The Role of Light Kappa and Lambda Chains in Heart Function Assessment in Patients with AL Amyloidosis
Abstract
1. Introduction
2. Materials and Methods
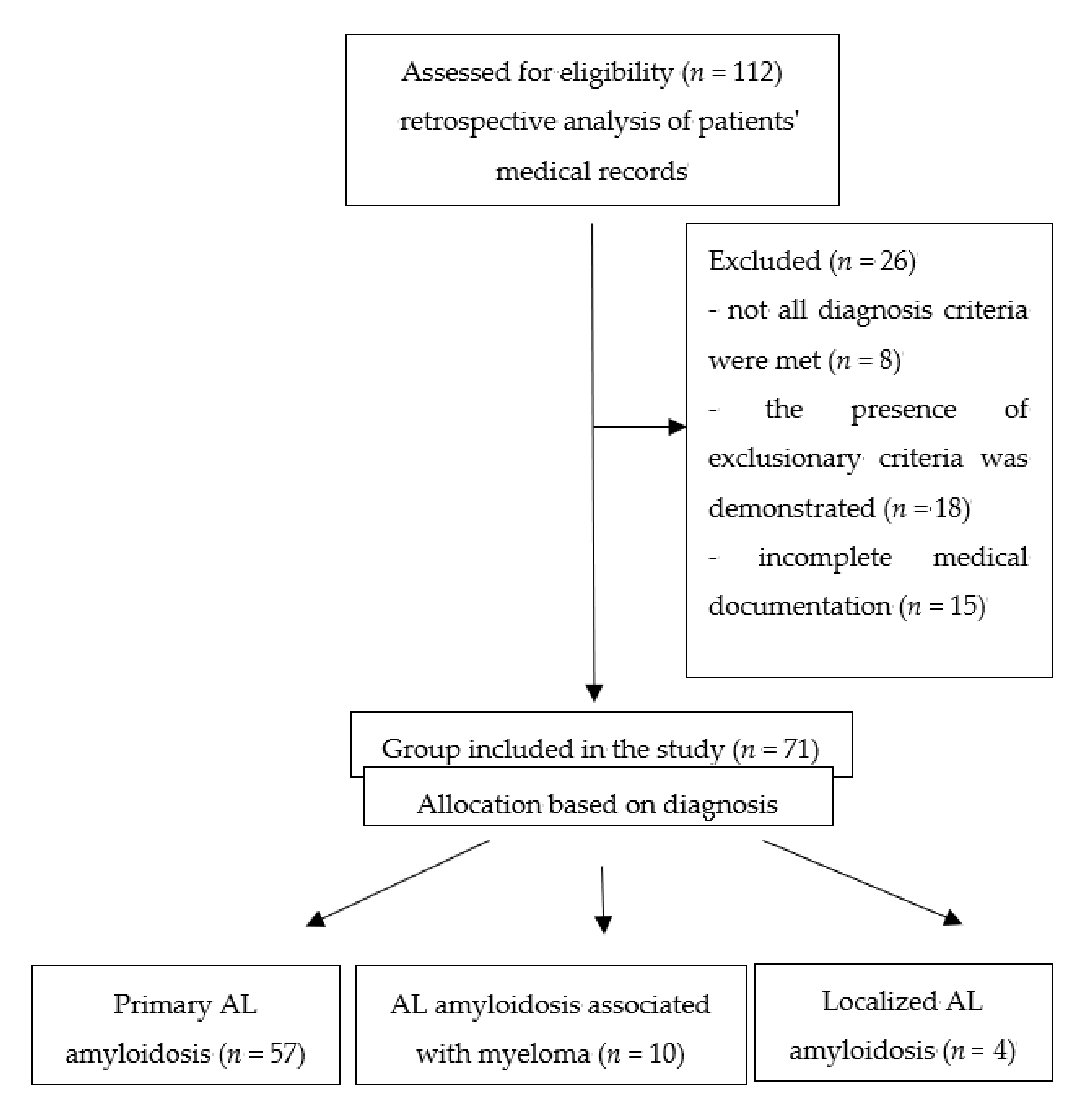
2.1. Study Group
- Amyloid presence confirmed by Congo Red staining of tissue biopsies (organ biopsy, adipose tissue, bone marrow, gingiva);
- Confirmation that amyloid is made of monoclonal immunoglobulin light chains (immunohistochemistry);
- Confirmation of the presence of plasmocyte dyscrasia (presence of monoclonal protein in the serum and/or urine, an incorrect value of the ratio of free κ and λ light chains in the serum, clonality of plasmocytes in bone marrow).
