Identification of Potential Bisphenol A (BPA) Exposure Biomarkers in Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis
2.1.1. Data Availability
2.1.2. Functional Analysis
2.1.3. Immunohistochemistry (IHC)
2.1.4. Statistical Analysis
3. Results
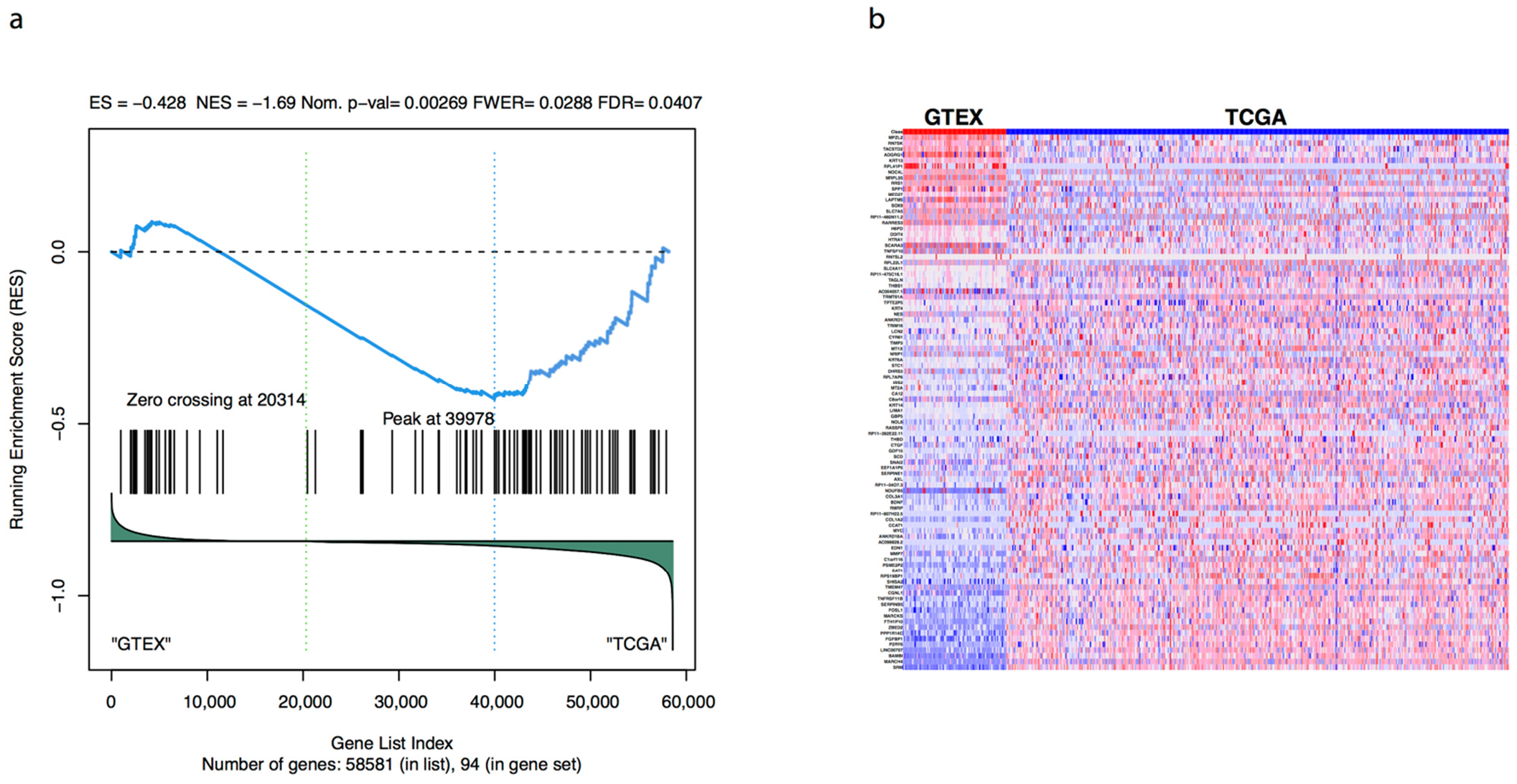
3.1. Transcriptional and Functional Characterisation
3.2. Evaluation of Prognosis and Diagnosis Potential
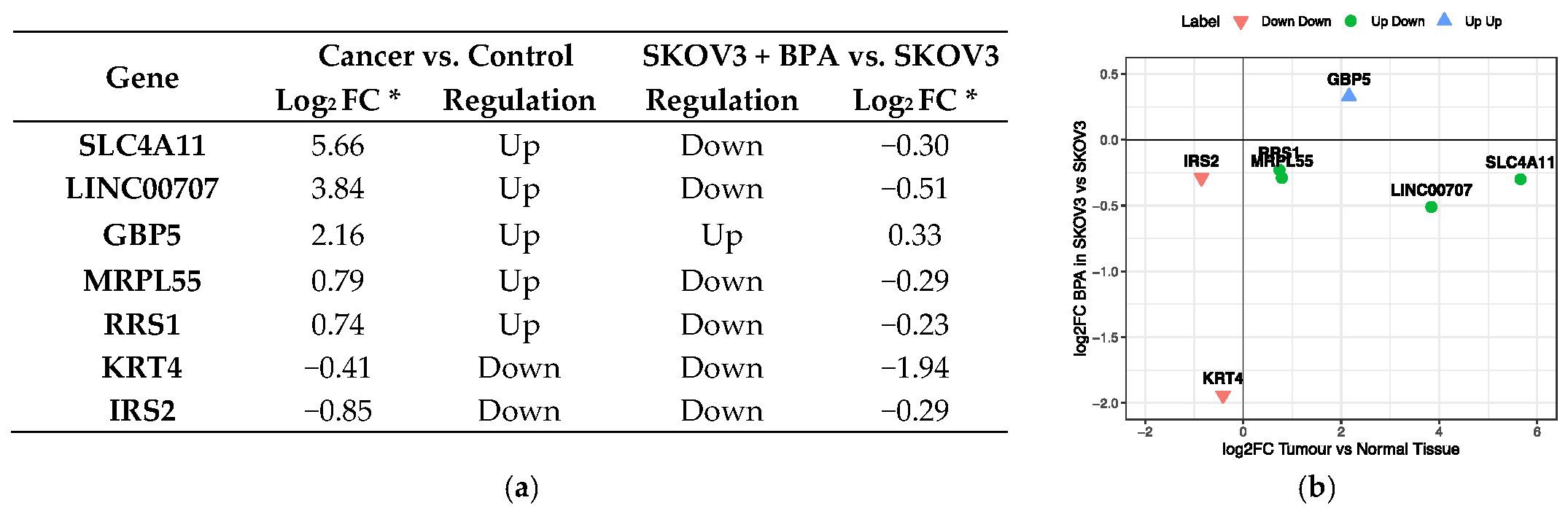
3.3. BPA Effect on Gene Function and Activity
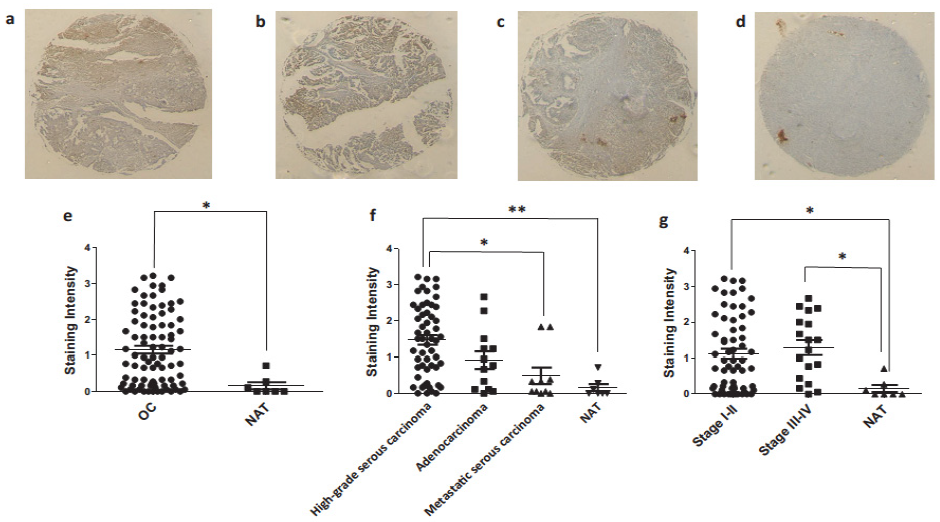
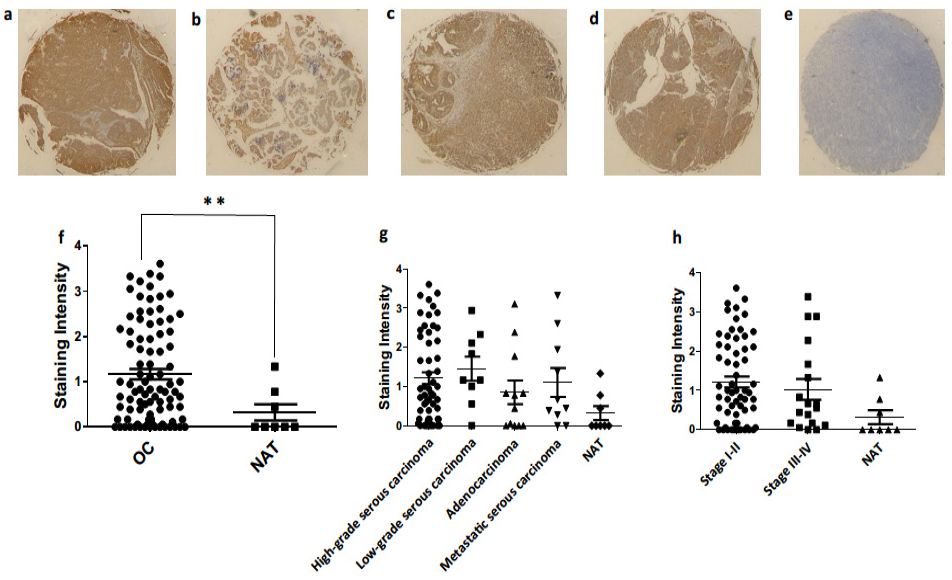
3.4. Ovarian Cancer Immunohistochemistry Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Liu, S. Low-Dose Bisphenol A Exposure: A Seemingly Instigating Carcinogenic Effect on Breast Cancer. Adv. Sci. 2016, 4, 1600248. [Google Scholar] [CrossRef] [PubMed]
- Global Bisphenol A Market Report 2018: Analysis 2013–2017 & Forecasts 2018–2023. Available online: https://www.prnewswire.com/news-releases/global-bisphenol-a-market-report-2018-analysis-2013-2017--forecasts-2018-2023-300757673.html (accessed on 31 August 2020).
- Alavian-Ghavanini, A.; Lin, P.-I.; Lind, P.M.; Rimfors, S.R.; Lejonklou, M.H.; Dunder, L.; Tang, M.; Lindh, C.; Bornehag, C.-G.; Rüegg, J. Prenatal Bisphenol A Exposure is Linked to Epigenetic Changes in Glutamate Receptor Subunit Gene Grin2b in Female Rats and Humans. Sci. Rep. 2018, 8, 11315. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Myers, J.P.; Shioda, T.; Soto, A.M.; Saal, F.S.V.; et al. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod. Toxicol. 2013, 38, 1–15. [Google Scholar] [CrossRef]
- Ottawa, C. Toxicological and Health Aspects of Bisphenol A Report of Joint FAO/WHO Expert Meeting and Report of Stakeholder Meeting on Bisphenol A Food and Agriculture Organization of the United Nations. Available online: www.who.int (accessed on 9 December 2020).
