Handgrip Strength Correlated with Falling Risk in Patients with Degenerative Cervical Myelopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Outcome Measures
2.3. HGS Measurement
2.4. Assessment of the Risk of Falling Using Four Functional Mobility Tests and an Actual Fall Diary
2.5. Statistical Analysis
3. Results
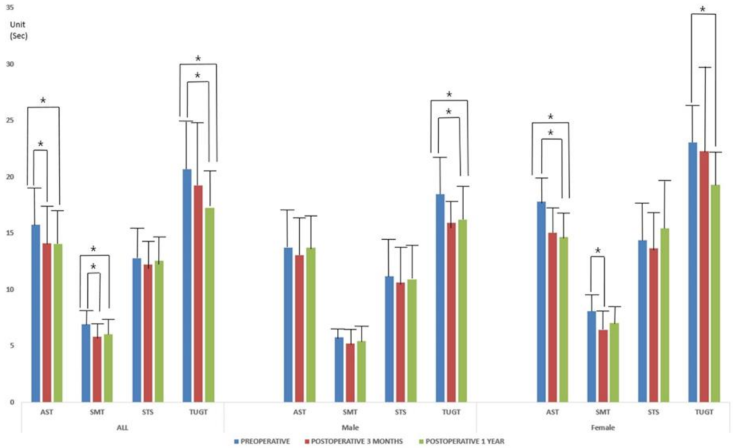
3.1. Functional Mobility Test Results and Actual Falls
3.2. QoL Outcomes: EQ-VAS, NDI, and mJOA Score and Grade
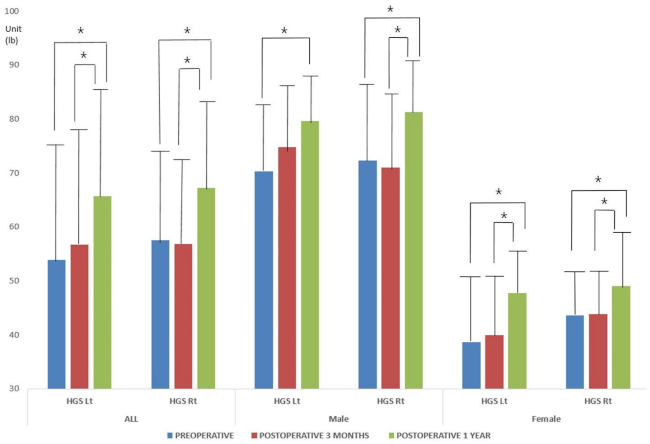
3.3. HGS
3.4. Multiple Regression Analyses of Parameters Associated with Falls and Fall-Related Mobility Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kimura, A.; Seichi, A.; Takeshita, K.; Inoue, H.; Kato, T.; Yoshii, T.; Furuya, T.; Koda, M.; Takeuchi, K.; Matsunaga, S. Fall-related deterioration of subjective symptoms in patients with cervical myelopathy. Spine 2017, 42, E398–E403. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kim, T.-H.; Park, M.-S.; Lim, S.; Park, J.-O.; Kim, H.-S.; Kim, H.-J.; Lee, H.-M.; Moon, S.-H. Comparison of effects of nonoperative treatment and decompression surgery on risk of patients with lumbar spinal stenosis falling: Evaluation with functional mobility tests. J. Bone Jt. Surg. 2014, 96, e110. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Yang, J.-H.; Kim, H.-S.; Suk, K.-S.; Lee, H.-M.; Park, J.-O.; Moon, S.-H. Effect of sagittal balance on risk of falling after lateral lumbar interbody fusion surgery combined with posterior surgery. Yonsei Med J. 2017, 58, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Park, J.-O.; Kim, H.-S.; Suk, K.-S.; Lee, S.-Y.; Lee, H.-M.; Yang, J.-H.; Moon, S.-H. Spinal sagittal balance status affects postoperative actual falls and quality of life after decompression and fusion in-situ surgery in patients with lumbar spinal stenosis. Clin. Neurol. Neurosurg. 2016, 148, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Takeshita, K.; Shiraishi, Y.; Inose, H.; Yoshii, T.; Maekawa, A.; Endo, K.; Miyamoto, T.; Furuya, T.; Nakamura, A.; et al. Effectiveness of surgical treatment for degenerative cervical myelopathy in preventing falls and fall-related neurological deterioration: A prospective multi-institutional study. Spine 2020, 45, E631–E638. [Google Scholar] [CrossRef] [PubMed]
- Bednarik, J.; Kadanka, Z.; Dusek, L.; Kerkovsky, M.; Vohanka, S.; Novotny, O.; Urbanek, I.; Kratochvilova, D. Presymptomatic spondylotic cervical myelopathy: An updated predictive model. Eur. Spine J. 2008, 17, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Bednarik, J.; Kadanka, Z.; Dusek, L.; Novotny, O.; Surelova, D.; Urbanek, I.; Prokes, B. Presymptomatic spondylotic cervical cord compression. Spine 2004, 29, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Bednařík, J.; Sládková, D.; Kadaňka, Z.; Dušek, L.; Keřkovský, M.; Voháňka, S.; Novotný, O.; Urbánek, I.; Němec, M. Are subjects with spondylotic cervical cord encroachment at increased risk of cervical spinal cord injury after minor trauma? J. Neurol. Neurosurg. Psychiatry 2011, 82, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Kadanka Jr, Z.; Adamova, B.; Kerkovsky, M.; Kadanka, Z.; Dusek, L.; Jurova, B.; Vlckova, E.; Bednarik, J. Predictors of symptomatic myelopathy in degenerative cervical spinal cord compression. Brain Behav. 2017, 7, e00797. [Google Scholar] [CrossRef] [PubMed]
