Radiological Cardiothoracic Ratio in Evidence-Based Medicine
Abstract
1. Introduction
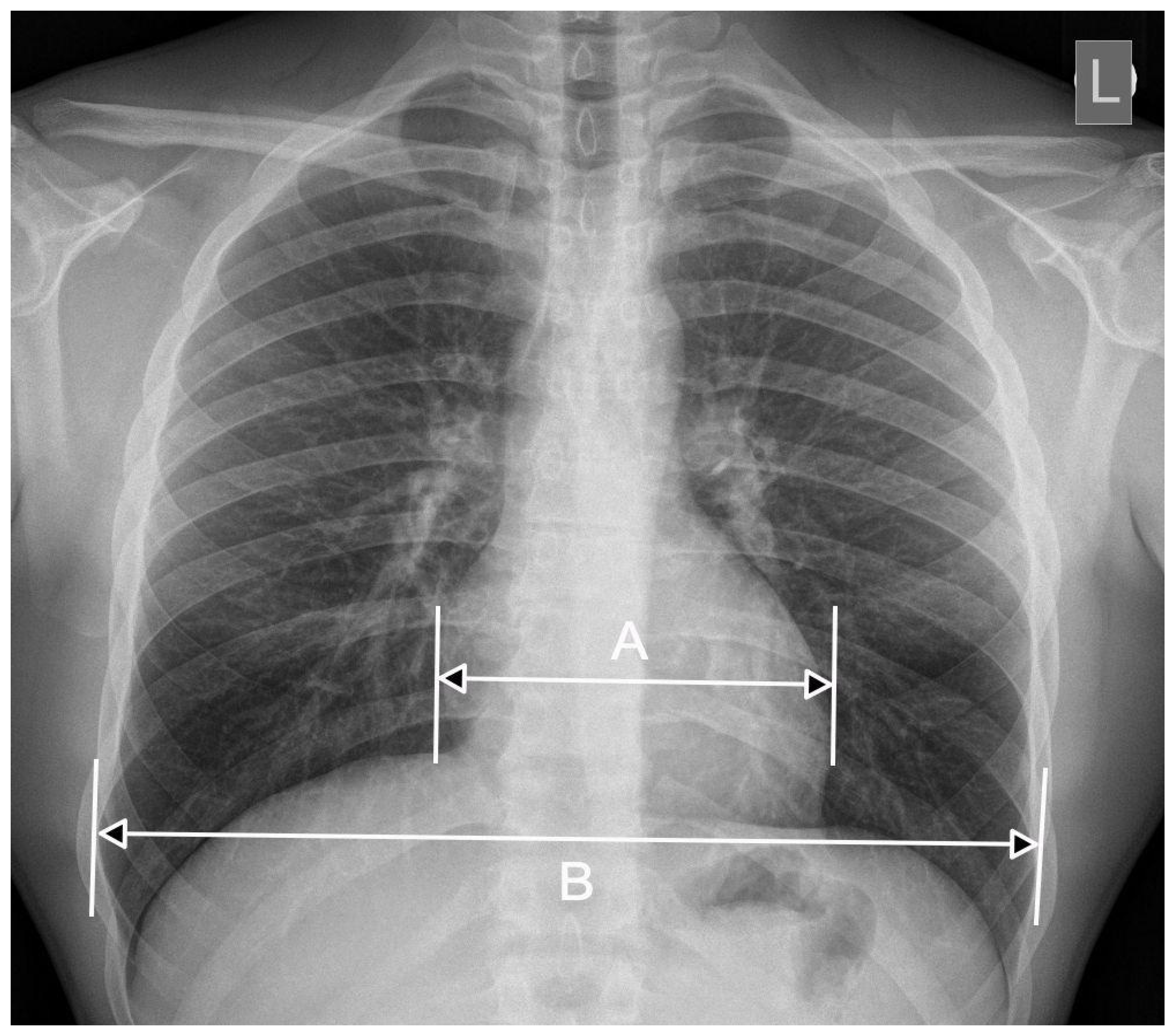
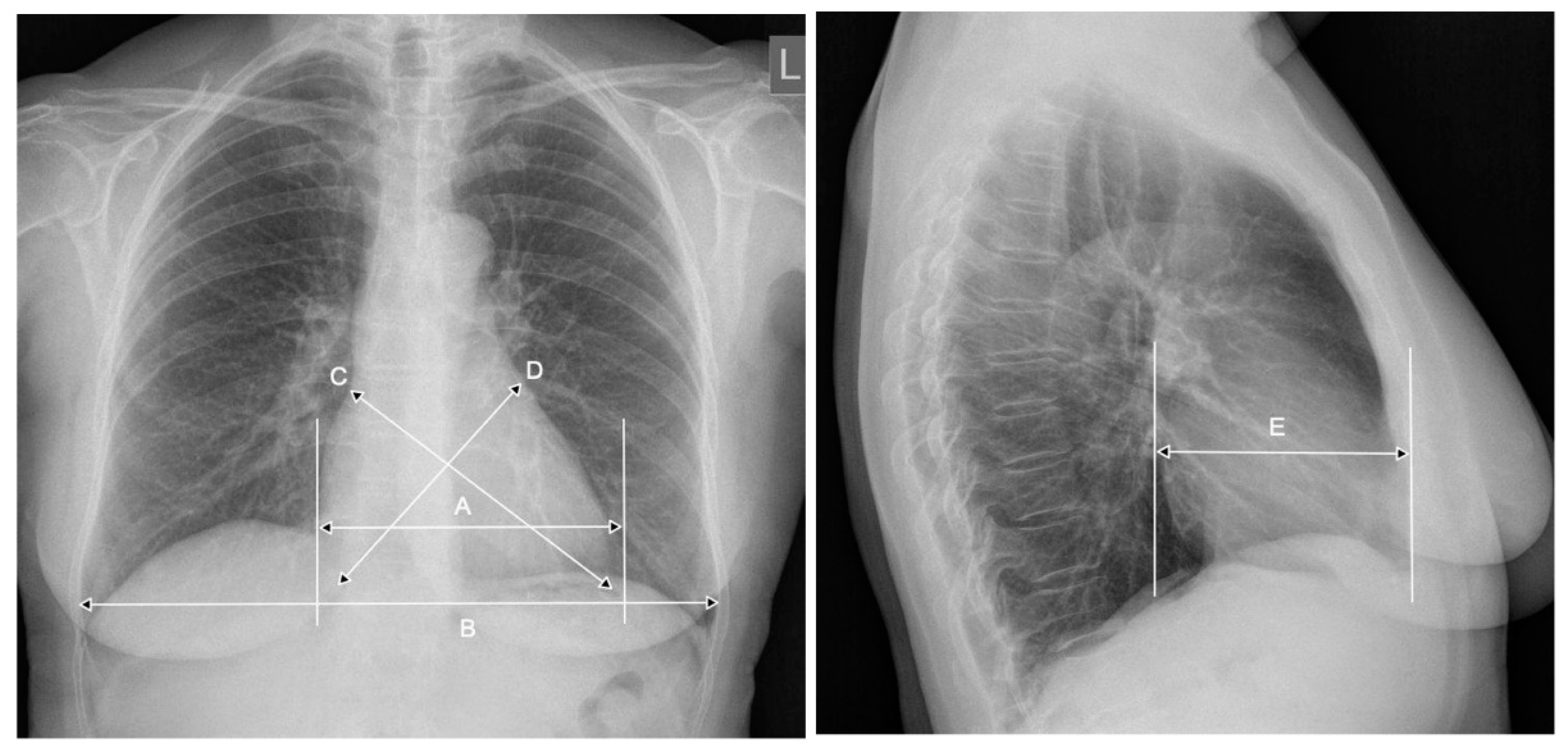
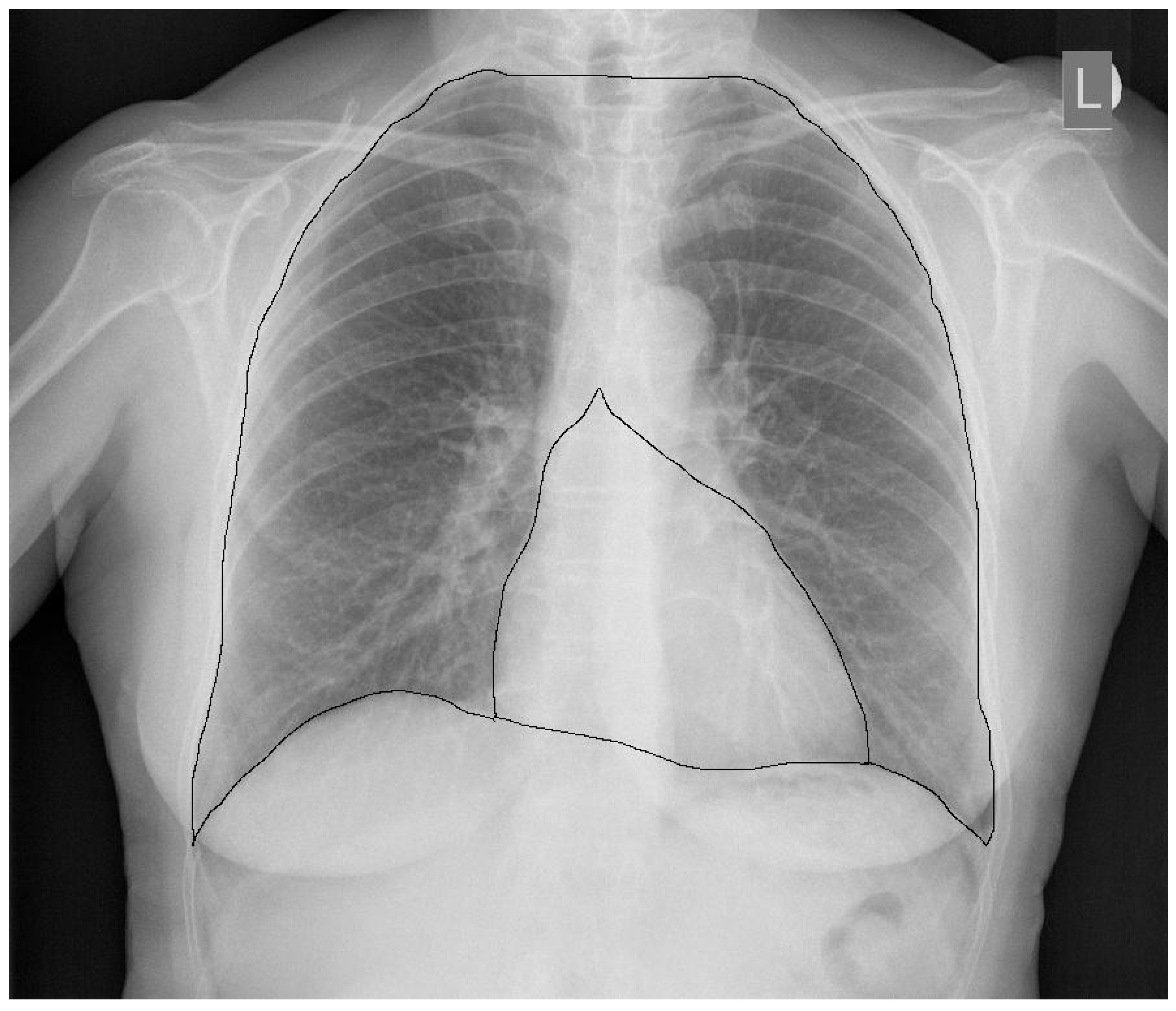
2. CTR—The Importance of Radiograph Projection
3. CTR—The Meaning of the Breathing Phase, the Anterior-Posterior Heart Dimension
4. CTR as a Prognostic Factor
5. CTR and Heart Function
6. CTR in the Pediatric Population
7. Post-Mortem Examinations—An Attempt to Evaluate CTR
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Danzer, C.S. The Cardiothoracic Ratio. Am. J. Med. Sci. 1919, 157, 513–554. [Google Scholar] [CrossRef]
- Nakayama, M.; Shibuya, A.; Inoue, R.; Kondo, Y. Automated measurement of cardiothoracic ratio using an R package. AMIA Annu. Symp. Proc. AMIA Symp. 2008, 6, 1064. [Google Scholar]
- Kearney, M.T.; Fox, K.A.; Lee, A.J.; Prescott, R.J.; Shah, A.M.; Batin, P.D.; Baig, W.; Lindsay, S.; Callahan, T.S.; Shell, W.E.; et al. Predicting death due to progressive heart failure in patients with mild-to-moderate chronic heart failure. J. Am. Coll. Cardiol. 2002, 40, 1801–1808. [Google Scholar] [CrossRef]
- Mensah, Y.B.; Mensah, K.; Asiamah, S.; Gbadamosi, H.; Idun, E.A.; Brakohiapa, W.; Oddoye, A. Establishing the cardiothoracic ratio using chest radiographs in an indigenous Ghanaian population: A simple tool for cardiomegaly screening. Ghana Med. J. 2015, 49, 159–164. [Google Scholar] [CrossRef] [PubMed]
- van der Jagt, E.J.; Smits, H.J. Cardiac size in the supine chest film. Eur. J. Radiol. 1992, 14, 173–177. [Google Scholar] [CrossRef]
- Milne, E.N.; Burnett, K.; Aufrichtig, D.; McMillan, J.; Imray, T.J. Assessment of cardiac size on portable chest films. J. Thorac. Imaging 1988, 3, 64–72. [Google Scholar] [CrossRef]
