 | 3 | 15.0 | 2 | 10.0 | 0 | 0 | 0.1591 |
| Intraoperative awareness or recall | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Time percentage of RE > 60 | 23.0 | 9.5, 50.6 (1.9, 77.3) | 26.4 | 17.1, 37.1 (5.0, 66.7) | 10.5 | 7.2, 18.8 (3.2, 51.5) | 12.5 | 7.4, 20.4 (2.5, 47.1) | 0.0049 |
| Time percentage of RE ranged 40–60 | 53.3 | 36.7, 71.8 (22.2, 90.7) | 71.1 | 52.2, 80.0 (27.8, 92.5) | 48.7 | 28.6, 67.2 (9.7, 89.5) | 82.9 | 75.0, 88.0 (52.9, 95.0) | <0.0001 |
| Time percentage of RE < 40 | 10.0 | 0, 30.2 (0, 55.6) | 1.3 | 0, 5.6 (0, 14.3) | 33.2 | 7.5, 61.7 (0, 87.1) | 0 | 0, 9.9 (0, 19.2) | <0.0001 |
| Average RE value | 52 | 48, 64 (42, 70) | 58 | 56, 61 (51, 68) | 46 | 41, 54 (36, 62) | 53 | 50, 55 (47, 61) | <0.0001 |
| Time percentage of SE > 60 | 20.6 | 9.2, 42.9 (1.9, 77.3) | 21.1 | 14.3, 32.6 (3.4, 61.1) | 9.0 | 5.3, 16.8 (3.2, 51.5) | 12.5 | 7.4, 16.7 (2.5, 41.2) | 0.0101 |
| Time percentage of SE ranged 40–60 | 49.1 | 39.6, 71.8 (22.2, 88.9) | 77.8 | 57.6, 81.9 (33.3, 92.5) | 51.3 | 25.0, 68.8 (9.7, 84.2) | 78.5 | 74.4, 84.6 (53.8, 95.0) | <0.0001 |
| Time percentage of SE < 40 | 10.7 | 0, 33.3 (0, 55.6) | 1.3 | 0, 5.6 (0, 14.3) | 34.9 | 11.1, 65.9 (0, 87.1) | 4.0 | 0, 14.3 (0, 28.6) | <0.0001 |
| Average SE value | 50 | 46, 62 (41, 68) | 56 | 54, 59 (49, 66) | 45 | 38, 53 (35, 60) | 50 | 48, 53 (45, 60) | 0.0001 |
| Average level of end-tidal SEVO, % | 1.66 | 1.57, 1.76 (1.21, 2.16) | 1.54 | 1.46, 1.83 (1.33, 2.29) | NA | NA | NA | NA | 0.4091 |
| Average level of end-tidal SEVO, aaMAC | 0.78 | 0.73, 0.83 (0.57, 0.99) | 0.73 | 0.69, 0.84 (0.63, 1.12) | NA | NA | NA | NA | 0.4165 |
| Average level of end-tidal DES, % | NA | NA | NA | NA | 4.85 | 4.60, 5.23 (3.65, 6.83) | 4.41 | 4.03, 5.09 (2.83, 5.95) | 0.0531 |
| Average level of end-tidal DES, aaMAC | NA | NA | NA | NA | 0.73 | 0.67, 0.80 (0.51, 0.95) | 0.64 | 0.59, 0.75 (0.42, 0.85) | 0.0358 |
| SEVO/EG vs. SEVO/NEG | DES/NEG vs. SEVO/NEG | DES/EG vs. SEVO/NEG | DES/NEG vs. SEVO/EG | DES/EG vs. SEVO/EG | DES/EG vs. DES/NEG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MD or RD (99.2% CI) | p | MD or RD (99.2% CI) | p | MD or RD (99.2% CI) | p | MD or RD (99.2% CI) | p | MD or RD (99.2% CI) | p | MD or RD (99.2% CI) | p | |
| Time to spontaneous eye opening, s | 4 (−179, 186) | 0.9532 | −55 (−237, 126) | 0.3966 | −198 (−357, −39) | 0.0014 | −59 (−222, 104) | 0.3145 | −202 (−338, −66) | 0.0002 | −142 (−276, −8) | 0.0052 |
| Time to obeying commands, s | −34 (−214, 146) | 0.5969 | −109 (−299, 81) | 0.1175 | −245 (−412, −79) | 0.0002 | −74 (−234, 85) | 0.1992 | −211 (−337, −85) | <0.0001 | −137 (−279, 6) | 0.0106 |
| Time to tracheal extubation, s | 14 (−168, 196) | 0.8302 | −65 (−272, 141) | 0.3810 | −265 (−422, −107) | <0.0001 | −79 (−279, 120) | 0.2726 | −279 (−425, −132) | <0.0001 | −199 (−379, −19) | 0.0037 |
| Time to leaving operating room, s | 47 (−144, 237) | 0.4946 | −30 (−223, 163) | 0.6681 | −220 (−383, −57) | 0.0005 | −77 (−271, 118) | 0.2762 | −267 (−432, −102) | <0.0001 | −190 (−358, −23) | 0.0029 |
| Emergence agitation, % | −35.0 (−71.4, 1.4) | 0.0181 | −15.0 (−56.0, 26.0) | 0.3373 | −35.0 (−71.4, 1.4) | 0.0181 | 20.0 (−15.3, 55.3) | 0.1441 | 0 (−30.0, 30.0) | >0.9999 | −20.0 (−55.3, 15.3) | 0.1441 |
| Drowsiness after tracheal extubation, % | 15.0 (−6.2, 36.2) | 0.2308 | 10.0 (−7.8, 27.8) | 0.4872 | NA | NA | −5.0 (−32.7, 22.7) | >0.9999 | −15.0 (−36.2, 6.2) | 0.2308 | −10.0 (−27.8, 7.8) | 0.4872 |
| Intraoperative awareness or recall | 0 (0, 0) | NA | 0 (0, 0) | NA | 0 (0, 0) | NA | 0 (0, 0) | NA | 0 (0, 0) | NA | 0 (0, 0) | NA |