- Artacho-Cordón, F.; Fernández, M.; Frederiksen, H.; Iribarne-Durán, L.; Jiménez-Díaz, I.; Vela-Soria, F.; Andersson, A.; Martin-Olmedo, P.; Peinado, F.; Olea, N.; et al. Environmental phenols and parabens in adipose tissue from hospitalized adults in Southern Spain. Environ. Int. 2018, 119, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, K.; Park, J.; Moon, H.-B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.-J.; Eun, S.-H.; Kim, G.-H.; et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother–neonate pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar] [CrossRef]
- Zbucka-Krętowska, M.; Łazarek, U.; Miltyk, W.; Sidorkiewicz, I.; Pierzyński, P.; Milewski, R.; Wołczyński, S.; Czerniecki, J. Simultaneous analysis of bisphenol A fractions in maternal and fetal compartments in early second trimester of pregnancy. J. Périnat. Med. 2019, 47, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Tateoka, Y. Bisphenol A Concentration in Breast Milk following Consumption of a Canned Coffee Drink. J. Hum. Lact. 2014, 31, 474–478. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Schantz, S.L. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ. Epigenetics 2018, 4, dvy022. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chen, Z.-J.; Zhang, K.-S.; Yang, X.-L.; Wu, Y.-M.; Chen, X.-H.; Huang, H.-B.; Liu, H.-L.; Cai, S.-H.; Du, J.; et al. The activation of G protein-coupled receptor 30 (GPR30) inhibits proliferation of estrogen receptor-negative breast cancer cells in vitro and in vivo. Cell Death Dis. 2014, 5, e1428. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Kim, T.-H.; Lee, H.-H. G-protein Coupled Estrogen Receptor (GPER/GPR30) and Women’s Health. J. Menopausal Med. 2015, 21, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Xuan, J.; Liu, Y.; Shi, G. Function of G-Protein-Coupled Estrogen Receptor-1 in Reproductive System Tumors. J. Immunol. Res. 2016, 2016, 7128702. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Prossnitz, E.R. GPER/GPR30 Knockout Mice: Effects of GPER on Metabolism. In Methods in Molecular Biology; Humana Press Inc.: Tortowa, NJ, USA, 2016; Volume 1366, pp. 489–502. [Google Scholar]
- Alonso-Magdalena, P.; Laribi, O.; Ropero, A.B.; Fuentes, E.; Ripoll, C.; Soria, B.; Nadal, A. Low Doses of Bisphenol A and Diethylstilbestrol Impair Ca2+ Signals in Pancreatic α-Cells through a Nonclassical Membrane Estrogen Receptor within Intact Islets of Langerhans. Environ. Health Perspect. 2005, 113, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Ropero, A.B.; Laribi, O.; Maillet, M.; Fuentes, E.; Soria, B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc. Natl. Acad. Sci. USA 2000, 97, 11603–11608. [Google Scholar] [CrossRef] [PubMed]
- Delfosse, V.; Grimaldi, M.; Pons, J.-L.; Boulahtouf, A.; Le Maire, A.; Cavailles, V.; Labesse, G.; Bourguet, W.; Balaguer, P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. USA 2012, 109, 14930–14935. [Google Scholar] [CrossRef]
- Ali, S.; Steinmetz, G.; Montillet, G.; Perrard, M.-H.; Loundou, A.; Durand, P.; Guichaoua, M.-R.; Prat, O. Exposure to Low-Dose Bisphenol A Impairs Meiosis in the Rat Seminiferous Tubule Culture Model: A Physiotoxicogenomic Approach. PLoS ONE 2014, 9, e106245. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Galbiati, V.; Maddalon, A.; Iulini, M.; Kenda, M.; Dolenc, M.S.; Marinovich, M.; Racchi, M.; Corsini, E. Effect of estrogen-active compounds on the expression of RACK1 and immunological implications. Arch. Toxicol. 2020, 94, 2081–2095. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Racchi, M.; Corsini, E. Endocrine-Disrupting Chemicals’ (EDCs) Effects on Tumour Microenvironment and Cancer Progression: Emerging Contribution of RACK1. Int. J. Mol. Sci. 2020, 21, 9229. [Google Scholar] [CrossRef]
- Huang, W.; Ai, W.; Lin, W.; Fang, F.; Wang, X.; Huang, H.; Dahlgren, R.A.; Wang, H. Identification of receptors for eight endocrine disrupting chemicals and their underlying mechanisms using zebrafish as a model organism. Ecotoxicol. Environ. Saf. 2020, 204, 111068. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Wen, X.; Xiong, Y.; Jin, L.; Zhang, M.; Huang, L.; Mao, Y.; Zhou, C.; Qiao, Y.; Zhang, Y. Bisphenol A Exposure Enhances Endometrial Stromal Cell Invasion and Has a Positive Association with Peritoneal Endometriosis. Reprod. Sci. 2020, 27, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, E.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. The Impact of Bisphenol A on Fertility, Reproductive System, and Development: A Review of the Literature. Int. J. Endocrinol. 2019, 2019, 4068717. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Rak, A.; Ptak, A. Bisphenol A and its derivatives decrease expression of chemerin, which reverses its stimulatory action in ovarian cancer cells. Toxicol. Lett. 2018, 291, 61–69. [Google Scholar] [CrossRef]
- Hui, L.; Li, H.; Lu, G.; Chen, Z.; Sun, W.; Shi, Y.; Fu, Z.; Huang, B.; Zhu, X.; Lu, W.; et al. Low Dose of Bisphenol A Modulates Ovarian Cancer Gene Expression Profile and Promotes Epithelial to Mesenchymal Transition via Canonical Wnt Pathway. Toxicol. Sci. 2018, 164, 527–538. [Google Scholar] [CrossRef]
- Caserta, D.; Di Segni, N.; Mallozzi, M.; Giovanale, V.; Mantovani, A.; Marci, R.; Moscarini, M. Bisphenol a and the female reproductive tract: An overview of recent laboratory evidence and epidemiological studies. Reprod. Biol. Endocrinol. 2014, 12, 37. [Google Scholar] [CrossRef]
- Ovarian Cancer Incidence Statistics. Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer/incidence#heading-Zero (accessed on 31 August 2020).