- Bontrager’s Handbook of Radiographic Positioning and Techniques, 9th ed.; Lampignano, J.P., Kendrick, L.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Chon, S.B.; Oh, W.S.; Cho, J.H.; Kim, S.S.; Lee, S.-J. Calculation of the Cardiothoracic Ratio from Portable Anteroposterior Chest Radiography. Korean Med. Sci. 2011, 26, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Kabala, J.E.; Wilde, P. The measurement of heart size in the antero-posterior chest radiograph. Br. J. Radiol. 1987, 60, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Yamashiro, T.; Matsuoka, S.; Matsushita, S.; Kurihara, Y.; Nakajima, Y. Changes in Cross-Sectional Area and Transverse Diameter of the Heart on Inspiratory and Expiratory Chest CT: Correlation with Changes in Lung Size and Influence on Cardiothoracic Ratio Measurement. PLoS ONE 2015, 10, e0131902. [Google Scholar] [CrossRef]
- Silverman, F.N. Heart. In Pediatric X-Ray Diagnosis, 7th ed.; Caffey, J., Ed.; Year Book Medical Publishers: Chicago, IL, USA, 1978; pp. 531–545. [Google Scholar]
- Dimopoulos, K.; Giannakoulas, G.; Bendayan, I.; Liodakis, E.; Petraco, R.; Diller, G.-P.; Piepoli, M.F.; Swan, L.; Mullen, M.; Best, N.; et al. Cardiothoracic ratio from postero-anterior chest radiographs: A simple, reproducible and independent marker of disease severity and outcome in adults with congenital heart disease. Int. J. Cardiol. 2013, 166, 453–457. [Google Scholar] [CrossRef]
- Kono, T.; Suwa, M.; Hanada, H.; Hirota, Y.; Kawamura, K. Clinical significance of normal cardiac silhouette in dilated cardiomyopathy. Evaluation Based Upon Echocardiography and Magnetic Resonance Imaging. Jpn. Circ. J. 1992, 56, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, H.; Shipley, M.; Christie, D.; Marmot, M. Cardiothoracic ratio and relative heart volume as predictors of coronary heart disease mortality. The Whitehall study 25 year follow-up. Eur. Heart J. 1998, 19, 859–869. [Google Scholar] [CrossRef]