2.2. Laboratory Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dispenzieri, A.; Gertz, M.A.; Buadi, F. What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Rev. 2012, 26, 137–154. [Google Scholar] [CrossRef]
- Charliński, G.; Juryszczyn, A.; Jędrzejczak, W.W. Light chain amyloidosis–clinical symptoms, updated diagnosis, and treatment. Med. Rev. 2014, 2, 102–109. [Google Scholar]
- Dmoszyńska, A.; Walter-Croneck, A.; Pieńkowska-Grela, B. Recommendations of Polish Myeloma Group concerning diagnosis and therapy of multiple myeloma and other plasmacytic dyscrasias for 2017. Acta Haematol. Pol. 2016, 2, 39–85. [Google Scholar] [CrossRef]
- Charliński, G.; Jędrzejczak, W.W. Primary systemic light-chain amyloidosis: Diagnosis and treatment. In Multiple Myeloma and other Plasma Cell Dyscrasias; Dmoszyńska, A., Giannopoulous, K., Eds.; Czelej: Lublin, Poland, 2015. [Google Scholar]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef]
- Desport, E.; Bridoux, F.; Sirac, C.; Delbes, S.; Bender, S.; Fernandez, B.; Quellard, N.; Lacombe, C.; Goujon, J.-M.; Lavergne, D.; et al. AL Amyloidosis. Orphanet J. Rare Dis. 2012, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Selvanayagam, J.B.; Hawkins, P.N.; Paul, B.; Myerson, S.G.; Neubauer, S. Evaluation and management of the cardiac amyloidosis. J. Am. Coll. Cardiol. 2007, 50, 2101–2110. [Google Scholar] [CrossRef]
- Jurczyszyn, A.; Engel, A.; Rajzer, M.; Czepiel, J.; Mazur, G. Progress in the diagnosis and treatment of cardiac amyloidosis. Med. Rev. 2014, 71, 340–345. [Google Scholar]
- Guan, J.; Mishra, S.; Falk, R.H.; Liao, R. Current perspectives on cardiac amyloidosis. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H544–H552. [Google Scholar] [CrossRef]
- Shah, K.B.; Inoue, Y.; Mehra, M.R. Amyloidosis and the heart: A comprehensive review. Arch. Intern. Med. 2006, 166, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.H. Diagnosis and management of the cardiac amyloidoses. Circulation 2005, 112, 2047–2060. [Google Scholar] [CrossRef]
- Kumar, S.; Dispenzieri, A.; Katzmann, J.A. Serum immunoglobulin free light chain measurement in AL amyloidosis: Prognostic value and correlations with clinical features. Blood 2010, 116, 5126–5129. [Google Scholar] [CrossRef]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.P.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef]
- Gertz, M.A.; Merlini, G. Definition of organ involvement and response to treatment in AL amyloidosis: An updated consensus opinion. Amyloid 2010, 17, 48–49. [Google Scholar]