| Time percentage of RE > 60 | −2.7 (−21.9, 16.5) | 0.6958 | −17.0 (−35.0, 0.9) | 0.0113 | −16.4 (−34.1, 1.3) | 0.0130 | −14.3 (−27.4, −1.3) | 0.0039 | −13.7 (−26.5, 1.0) | 0.0047 | 0.6 (−9.2, 10.4) | 0.8630 |
| Time percentage of RE ranged 40–60 | 14.5 (−2.4, 31.4) | 0.0210 | −4.9 (−24.2, 14.4) | 0.4832 | 27.0 (12.4, 41.6) | <0.0001 | −19.4 (−37.7, −1.1) | 0.0052 | 12.5 (−0.6, 25.6) | 0.0113 | 31.9 (15.5, 48.3) | <0.0001 |
| Time percentage of RE < 40 | −11.8 (−23.6, 0.1) | 0.0083 | 21.9 (0.6, 43.3) | 0.0066 | −10.6 (−22.7, 1.6) | 0.0186 | 33.7 (14.5, 52.8) | <0.0001 | 1.2 (−3.6, 6.0) | 0.4890 | −32.5 (−51.8, −13.2) | <0.0001 |
| Average RE value | 4 (−3, 10) | 0.1121 | −8 (−15, −0.2) | 0.0067 | −2 (−8, 4) | 0.3564 | −11 (−17, −5) | <0.0001 | −6 (−9, −2) | <0.0001 | 6 (−0.04, 11) | 0.0084 |
| Time percentage of SE > 60 | −3.9 (−21.8, 14.1) | 0.5499 | −15.6 (−32.6, 1.3) | 0.0137 | −14.6 (−31.1, 1.9) | 0.0173 | −11.8 (−24.2, 0.7) | 0.0119 | −10.7 (−22.5, 1.0) | 0.0146 | 1.1 (−8.4, 10.5) | 0.7558 |
| Time percentage of SE ranged 40–60 | 17.7 (1.4, 34.0) | 0.0042 | −6.1 (−25.3, 13.1) | 0.3791 | 24.0 (9.5, 38.5) | <0.0001 | −23.8 (−41.6, −6.0) | 0.0006 | 6.3 (−6.0, 18.6) | 0.1583 | 30.1 (13.8, 46.5) | <0.0001 |
| Time percentage of SE < 40 | −13.8 (−27.3, −0.4) | 0.0046 | 21.8 (−0.5, 44.0) | 0.0094 | −9.5 (−23.6, 4.7) | 0.0656 | 41.2 (19.5, 63.0) | <0.0001 | 4.4 (−2.2, 10.9) | 0.0669 | −31.2 (−51.2, −11.2) | 0.0002 |
| Average SE value | 3 (−3, 10) | 0.1253 | −7 (−15, −0.03) | 0.0078 | −2 (−8, 4) | 0.3404 | −11 (−16, −5) | <0.0001 | −5 (−9, −2) | 0.0001 | 5 (−0.3, 11) | 0.0112 |
| Average level of end-tidal SEVO, % | 0.001 (−0.23, 0.23) | 0.9904 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Average level of end-tidal SEVO, aaMAC | −0.001 (−0.11, 0.11) | 0.9895 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Average level of end-tidal DES, % | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | −0.46 (−1.13, 0.22) | 0.0673 |
| Average level of end-tidal DES, aaMAC | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | −0.08 (−0.18, 0.02) | 0.0231 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-M.; Su, Y.-H.; Huang, S.-Y.; Lo, P.-H.; Chen, J.-T.; Chang, H.-C.; Yang, Y.-L.; Cherng, Y.-G.; Wu, H.-L.; Tai, Y.-H. Recovery Profiles of Sevoflurane and Desflurane with or without M-Entropy Guidance in Obese Patients: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 162. https://doi.org/10.3390/jcm11010162
Wu Y-M, Su Y-H, Huang S-Y, Lo P-H, Chen J-T, Chang H-C, Yang Y-L, Cherng Y-G, Wu H-L, Tai Y-H. Recovery Profiles of Sevoflurane and Desflurane with or without M-Entropy Guidance in Obese Patients: A Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(1):162. https://doi.org/10.3390/jcm11010162
Chicago/Turabian StyleWu, Yu-Ming, Yen-Hao Su, Shih-Yu Huang, Po-Han Lo, Jui-Tai Chen, Hung-Chi Chang, Yun-Ling Yang, Yih-Giun Cherng, Hsiang-Ling Wu, and Ying-Hsuan Tai. 2022. "Recovery Profiles of Sevoflurane and Desflurane with or without M-Entropy Guidance in Obese Patients: A Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 1: 162. https://doi.org/10.3390/jcm11010162
APA StyleWu, Y.-M., Su, Y.-H., Huang, S.-Y., Lo, P.-H., Chen, J.-T., Chang, H.-C., Yang, Y.-L., Cherng, Y.-G., Wu, H.-L., & Tai, Y.-H. (2022). Recovery Profiles of Sevoflurane and Desflurane with or without M-Entropy Guidance in Obese Patients: A Randomized Controlled Trial. Journal of Clinical Medicine, 11(1), 162. https://doi.org/10.3390/jcm11010162