- Yotsueda, R.; Taniguchi, M.; Tanaka, S.; Eriguchi, M.; Fujisaki, K.; Torisu, K.; Masutani, K.; Hirakata, H.; Kitazono, T.; Tsuruya, K. Cardiothoracic Ratio and All-Cause Mortality and Cardiovascular Disease Events in Hemodialysis Patients: The Q-Cohort Study. AJKD 2017, 70, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-J.; Wu, I.-W.; Hsu, K.-H.; Sun, C.-Y.; Chen, C.-Y.; Lee, C.-C. Vitamin D deficiency, cardiothoracic ratio, and long-term mortality in hemodialysis patients. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Ito, K.; Ookawara, S.; Ueda, Y.; Miyazawa, H.; Yamada, H.; Goto, S.; Ishii, H.; Shindo, M.; Kitano, T.; Hirai, K.; et al. A Higher Cardiothoracic Ratio Is Associated with 2-Year Mortality after Hemodialysis Initiation. Nephron Extra 2015, 5, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, W.-G.; Geng, Q.-S.; Du, G.; He, P.-C.; Feng, D.; Qin, T.-H.; Wei, X.B. The cardiothoracic ratio: A neglected preoperative risk-stratified method for patientswith rheumatic heart disease undergoing valve replacement surgery. Eur. J. Cardiothorac. Surg. 2018, 55, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Chana, H.S.; Martin, C.A.; Cakebread, H.E.; Adjei, F.D.; Gajendragadkar, P.R. Diagnostic accuracy of cardiothoracic ratio on admission chest radiography to detect left or right ventricular systolic dysfunction: A retrospective study. J. R. Soc. Med. 2015, 108, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Breur, J.M.; Cate, F.E.U.T.; Kapusta, L.; Cohen, M.I.; Crosson, J.E.; Boramanand, N.; Lubbers, L.J.; Friedman, A.H.; Brenner, J.I.; Vetter, V.L.; et al. Pacemaker therapy in isolated congenital complete atrioventricular block. Pacing Clin. Electrophysiol. 2002, 25, 1685–1691. [Google Scholar] [CrossRef]