- Menon, U.; McGuire, A.J.; Raikou, M.; Ryan, A.; Davies, S.K.; Burnell, M.; Gentry-Maharaj, A.; Kalsi, J.K.; Singh, N.; Amso, N.N.; et al. The cost-effectiveness of screening for ovarian cancer: Results from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Br. J. Cancer 2017, 117, 619–627. [Google Scholar] [CrossRef]
- Susiarjo, M.; Hassold, T.J.; Freeman, E.; Hunt, P.A. Bisphenol A Exposure in Utero Disrupts Early Oogenesis in the Mouse. PLoS Genet. 2007, 3, e5. [Google Scholar] [CrossRef]
- Hwang, K.-A.; Park, S.-H.; Yi, B.-R.; Choi, K.-C. Gene Alterations of Ovarian Cancer Cells Expressing Estrogen Receptors by Estrogen and Bisphenol A Using Microarray Analysis. Lab. Anim. Res. 2011, 27, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ptak, A.; Hoffmann, M.; Gruca, I.; Barć, J. Bisphenol A induce ovarian cancer cell migration via the MAPK and PI3K/Akt signalling pathways. Toxicol. Lett. 2014, 229, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Ptak, A.; Wróbel, A.; Gregoraszczuk, E.L. Effect of bisphenol-A on the expression of selected genes involved in cell cycle and apoptosis in the OVCAR-3 cell line. Toxicol. Lett. 2011, 202, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Craft, B.; Brooks, A.; Zhu, J.; Haussler, D. The UCSC Xena platform for public and private cancer genomics data visualization and interpretation. bioRxiv 2018, 326470. [Google Scholar] [CrossRef]
- Vivian, J.; Rao, A.A.; Nothaft, F.A.; Ketchum, C.; Armstrong, J.; Novak, A.; Pfeil, J.; Narkizian, J.; DeRan, A.D.; Musselman-Brown, A.; et al. Toil enables reproducible, open source, big biomedical data analyses. Nat. Biotechnol. 2017, 35, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Ashburner, M. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zheng, L.; Tao, L. Downregulation of IQGAP2 Correlates with Prostate Cancer Recurrence and Metastasis. Transl. Oncol. 2019, 12, 236–244. [Google Scholar] [CrossRef]
- Luan, F.; Li, X.; Cheng, X.; Huangfu, L.; Han, J.; Guo, T.; Du, H.; Wen, X.; Ji, J. TNFRSF11B activates Wnt/β-catenin signaling and promotes gastric cancer progression. Int. J. Biol. Sci. 2020, 16, 1956–1971. [Google Scholar] [CrossRef]
- Morales, M.; Arenas, E.J.; Urosevic, J.; Guiu, M.; Fernández, E.; Planet, E.; Fenwick, R.B.; Fernández-Ruiz, S.; Salvatella, X.; Reverter, D.; et al. RARRES 3 suppresses breast cancer lung metastasis by regulating adhesion and differentiation. EMBO Mol. Med. 2014, 6, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sun, W.; Ye, G.; Ding, X.; Liu, Z.; Zhang, S.; Xia, T.; Xiao, B.; Xi, Y.; Guo, J. Long non-coding RNA expression profile in human gastric cancer and its clinical significances. J. Transl. Med. 2013, 11, 225. [Google Scholar] [CrossRef]