- Kadaňka, Z.; Bednařík, J.; Novotný, O.; Urbánek, I.; Dušek, L. Cervical spondylotic myelopathy: Conservative versus surgical treatment after 10 years. Eur. Spine J. 2011, 20, 1533–1538. [Google Scholar] [CrossRef]
- Kadaňka, Z.; Bednařík, J.; Voháňka, S.; Vlach, O.; Stejskal, L.; Chaloupka, R.; Filipovičová, D.; Šurelová, D.; Adamová, B.; Novotný, O. Conservative treatment versus surgery in spondylotic cervical myelopathy: A prospective randomised study. Eur. Spine J. 2000, 9, 538–544. [Google Scholar] [CrossRef]
- Kadaňka, Z.; Mareš, M.; Bednařík, J.; Smrčka, V.; Krbec, M.; Chaloupka, R.; Dušek, L. Predictive factors for mild forms of spondylotic cervical myelopathy treated conservatively or surgically. Eur. J. Neurol. 2005, 12, 16–24. [Google Scholar] [CrossRef]
- Kadanka, Z.; Mareš, M.; Bednarík, J.; Smrcka, V.; Krbec, M.; Stejskal, L.; Chaloupka, R.; Dagmar, S.; Novotný, O.; Urbánek, I. Approaches to spondylotic cervical myelopathy: Conservative versus surgical results in a 3-year follow-up study. Spine 2002, 27, 2205–2210. [Google Scholar] [CrossRef]
- Kalsi-Ryan, S.; Riehm, L.E.; Tetreault, L.; Martin, A.R.; Teoderascu, F.; Massicotte, E.; Curt, A.; Verrier, M.C.; Velstra, I.-M.; Fehlings, M.G. Characteristics of upper limb impairment related to degenerative cervical myelopathy: Development of a sensitive hand assessment (graded redefined assessment of strength, sensibility, and prehension version myelopathy). Neurosurgery 2019, 86, E292–E299. [Google Scholar] [CrossRef]
- Kalsi-Ryan, S.; Rienmueller, A.C.; Riehm, L.; Chan, C.; Jin, D.; Martin, A.R.; Badhiwala, J.H.; Akbar, M.A.; Massicotte, E.M.; Fehlings, M.G. Quantitative assessment of gait characteristics in degenerative cervical myelopathy: A prospective clinical study. J. Clin. Med. 2020, 9, 752. [Google Scholar] [CrossRef]
- Kerkovský, M.; Bednarík, J.; Dušek, L.; Šprláková-Puková, A.; Urbánek, I.; Mechl, M.; Válek, V.; Kadanka, Z. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: Correlations between clinical and electrophysiological findings. Spine 2012, 37, 48–56. [Google Scholar] [CrossRef]
- Kovalova, I.; Kerkovsky, M.; Kadanka, Z.; Kadanka Jr, Z.; Nemec, M.; Jurova, B.; Dusek, L.; Jarkovsky, J.; Bednarik, J. Prevalence and imaging characteristics of nonmyelopathic and myelopathic spondylotic cervical cord compression. Spine 2016, 41, 1908–1916. [Google Scholar] [CrossRef]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Kalsi-Ryan, S.; Cadotte, D.W.; Wilson, J.R.; Tetreault, L.; Nouri, A.; Crawley, A.; Mikulis, D.J. Monitoring for myelopathic progression with multiparametric quantitative mri. PLoS ONE 2018, 13, e0195733. [Google Scholar]