- Bochtler, T.; Hegenbart, U.; Heiss, C.; Benner, A.; Cremer, F.; Volkmann, M.; Ludwig, J.; Perz, J.B.; Ho, A.D.; Goldschmidt, H.; et al. Evaluation of the serum-free light chain test in untreated patients with AL amyloidosis. Haematologica 2008, 93, 459–462. [Google Scholar] [CrossRef]
- Snozek, C.L.; Katzmann, J.A.; Kyle, R.A.; Dispenzieri, A.; Larson, D.R.; Therneau, T.M.; Melton, L.J.; Kumar, S.; Greipp, P.R.; Clark, R.J.; et al. Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: Proposed incorporation into the international staging system. Leukemia 2008, 22, 1933–1937. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A. Clinical and laboratoty features in 474 cases. Semin. Hematol. 1995, 32, 45–59. [Google Scholar]
- Merlini, G.; Sanchorawala, V.; Zonder, J.A.; Kukreti, V.; Schonland, O.S.; Jaccard, A.; Dispenzieri, A.; Cohen, A.D.; Berg, R.D.; Liu, G.; et al. MLN9708, a novel, investigational oral proteasome inhibitor, in patients with relapsed or refractory light chain amyloidosis (AL): Results of a phase 1 study. Blood 2012, 120, 731. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, S.P.; Kim, Y.J.; Sohn, D.W. Incidence, Diagnosis and Prognosis of Cardiac Amyloidosis. Korean Circ. J. 2013, 43, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Q.; Jiang, S.; Chen, W.; Zeng, C.; Liu, Z. The clinical features and outcomes of systemic AL amyloidosis: A cohort of 231 Chinese patients. Clin. Kidney J. 2015, 8, 120–126. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Gertz, M.A.; Kumar, S.K.; Lacy, M.Q.; Kyle, A.R.; Saenger, A.K.; Grogan, M.; Zeldenrust, S.R.; Hayman, S.R.; Buadi, F.; et al. High sensitivity cardiac troponin T in patients with immunoglobulin light chain amyloidosis. Heart 2014, 100, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Lacy, M.Q.; Katzmann, J.A.; Rajkumar, S.V.; Abraham, R.S.; Hayman, S.R.; Kumar, S.K.; Clark, R.; Kyle, R.A.; Litzow, M.R.; et al. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood 2006, 107, 3378–3383. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Merlini, G.; Quarta, C.C.; Riva, L.; Longhi, S.; Leone, O.; Salvi, F.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; et al. Systemic cardiac amyloidosis: Disease profiles and clinical courses of the 3 main types. Circulation 2009, 120, 1203–1212. [Google Scholar] [CrossRef]
- Palladini, G.; Lavatelli, F.; Russo, P.; Perlini, S.; Perfetti, V.; Bosoni, T.; Obici, L.; Bradwell, A.R.; D’Eril, G.M.; Fogari, R.; et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood 2006, 107, 3854–3858. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.A.; Jain, M.; Pimentel, D.R.; Wang, B.; Connors, L.H.; Skinner, M.; Apstein, C.S.; Liao, R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ. Res. 2004, 94, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guan, J.; Jiang, B.; Brenner, D.A.; Del Monte, F.; Ward, J.E.; Connors, L.H.; Sawyer, D.B.; Semigran, M.J.; MacGillivray, T.E.; et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4188–4193. [Google Scholar] [CrossRef] [PubMed]