- Lu, X.; Lu, J.; Liao, B.; Li, X.; Qian, X.; Li, K. Driver pattern identification over the gene co-expression of drug response in ovarian cancer by integrating high throughput genomics data. Sci. Rep. 2017, 7, 16188. [Google Scholar] [CrossRef] [PubMed]
- Gagné, A.; Têtu, B.; Orain, M.; Turcotte, S.; Plante, M.; Grégoire, J.; Renaud, M.-C.; Bairati, I.; Trudel, D. HtrA1 expression and the prognosis of high-grade serous ovarian carcinoma: A cohort study using digital analysis. Diagn. Pathol. 2018, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Stavnes, H.T.; Holth, A.; Chen, X.; Yang, Y.; Shih, I.M.; Wang, T.L. Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from breast carcinoma in effusions. J. Cell. Mol. Med. 2011, 15, 535–544. [Google Scholar] [CrossRef]
- Hao, S.; Lv, J.; Yang, Q.; Wang, A.; Li, Z.; Guo, Y.; Zhang, G. Identification of Key Genes and Circular RNAs in Human Gastric Cancer. Med. Sci. Monit. 2019, 25, 2488–2504. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Pan, J. PACE4 regulates proliferation, migration and invasion in human breast cancer MDA-MB-231 cells. Mol. Med. Rep. 2014, 11, 698–704. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Liang, Q.; Wang, S.; Xiwen, L.; Pan, F.; Chen, H.; Li, D. Distinct prognostic value of mRNA expression of guanylate-binding protein genes in skin cutaneous melanoma. Oncol. Lett. 2018, 15, 7914–7922. [Google Scholar] [CrossRef]
- Godoy, P.; Cadenas, C.; Hellwig, B.; Marchan, R.; Stewart, J.; Reif, R.; Lohr, M.; Gehrmann, M.; Rahnenführer, J.; Schmidt, M.; et al. Interferon-inducible guanylate binding protein (GBP2) is associated with better prognosis in breast cancer and indicates an efficient T cell response. Breast Cancer 2012, 21, 491–499. [Google Scholar] [CrossRef]
- Tretina, K.; Park, E.-S.; Maminska, A.; MacMicking, J.D. Interferon-induced guanylate-binding proteins: Guardians of host defense in health and disease. J. Exp. Med. 2019, 216, 482–500. [Google Scholar] [CrossRef]