- Kwon, J.-W.; Lee, B.H.; Lee, S.-B.; Sung, S.; Lee, C.-U.; Yang, J.-H.; Park, M.-S.; Byun, J.; Lee, H.-M.; Moon, S.-H. Hand grip strength can predict clinical outcomes and risk of falls after decompression and instrumented posterolateral fusion for lumbar spinal stenosis. Spine J. 2020, 20, 1960–1967. [Google Scholar] [CrossRef]
- Foster, J. Handbook of clinical neurology, vol. 26 (injuries of the spine and spinal cord, part ii): By pj vinken and gw bruyn (eds.), in collaboration with r. Braakman, associate editor hl klawans, jr., xii+ 550 pages, 310 illustrations, 71 tables, north-holland publishing company, amsterdam, 1976, US $78.75, dfl 205.00, subscription price us $66.95, dfl 174.25. J. Neurol. Sci. 1977, 34, 299. [Google Scholar]
- Nardone, R.; Höller, Y.; Brigo, F.; Frey, V.; Lochner, P.; Leis, S.; Golaszewski, S.; Trinka, E. The contribution of neurophysiology in the diagnosis and management of cervical spondylotic myelopathy: A review. Spinal Cord 2016, 54, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.S.; Almefty, K.K.; Godzik, J.; Muma, A.H.; Hlubek, R.J.; Martinez-del-Campo, E.; Theodore, N.; Kakarla, U.K.; Turner, J.D. Functional improvement in hand strength and dexterity after surgical treatment of cervical spondylotic myelopathy: A prospective quantitative study. J. Neurosurg. Spine 2020, 32, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Al-Mefty, O.; Harkey, L.H.; Middleton, T.H.; Smith, R.R.; Fox, J.L. Myelopathic cervical spondylotic lesions demonstrated by magnetic resonance imaging. J. Neurosurg. 1988, 68, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kang, C.-N. Degenerative cervical myelopathy: Pathophysiology and current treatment strategies. Asian Spine J. 2020, 14, 710–720. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.A.; Riew, K.D.; Middleton, J.W.; Aarabi, B.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Carette, S.; Chen, R.; et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: Recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Glob. Spine J. 2017, 7, 70S–83S. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chun, H.-J.; Han, C.-D.; Moon, S.-H.; Kang, K.-T.; Kim, H.-S.; Park, J.-O.; Moon, E.-S.; Kim, B.-R.; Sohn, J.-S. The risk assessment of a fall in patients with lumbar spinal stenosis. Spine 2011, 36, E588–E592. [Google Scholar] [CrossRef]
- Bohannon, R.W. Hand-grip dynamometry predicts future outcomes in aging adults. J. Geriatr. Phys. Ther. 2008, 31, 3–10. [Google Scholar] [CrossRef]
- Vernon, H.; Mior, S. The neck disability index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Kato, S.; Oshima, Y.; Oka, H.; Chikuda, H.; Takeshita, Y.; Miyoshi, K.; Kawamura, N.; Masuda, K.; Kunogi, J.; Okazaki, R. Comparison of the japanese orthopaedic association (joa) score and modified joa (mjoa) score for the assessment of cervical myelopathy: A multicenter observational study. PLoS ONE 2015, 10, e0123022. [Google Scholar] [CrossRef]