- Sikkink, L.A.; Ramirez-Alvarado, M. Cytotoxicity of amyloidogenic immunoglobulin light chain in cell culture. Cell Death Dis. 2010, 1, e98. [Google Scholar] [CrossRef]
| Organ | Criteria for Organ Involvement |
|---|---|
| Heart | Echocardiographic examination: cardiac wall thickness >12 mm, excluding any other cardiological causes |
| Kidney | Daily urine collection: proteinuria >0.5 g/day (predominantly albuminuria) |
| Liver | Total liver size >15 cm, excluding accompanying heart failure, or alkaline phosphatase activity >1.5 upper reference range |
| Autonomic nervous system | Abnormal gastric peristalsis, micturition disorders, or pseudoconstipation (excluding other organic causes) |
| Peripheral nervous system | Clinical symptoms: sensorimotor, symmetrical, peripheral neuropathy of the lower extremities |
| Gastrointestinal tract | Direct biopsy confirmation of the presence of amyloid deposits in conjunction with clinical symptoms |
| Lung | Biopsy confirmation of the presence of amyloids in conjunction with clinical symptoms; X-ray shows interstitial lesions in the lungs |
| Soft tissue | Tongue enlargement, intermittent claudication due to the presence of amyloids in the vessels, arthropathy, carpal tunnel syndrome, biopsy, myopathy or lymphadenopathy, skin lesions |
| Study Group 57 (80%) Primary Systemic Light Chain Amyloidosis 10 (14%) Primary Systemic Light Chain Amyloidosis Accompanying Myeloma 4 (6%) Localized Primary Light Chain Amyloidosis |
|---|
| Amyloid structure |
| 43 (61%) λ chains |
| 28 (39%) ĸ chains |
| Amyloid location by number of sites |
| 22 (31%) one organ |
| 28 (40%) two organs |
| 18 (25%) three or more organs |
| Amyloid location in organs |
| 51 (72%) heart |
| 41 (58%) kidneys |
| 20 (28%) gastrointestinal tract |
| 10 (14%) liver |
| 3 (4%) bone marrow or in adipose tissue only |
| 14 (20%) others (bronchi, lungs, peripheral nervous system, nasopharynx, tongue, skin) |
| Involved Chain | All Me (Q25–75) | Λ Me (Q25–75) | Κ Me (Q25–75) | p-Value |
|---|---|---|---|---|
| n | 71 | 43 | 28 | |
| Age (years) | 57 (54–65) | 60 (56–64) | 55 (51–67) | 0.003 |
| Plasmocytes (%) | 11 (6–15) | 10 (5–15) | 15 (6–15) | 0.084 |
| TnI (ng/mL) | 0.019 (0.000–0.072) | 0.030 (0.002–0.100) | 0.010 (0.000–0.045) | 0.002 |
| NT-proBNP (pg/mL) | 1958 (362–7393) | 2705 (731–8170) | 657 (286–6247) | <0.001 |