- Fleming, D.S.; Miller, L.C. Leading edge analysis of transcriptomic changes during pseudorabies virus infection. Genom. Data 2016, 10, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Steckling, N.; Gotti, A.; Bose-O’Reilly, S.; Chapizanis, D.; Costopoulou, D.; De Vocht, F.; Garí, M.; Grimalt, J.O.; Heath, E.; Hiscock, R.; et al. Biomarkers of exposure in environment-wide association studies—Opportunities to decode the exposome using human biomonitoring data. Environ. Res. 2018, 164, 597–624. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Fan, L.; Zhong, X.; Yang, H.; Xie, R.; Lv, Z.; Fu, D.; Luo, P.; Ma, Y. Dysregulation of TMPRSS3 and TNFRSF11B correlates with tumorigenesis and poor prognosis in patients with breast cancer. Oncol. Rep. 2017, 37, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Rogers-Broadway, K.; Kumar, J.; Sisu, C.; Wander, G.; Mazey, E.; Jeyaneethi, J.; Pados, G.; Tsolakidis, D.; Klonos, E.; Grunt, T.; et al. Differential expression of mTOR components in endometriosis and ovarian cancer: Effects of rapalogues and dual kinase inhibitors on mTORC1 and mTORC2 stoichiometry. Int. J. Mol. Med. 2018, 43, 47–56. [Google Scholar] [CrossRef]
- Rogers-Broadway, K.-R.; Chudasama, D.; Pados, G.; Tsolakidis, D.; Goumenou, A.; Hall, M.; Karteris, E. Differential effects of rapalogues, dual kinase inhibitors on human ovarian carcinoma cells in vitro. Int. J. Oncol. 2016, 49, 133–143. [Google Scholar] [CrossRef][Green Version]
- Xiao, Y.; Yu, Y.; Jiang, P.; Li, Y.; Wang, C.; Zhang, R. The PI3K/mTOR dual inhibitor GSK458 potently impedes ovarian cancer tumorigenesis and metastasis. Cell. Oncol. 2020, 43, 669–680. [Google Scholar] [CrossRef]
- Langdon, S.P.; Herrington, C.S.; Hollis, R.L.; Gourley, C. Estrogen Signaling and Its Potential as a Target for Therapy in Ovarian Cancer. Cancers 2020, 12, 1647. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Arterburn, J.B.; Smith, H.O.; Oprea, T.I.; Sklar, L.A.; Hathaway, H.J. Estrogen Signaling through the Transmembrane G Protein–Coupled Receptor GPR30. Annu. Rev. Physiol. 2008, 70, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, S.A. The Endocrine Disruptor Bisphenol A (BPA) Exerts a Wide Range of Effects in Carcinogenesis and Response to Therapy. Curr. Mol. Pharmacol. 2019, 12, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Kwiatkowski, N.P.; Zhang, T.; Nabet, B.; Xu, M.; Liang, Y.; Quan, C.; Wang, J.; Hao, M.; Palakurthi, S.; et al. Targeting MYC dependency in ovarian cancer through inhibition of CDK7 and CDK12/13. eLife 2018, 7, e39030. [Google Scholar] [CrossRef]