- Whynes, D.K.; Group, T. Correspondence between eq-5d health state classifications and eq vas scores. Health Qual. Life Outcomes 2008, 6, 94. [Google Scholar] [CrossRef]
- Ali, R.; Schwalb, J.M.; Nerenz, D.R.; Antoine, H.J.; Rubinfeld, I. Use of the modified frailty index to predict 30-day morbidity and mortality from spine surgery. J. Neurosurg. Spine 2016, 25, 537–541. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Wilson, J.R.; Kopjar, B.; Yoon, S.T.; Arnold, P.M.; Massicotte, E.M.; Vaccaro, A.R.; Brodke, D.S.; Shaffrey, C.I.; Smith, J.S. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: Results of the aospine north america prospective multi-center study. J. Bone Jt. Surg. 2013, 95, 1651–1658. [Google Scholar] [CrossRef]
- Cheung, J.P.Y.; Cheung, P.W.H.; Chiu, C.K.; Chan, C.Y.W.; Kwan, M.K. Variations in practice among asia–pacific surgeons and recommendations for managing cervical myelopathy: The first asia–pacific spine society collaborative study. Asian Spine J. 2019, 13, 45. [Google Scholar] [CrossRef]
- Omori, M.; Shibuya, S.; Nakajima, T.; Endoh, T.; Suzuki, S.; Irie, S.; Ariyasu, R.; Unenaka, S.; Sano, H.; Igarashi, K. Hand dexterity impairment in patients with cervical myelopathy: A new quantitative assessment using a natural prehension movement. Behav. Neurol. 2018. [Google Scholar] [CrossRef]
- Doita, M.; Sakai, H.; Harada, T.; Nishida, K.; Miyamoto, H.; Kaneko, T.; Kurosaka, M. Evaluation of impairment of hand function in patients with cervical myelopathy. Clin. Spine Surg. 2006, 19, 276–280. [Google Scholar] [CrossRef]
- Yoo, J.S.; Ahn, J.; Mayo, B.C.; Bohl, D.D.; Ahn, J.; Hrynewycz, N.M.; Brundage, T.S.; Park, D.D.; Colman, M.W.; Phillips, F.M. Improvements in grip and pinch strength and patient-reported outcomes after anterior cervical discectomy and fusion. Clin. Spine Surg. 2019, 32, 403–408. [Google Scholar] [CrossRef]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O. Sarcopenia in asia: Consensus report of the asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Matsumoto, M.; Toyama, Y.; Ishikawa, M.; Chiba, K.; Suzuki, N.; Fujimura, Y. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy: Does it predict the outcome of conservative treatment? Spine 2000, 25, 677–682. [Google Scholar] [CrossRef]
- Dolan, R.T.; Butler, J.S.; O’Byrne, J.M.; Poynton, A.R. Mechanical and cellular processes driving cervical myelopathy. World J. Orthop. 2016, 7, 20. [Google Scholar] [CrossRef]
- Pandita, N.; Gupta, S.; Raina, P.; Srivastava, A.; Hakak, A.Y.; Singh, O. Neurological recovery pattern in cervical spondylotic myelopathy after anterior surgery: A prospective study with literature review. Asian Spine J. 2019, 13, 423. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Watson, W.L.; Milat, A.; Chung, A.Z.; Lord, S. Health and lifestyle risk factors for falls in a large population-based sample of older people in australia. J. Saf. Res. 2013, 45, 7–13. [Google Scholar] [CrossRef]