- Browne, R.F.J.; O’Reilly, G.; McInerney, D. Extraction of the Two-Dimensional Cardiothoracic Ratio from Digital PA Chest Radiographs: Correlation with Cardiac Function and the Traditional Cardiothoracic Ratio. J. Digit. Imaging 2004, 17, 120–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlett, C.L.; Kwait, D.C.; Mahabadi, A.A.; Bamberg, F.; O’Donnell, C.J.; Fox, C.S.; Hoffmann, U. Simple area-based measurement for multidetector computed tomography to predict left ventricular size. Eur. Radiol. 2010, 20, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Giamouzis, G.; Sui, X.; Love, T.E.; Butler, J.; Young, J.B.; Ahmed, A. A Propensity-Matched Study of the Association of Cardiothoracic Ratio With Morbidity and Mortality in Chronic Heart Failure. Am. J. Cardiol. 2008, 101, 343–347. [Google Scholar] [CrossRef]
- Grotenhuis, H.B.; Zhou, C.; Tomlinson, G.; Isaac, K.V.; Seed, M.; Grosse-Wortmann, L.; Yoo, S.-J. Cardiothoracic ratio on chest radiograph in pediatric heart disease: How does it correlate with heart volumes at magnetic resonance imaging? Pediatr. Radiol. 2015, 45, 1616–1623. [Google Scholar] [CrossRef]
- Nakamura, T.; Noma, S. Follow-up of isolated congenital complete atrioventricular block with longitudinal measurements of serum NT-proBNP and cardiothoracic ratio. Fukushima J. Med. Sci. 2020, 66, 37–40. [Google Scholar] [CrossRef]
- Wanapirak, C.; Sirichotiyakul, S.; Luewan, S.; Srisupundit, K.; Tongprasert, F.; Tongsong, T. Appearance of Abnormal Cardiothoracic Ratio of Fetuses with Hemoglobin Bart’s Disease: Life Table Analysis. Ultraschall der Med. Eur. J. Ultrasound 2017, 38, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Emi, M.; Inamura, N. Cardiothoracic Area Ratio Predicts Lethal Pulmonary Venous Obstruction in Patients with Single Ventricle and Total Anomalous Pulmonary Venous Connection. Am. J. Perinatol. Rep. 2018, 8, e174–e179. [Google Scholar] [CrossRef]
- Jotterand, M.; Faouzi, M.; Dédouit, F.; Michaud, K. New formula for cardiothoracic ratio for the diagnosis of cardiomegaly on post-mortem CT. Int. J. Leg. Med. 2019, 134, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Jotterand, M.; Doenz, F.; Grabherr, S.; Faouzi, M.; Boone, S.; Mangin, P.; Michaud, K. The cardiothoracic ratio on post-mortem computer tomography. Int. J. Leg. Med. 2016, 130, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Francone, M.; Di Cesare, E.; Cademartiri, F.; Pontone, G.; Lovato, L.; Matta, G.; Secchi, F.; Maffei, E.; Pradella, S.; Carbone, I.; et al. Italian registry of cardiac magnetic resonance. Eur. J. Radiol. 2014, 83, e15–e22. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truszkiewicz, K.; Poręba, R.; Gać, P. Radiological Cardiothoracic Ratio in Evidence-Based Medicine. J. Clin. Med. 2021, 10, 2016. https://doi.org/10.3390/jcm10092016
Truszkiewicz K, Poręba R, Gać P. Radiological Cardiothoracic Ratio in Evidence-Based Medicine. Journal of Clinical Medicine. 2021; 10(9):2016. https://doi.org/10.3390/jcm10092016
Chicago/Turabian StyleTruszkiewicz, Krystian, Rafał Poręba, and Paweł Gać. 2021. "Radiological Cardiothoracic Ratio in Evidence-Based Medicine" Journal of Clinical Medicine 10, no. 9: 2016. https://doi.org/10.3390/jcm10092016
APA StyleTruszkiewicz, K., Poręba, R., & Gać, P. (2021). Radiological Cardiothoracic Ratio in Evidence-Based Medicine. Journal of Clinical Medicine, 10(9), 2016. https://doi.org/10.3390/jcm10092016