- Au, C.W.; Siu, M.K.; Liao, X.; Wong, E.S.; Ngan, H.Y.; Tam, K.F.; Chan, D.C.; Chan, Q.K.; Cheung, A.N. Tyrosine kinase B receptor and BDNF expression in ovarian cancers—Effect on cell migration, angiogenesis and clinical outcome. Cancer Lett. 2009, 281, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Cai, M.; Zhao, S.; Wang, H.; Li, M.; Yao, S.; Jiang, N. CYR61 overexpression associated with the development and poor prognosis of ovarian carcinoma. Med. Oncol. 2014, 31, 117. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.J.M.; Macleod, K.G.; Burns, D.J.; Smyth, J.F.; Langdon, S.P. Estrogen receptor-α mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr. Relat. Cancer 2005, 12, 851–866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, G.; MacLeod, K.; Williams, A.R.; Cameron, D.A.; Smyth, J.F.; Langdon, S.P. Estrogen-regulated gene expression predicts response to endocrine therapy in patients with ovarian cancer. Gynecol. Oncol. 2007, 106, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Schüler-Toprak, S.; Häring, J.; Inwald, E.C.; Moehle, C.; Ortmann, O.; Treeck, O. Agonists and knockdown of estrogen receptor β differentially affect invasion of triple-negative breast cancer cells in vitro. BMC Cancer 2016, 16, 951. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, M.; Edwards, D.; Creighton, C.J.; Medina, D.; Conneely, O.M. Reprogramming of the estrogen responsive transcriptome contributes to tamoxifen-dependent protection against tumorigenesis in the p53 null mammary epithelial cells. PLoS ONE 2018, 13, e0194913. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Vendrell, J.A.; Poulard, C.; Győrffy, B.; Goddard-Leon, S.; Bieche, I.; Corbo, L.; Le Romancer, M.; Bachelot, T.; Treilleux, I.; et al. A functional interplay between ZNF217 and Estrogen Receptor alpha exists in luminal breast cancers. Mol. Oncol. 2014, 8, 1441–1457. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, G.; Qin, J.; Qiu, Y.; Gao, Y.; Yu, Y.; Deng, Q. Microarray analysis of gene expression in the ovarian cancer cell line HO-8910 with silencing of the ZNF217 gene. Mol. Med. Rep. 2009, 2, 851–855. [Google Scholar] [CrossRef]
- Jorgensen, E.M.; Alderman, M.H.; Taylor, H.S. Preferential epigenetic programming of estrogen response after in utero xenoestrogen (bisphenol-A) exposure. FASEB J. 2016, 30, 3194–3201. [Google Scholar] [CrossRef]
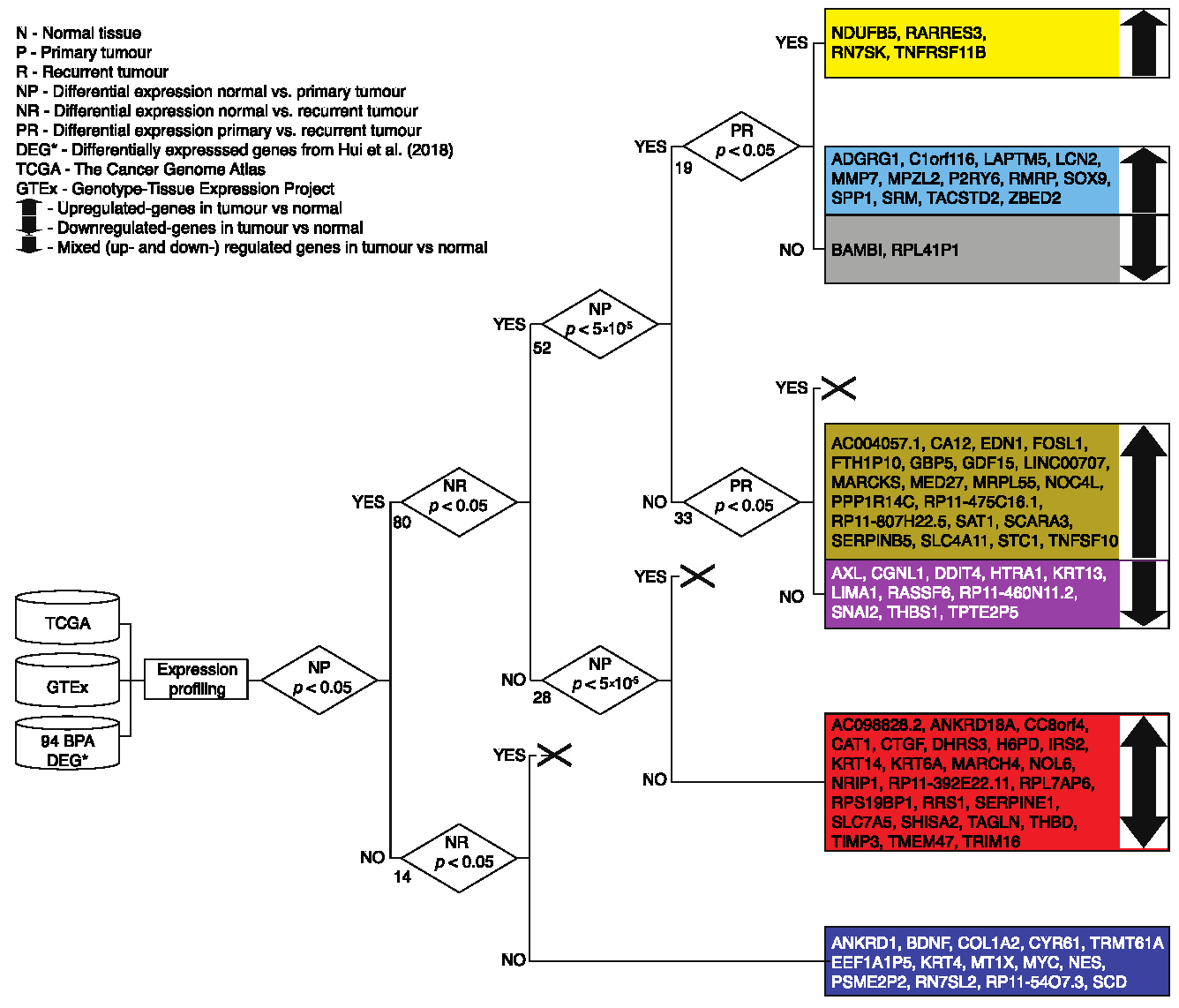
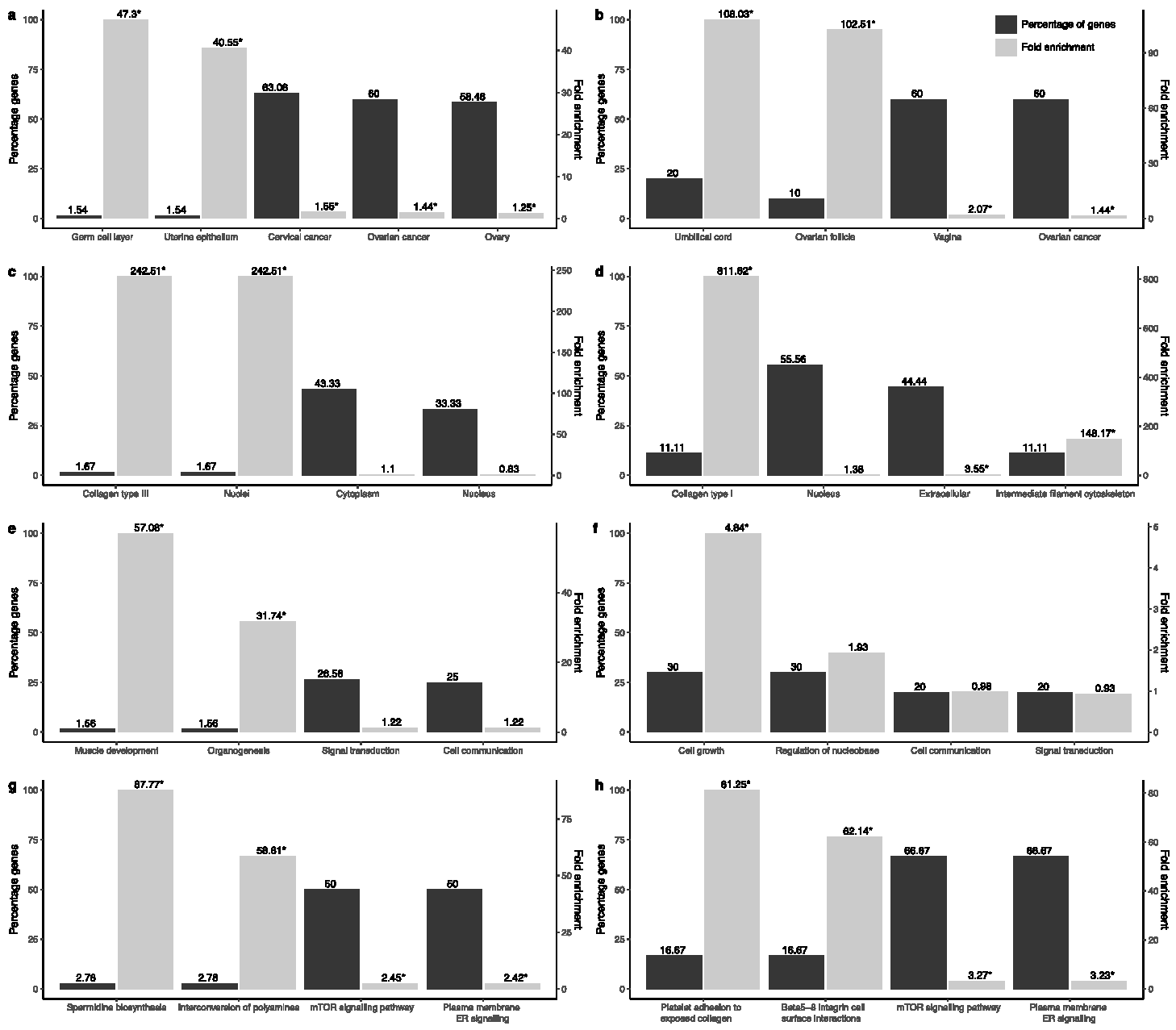

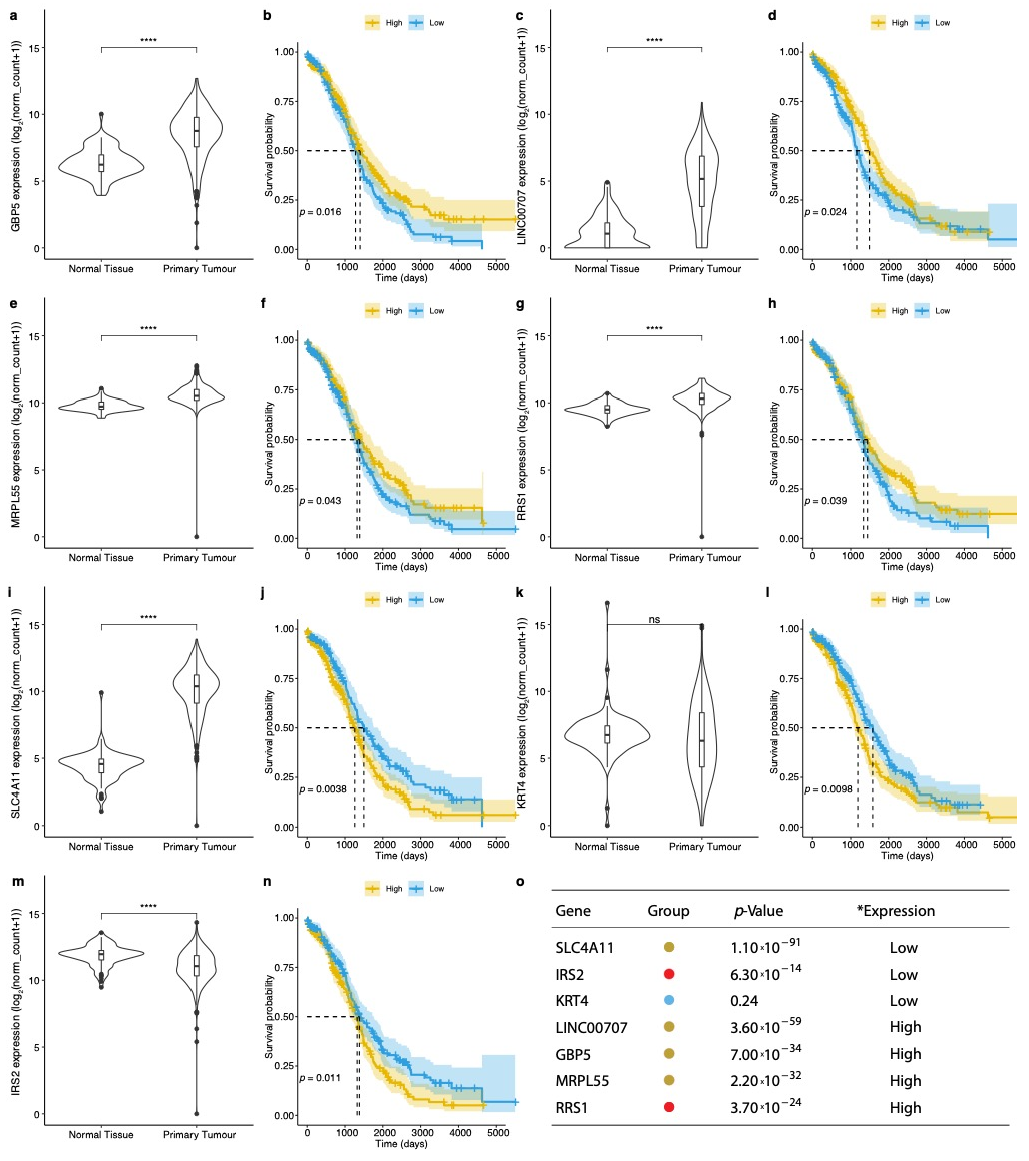