| LV (mm) | 45 (41–50) | 45 (40–51) | 45 (40–50) | 0.759 |
| IVS (mm) | 13 (11–14) | 13 (11–14) | 12 (11–14) | 0.309 |
| PW (mm) | 12 (11–14) | 13 (11–15) | 12 (10–14) | 0.246 |
| RV (mm) | 30 (27–33) | 29 (26–35) | 30 (28–32) | 0.589 |
| EF (%) | 60 (55–65) | 60 (55–64) | 61 (55–65) | 0.203 |
| κ (mg/L) | 28.7 (16.0–70.2) | 22.5 (15.3–45.5) | 41.7 (21.1–103.5) | <0.001 |
| λ (mg/L) | 24.8 (15.1–63.9) | 42.6 (21.4–99.2) | 16.7 (11.8- 24.8) | <0.001 |
| dFLC (mg/L) | 12.2 (−0.3–50.7) | 4.3 (−6.8–48.1) | 17.3 (5.2–51.8) | <0.001 |
| κ/λ | 1.21 (0.62–1.94) | 0.80 (0.32–1.30) | 1.76 (1.29–2.80) | <0.001 |
| κ/λ | <0.26 Me (Q25–75) | >1.65 Me (Q25–75) | 0.26–1.65 Me (Q25–75) | p-Value |
|---|---|---|---|---|
| n | 51 | 146 | 259 | |
| Age (years) | 58 (49–65) | 59 (54–63) | 56 (51–64) | 0.667 |
| Plasmocytes (%) | 20 (12–25) | 15 (7–15) | 10 (5–15) | <0.001 |
| TnI (ng/mL) | 0.092 (0.036–0.200) | 0.006 (0.000–0.030) | 0.002 (0.000–0.039) | <0.001 |
| NT-proBNP (pg/mL) | 0.092 (0.036–0.200) | 0.006 (0.000–0.030) | 0.002 (0.000–0.039) | <0.001 |
| κ (mg/L) | 14.5 (10.2–18.3) | 76.2 (39.9–148.6) | 21.9 (13.6–40.3) | <0.001 |
| λ (mg/L) | 188.7 (112.1–280.5) | 21.2 (15.1–30.3) | 23.1 (13.9–59.9) | <0.001 |
| dFLC | 160.2 (92.3–270.0) | 23.0 (−13.4–79.3) | 3.4 (−1.2–18.6) | <0.001 |
| κ/λ | 0.10 (0.04–0.15) | 2.47 (1.98–5.08) | 0.98 (0.64–1.30) | <0.001 |
| LV (mm) | 44 (40–51) | 47 (44–52) | 46 (43–48) | 0.338 |
| IVS (mm) | 14 (13–14) | 13 (12–13) | 12 (11–14) | 0.028 |
| PW (mm) | 13 (12–15) | 13 (12–14) | 12 (10–14) | 0.158 |
| RV (mmm) | 31 (27–33) | 31 (28–32) | 26 (25–30) | 0.063 |
| EF (%) | 59 (41–60) | 60 (55–63) | 60 (55–65) | 0.318 |
| dFLC [mg/L] | ≥180 Me (Q25–75) | <180 Me (Q25–75) | p-Value |
|---|---|---|---|
| Age (years) | 59 (50–66) | 56 (52–64) | 0.425 |
| Plasmocytes (%) | 11.5 (8.0–20.0) | 12.0 (6.0–15.0) | 0.083 |
| TnI (ng/mL) | 0.079 (0.035–0.297) | 0.003 (0.000–0.039) | <0.001 |
| NT-proBNP (pg/mL) | 4425 (724–10577) | 1602 (337–4466) | 0.014 |
| κ (mg/L) | 36.1 (11.8–613.3) | 28.9 (16.3–63.7) | 0.153 |
| λ (mg/L) | 219.4 (10.7–312.8) | 24.4 (15.5–59.9) | 0.007 |
| κ “i” | 616.3 (520.8–775.3) | 36.3 (18.7–80.2) | <0.001 |
| λ “i” | 289.3 (232.3–617.3) | 36.5 (20.4–73.8) | <0.001 |
| κ “ui” | 12.2 (10.3–16.9) | 25.0 (16.1–46.8) | <0.001 |
| λ “ui” | 9.2 (8.1–14.9) | 17.8 (12.7–25.5) | <0.001 |
| dFLC (mg/L) | 538.8 (236.7–682.4) | 8.4 (−1.5–35.4) | <0.001 |
| κ/λ | 0.095 (0.04–53.5) | 1.23 (0.75–1.90) | 0.224 |
| LV (mm) | 45 (43–51) | 46 (41–51) | 0.849 |
| IVS (mm) | 14.0 (13–16) | 12 (11–13) | 0.019 |
| PW (mm) | 14 (13–16) | 12 (11–14) | 0.020 |
| RV (mm) | 32 (30–35) | 28 (26–32) | 0.070 |