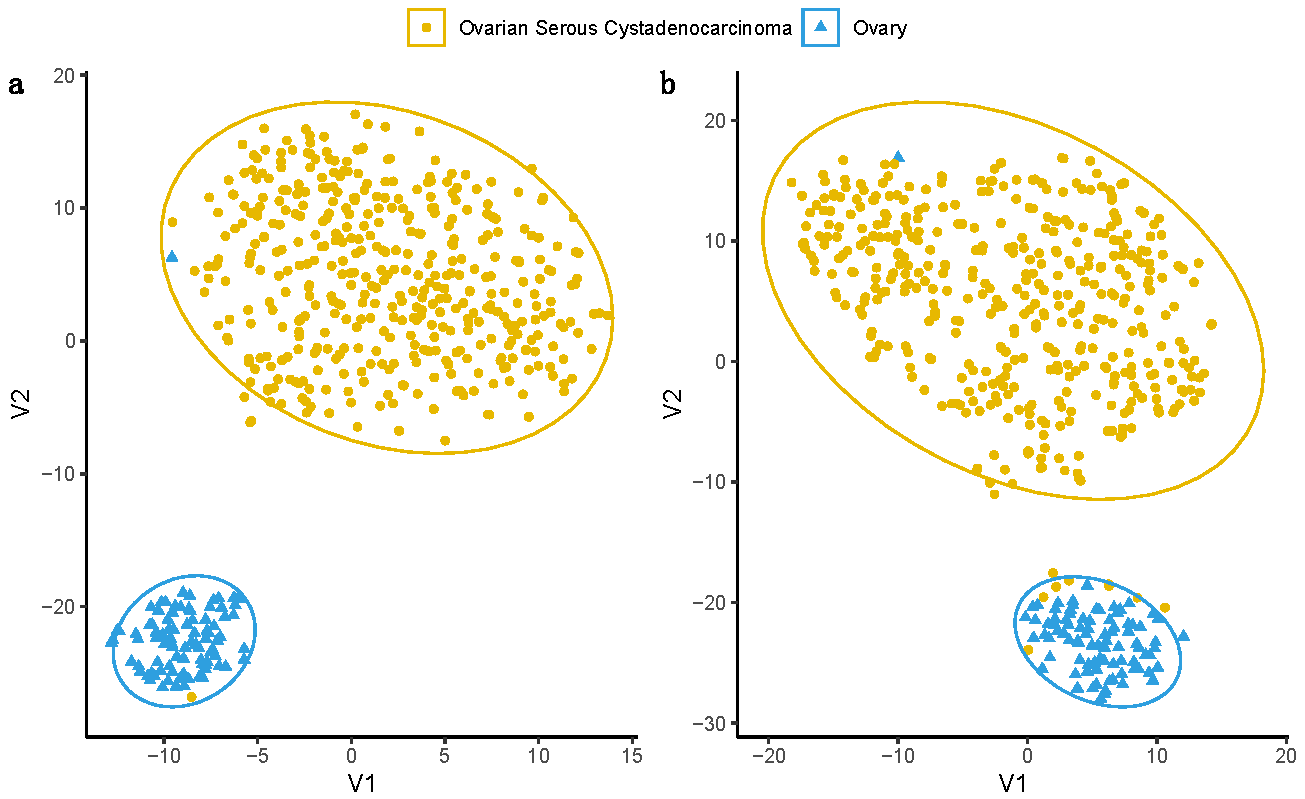
| Phenotype | TCGA | GTEx |
|---|---|---|
| Total Samples | 427 | 88 |
| Normal tissue | - | 88 (100%) |
| Primary tumour | 419 (98.13%) | - |
| Recurrent tumour | 8 (1.87%) | - |
| Category | ||
| Normal ovary | - | |
| Ovarian serous | 427 (100%) | 88 (100%) |
| Cystadenocarcinoma | NA | |
| Primary diagnosis | NA | |
| Serous cystadenocarcinoma, NOS | 422 (98.83%) | |
| Papillary serous cystadenocarcinoma | 4 (0.94%) | |
| Cystadenocarcinoma, NOS | 1 (0.23%) | |
| Clinical stage | NA | |
| Stage I | 1 (0.23%) | |
| Stage II | 26 (6.09%) | |
| Stage III | 334 (78.22%) | |
| Stage IV | 63 (14.75%) | |
| Overall survival (days) | Min 8 Max 5481 | NA |
| Age range (years) | 30–87 | 20–69 |
| Age < 50 | 103 (24.12%) | 39 (44.31%) |
| Age > 50 | 324 (75.88%) | 49(55.68%) |
| Mortality | NA | |
| Living | 162 (37.94%) | |
| Deceased | 265 (62.06%) | |
| Initial Diagnosis Methods | NA | |
| Cytology (e.g., pleural fluid) | 54 (12.65%) | |
| Excisional biopsy | 5 (1.17%) | |
| FNA biopsy | 9 (2.11%) | |
| Incisional biopsy | 6 (1.41%) | |
| Tumour resection | 347 (81.26%) | |
| Unspecified method | 6 (1.41%) | |
| Neoplasm Histologic Grade | NA | |
| G1 | 1 (0.23%) | |
| G2 | 52 (12.18%) | |
| G3 | 363 (85.01%) | |
| G4 | 1 (0.23%) | |
| GB | 2 (0.47%) | |
| GX | 6 (1.41%) | |
| Unspecified grade | 2 (0.47%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, A.; Dong, Q.; Hall, M.; Jeyaneethi, J.; Silva, E.; Karteris, E.; Sisu, C. Identification of Potential Bisphenol A (BPA) Exposure Biomarkers in Ovarian Cancer. J. Clin. Med. 2021, 10, 1979. https://doi.org/10.3390/jcm10091979
Zahra A, Dong Q, Hall M, Jeyaneethi J, Silva E, Karteris E, Sisu C. Identification of Potential Bisphenol A (BPA) Exposure Biomarkers in Ovarian Cancer. Journal of Clinical Medicine. 2021; 10(9):1979. https://doi.org/10.3390/jcm10091979
Chicago/Turabian StyleZahra, Aeman, Qiduo Dong, Marcia Hall, Jeyarooban Jeyaneethi, Elisabete Silva, Emmanouil Karteris, and Cristina Sisu. 2021. "Identification of Potential Bisphenol A (BPA) Exposure Biomarkers in Ovarian Cancer" Journal of Clinical Medicine 10, no. 9: 1979. https://doi.org/10.3390/jcm10091979
APA StyleZahra, A., Dong, Q., Hall, M., Jeyaneethi, J., Silva, E., Karteris, E., & Sisu, C. (2021). Identification of Potential Bisphenol A (BPA) Exposure Biomarkers in Ovarian Cancer. Journal of Clinical Medicine, 10(9), 1979. https://doi.org/10.3390/jcm10091979