| EF (%) | 56 (41–65) | 60 (55–64) | 0.312 |
| Stage | I Me (Q25–75) | II Me (Q25–75) | III Me (Q25–75) | IV Me (Q25–75) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 56 (49–60) | 56 (55–64) | 61 (44–65) | 57 (53–61) | 0.382 |
| lasmocytes (%) | 10 (6–15) | 12 (5–20) | 12 (6–25) | 15 (12–20) | 0.050 |
| TnI (ng/mL) | 0.000 | 0.034 (0.009–0.043) | 0.170 (0.086–0.434) | 0.249 (0.120–0.734) | <0.001 |
| NT-proBNP (pg/mL) | 313 (98–543) | 2960 (1986–7820) | 7401 (2985–10500) | 10931 (7849–13773) | <0.001 |
| κ (mg/L) | 18.1 (11.7–39.3) | 48.2 (18.5–130.2) | 25.5 (17.2–42.9) | 11.3 (9.8–16.7) | <0.001 |
| λ (mg/L) | 17.3 (13.1–21.9) | 47.3 (24.3–86.9) | 65.3 (44.2–116.0) | 363.2 (230.1–617.3) | <0.001 |
| dFLC (mg/L) | 3.39 (0.74–23.74) | 36.69 (1.0–104.81) | 49.41 (31.31–100.31) | 349.50 (211.36–606.34) | <0.001 |
| κ/λ | 1.18 (0.86–1.94) | 1.17 (0.40–2.66) | 0.48 (0.26–1.21) | 0.04 (0.02–0.07) | <0.001 |
| κ “i” (mg/L) | 33.6 (11.2–49.5) | 130.4 (96.0–182.5) | 26.7 (21.3–140.3) | - | <0.001 |
| λ “ui” (mg/L) | 16.4 (11.3–20.5) | 22.8 (16.0–46.2) | 22.1 (20.3–63.3) | - | 0.003 |
| κ “ui” (mg/L) | 16.7 (12.1–23.7) | 27.4 (16.8–77.3) | 21.6 (12.6–42.9) | 11.3 (9.8–16.7) | 0.002 |
| λ “i” | 18.8 (13.8–26.8) | 63.8 (37.5–105.3) | 95.2 (65.2–162.4) | 363.2 (230.1–617.3) | <0.001 |
| LV (mm) | 44 (44–47) | 45 (40–50) | 37 (36–47) | 48 (41–52) | 0.556 |
| IVS (mm) | 12 (11–12) | 13 (11–14) | 15 (11–17) | 14 (13–17) | 0.015 |
| PW (mm) | 12 (10–12) | 12 (11–14) | 14 (13–14) | 15 (13–16) | 0.010 |
| RV (mm) | 30 (25–32) | 27 (27–28) | 26 (26–27) | 33 (32–35) | 0.038 |
| EF (%) | 60 (55–63) | 60 (59–65) | 59 (50–60) | 39 (28–56) | 0.025 |
| Diagnosis | AL Amyloidosis | AL Amyloidosis Associated with Myeloma | Localized AL Amyloidosis | |
|---|---|---|---|---|
| Me (Q25–75) | Me (Q25–75) | Me (Q25–75) | p-Value | |
| Age (years) | 56 (54–64) | 63 (41–65) | 70 (44–70) | <0.001 |
| Plasmocytes (%) | 10 (5–15) | 15 (15–25) | 6 (6–8) | <0.001 |
| TnI (ng/mL) | 0.021 (0.000–0.076) | 0.039 (0.007–0.083) | 0.000 (0.000–0.000) | <0.001 |
| NT-proBNP (pg/mL) | 1932 (382–7401) | 4499 (1958–8711) | 106 (88–271) | <0.001 |
| κ (mg/L) | 29.83 (16.18–75.70) | 29.27 (18.46–65.65) | 12.62 (9.95–24.55) | 0.009 |
| λ (mg/L) | 23.83 (15.10–61.77) | 47.34 (23.39–112.06) | 13.98 (12.26–15.13) | <0.001 |
| κ “i” | 43.95 (21.78–103.00) | 562.40 (429.60–787.00) | NA | <0.001 |
| λ “i” | 39.51 (21.35–95.06) | 53.10 (26.17–113.13) | NA | 0.397 |
| κ “ui” | 22.02 (14.62–45.73) | 25.32 (17.46–48.16) | NA | 0.204 |
| λ “ui” | 18.26 (11.39–25.51) | 15.50 (13.40–47.34) | NA | 0.132 |
| dFLC (mg/L) | 13.85 (0.22–48.73) | 8.42 (−2.40–102.50) | NA | 0.008 |
| κ/λ | 1.32 (0.75–2.02) | 0.87 (0.31–1.34) | 0.94 (0.80–1.64) | <0.001 |
| LV (mm) | 46 (43–50) | 42 (40–46) | 50 (43–55) | 0.087 |
| IVS (mm) | 13 (11–14) | 14 (13–17) | 10 (8–11) | 0.162 |
| PW (mm) | 12 (11–14) | 15 (12–15) | 9 (8–10) | 0.029 |
| RV (mm) | 30 (27–35) | 28 (27–32) | 28 (26–30) | 0.319 |
| EF (%) | 60 (56–65) | 60 (55–64) | 55 (55–60) | 0.517 |
| Variable | Effect | SE | 95% CI | p | R2 | Beta |
|---|---|---|---|---|---|---|
| TnI | 0.003 | 0.130 | ||||
| λ | 0.0005 | 0.0001 | 28.01 to 54.59 | 0.003 | 0.360 | |
| NT pro-BNP | <0.001 | 0.340 | ||||
| λ | 41.302 | 6.708 | 28.01 to 54.59 | <0.001 | 0.475 | |
| eGFR | –63.396 | 32.849 | –128.48 to 1.69 | 0.042 | –0.171 | |
| κ | 114.051 | 37.495 | 39.76 to 188.34 | 0.003 | 0.270 |
| Variable | Effect | SE | 95% CI | p | R2 | Beta |
|---|---|---|---|---|---|---|
| NT pro-BNP | <0.001 | 0.370 | ||||
| λ | 204.254 | 31.603 | 141.24 to 267.269 | <0.001 | 0.609 |
| Monoclonal λ Chains | Monoclonal κ Chains | |||||||
|---|---|---|---|---|---|---|---|---|
| κ | λ | dFLC | κ/λ | κ | λ | dFLC | κ/λ | |
| LV | 0.280 | −0.238 | −0.438 * | 0.431 * | −0.051 | −0.018 | −0.113 | −0.188 |
| IVS | −0.358 | 0.496 * | 0.434 * | −0.465 * | 0.111 | 0.211 | −0.193 | −0.247 |
| PW | −0.060 | 0.323 | 0.092 | −0.132 | 0.016 | 0.333 | −0.202 | −0.244 |
| EF | 0.297 | −0.107 | −0.077 | 0.130 | −0.052 | 0.196 | −0.063 | −0.128 |
| TnI | −0.278 | 0.688 * | 0.669 * | −0.694 * | −0.215 | 0.215 | −0.269 | −0.398 * |
| NTpro-BNP | 0.263 * | 0.449 * | 0.053 | −0.080 | 0.324 * | 0.695 * | −0.027 | −0.314 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czyżewska, E.; Wiśniewska, A.; Waszczuk-Gajda, A.; Ciepiela, O. The Role of Light Kappa and Lambda Chains in Heart Function Assessment in Patients with AL Amyloidosis. J. Clin. Med. 2021, 10, 1274. https://doi.org/10.3390/jcm10061274
Czyżewska E, Wiśniewska A, Waszczuk-Gajda A, Ciepiela O. The Role of Light Kappa and Lambda Chains in Heart Function Assessment in Patients with AL Amyloidosis. Journal of Clinical Medicine. 2021; 10(6):1274. https://doi.org/10.3390/jcm10061274
Chicago/Turabian StyleCzyżewska, Emilia, Agnieszka Wiśniewska, Anna Waszczuk-Gajda, and Olga Ciepiela. 2021. "The Role of Light Kappa and Lambda Chains in Heart Function Assessment in Patients with AL Amyloidosis" Journal of Clinical Medicine 10, no. 6: 1274. https://doi.org/10.3390/jcm10061274
APA StyleCzyżewska, E., Wiśniewska, A., Waszczuk-Gajda, A., & Ciepiela, O. (2021). The Role of Light Kappa and Lambda Chains in Heart Function Assessment in Patients with AL Amyloidosis. Journal of Clinical Medicine, 10(6), 1274. https://doi.org/10.3390/jcm10061274