- Leven, D.M.; Lee, N.J.; Kothari, P.; Steinberger, J.; Guzman, J.; Skovrlj, B.; Shin, J.I.; Caridi, J.M.; Cho, S.K. Frailty index is a significant predictor of complications and mortality after surgery for adult spinal deformity. Spine 2016, 41, E1394–E1401. [Google Scholar] [CrossRef]
- Shin, J.I.; Kothari, P.; Phan, K.; Kim, J.S.; Leven, D.; Lee, N.J.; Cho, S.K. Frailty index as a predictor of adverse postoperative outcomes in patients undergoing cervical spinal fusion. Spine 2017, 42, 304–310. [Google Scholar] [CrossRef]
- Fiore, S.; Labiak, J.J.; Davis, R.P. Combined anterior-posterior decompression and fusion for cervical spondylotic myelopathy. Am. J. Orthop. 2017, 46, E97–E104. [Google Scholar]
- Kang, K.-C.; Lee, H.S.; Lee, J.-H. Cervical radiculopathy focus on characteristics and differential diagnosis. Asian Spine J. 2020, 14, 921–930. [Google Scholar] [CrossRef]
- Suk, K.-S.; Jimenez, K.A.; Jo, J.H.; Kim, H.-S.; Lee, H.-M.; Moon, S.-H.; Lee, B.H. Anterior plate-screws and lower postoperative t1 slope affect cervical allospacer failures in multi-level acdf surgery: Anterior versus posterior fixation. Glob. Spine J. 2021, 2192568221991515. [Google Scholar] [CrossRef]
| All | Male | Female | p Value | |
|---|---|---|---|---|
| (N = 203) | (N = 98) | (N = 105) | ||
| Age (years) | 63.76 ± 10.56 | 59.93 ± 10.26 | 67.33 ± 9.56 | 0.000 |
| Symptom duration (months) | 37.17 ± 40.87 | 29.92 ± 33.22 | 46.73 ± 44.98 | 0.000 |
| Body mass index (kg/m2) | 24.30 ± 3.82 | 23.69 ± 2.45 | 24.86 ± 4.71 | 0.026 |
| Waist circumference (cm) | 89.43 ± 10.0 | 88.82 ± 9.30 | 90.00 ± 1.04 | N.S |
| Modified frailty index | 1.37 ± 1.27 | 1.14 ± 1.13 | 1.60 ± 1.36 | 0.010 |
| Smoker:non-smoker * | 42:161 | 35:63 | 7:98 | 0.000 |
| Spinal cord signal change (+):(−) * | 133:70 | 77:21 | 56:49 | 0.000 |
| Operation length (fusion level) * | 2.96 ± 0.93 | 2.85 ± 0.91 | 3.06 ± 0.93 | 0.000 |
| 1 level | 7 (3.4%) | 0 | 7 (6.7%) | |
| 2 level | 63 (31%) | 42 (42.9%) | 21 (20%) | |
| 3 level | 70 (34.5%) | 35 (35.7%) | 35 (33.3%) | |
| 4 level | 56 (27.6%) | 14 (14.3%) | 42 (40.0%) | |
| 5 level | 7 (3.4%) | 7 (7.1%) | 0 | |
| Surgery type * | 0.002 | |||
| Anterior | 91 (44.8%) | 56 (57.1%) | 35 (33.3%) | |
| Posterior | 42 (20.7%) | 14 (14.3%) | 28 (26.7%) | |
| Combined anterior-posterior | 70 (34.5%) | 28 (28.6%) | 42 (40.0%) |
| All | Males | Females | p Value | |
|---|---|---|---|---|
| Preoperative (unit: seconds) | ||||
| Alternate-Step Test | 15.74 ± 4.38 | 13.71 ± 3.2 | 17.78 ± 4.46 | 0.000 |
| Six-Meter-Walk Test | 6.91 ± 2.82 | 5.74 ± 2.52 | 8.08 ± 2.61 | 0.000 |
| Sit-to-Stand test | 12.78 ± 3.82 | 11.18 ± 3.46 | 14.38 ± 3.49 | 0.000 |
| Time Up and Go Test | 20.66 ± 5.12 | 18.47 ± 5.18 | 23.02 ± 3.87 | 0.000 |
| Actual fall * | ||||
| No fall:fall | 168:35 | 84:14 | 84:21 | NS |
| Single:multiple | 20:15 | 8:6 | 12:9 | NS |
| Postoperative 3 months(unit: seconds) | ||||
| Alternate-Step Test | 14.12 ± 3.52 | 13.06 ± 3.72 | 15.04 ± 3.07 | 0.000 |
| Six-Meter-Walk Test | 5.84 ± 1.96 | 5.19 ± 0.85 | 6.40 ± 2.42 | 0.000 |
| Sit-to-Stand test | 12.24 ± 3.55 | 10.63 ± 2.10 | 13.64 ± 3.94 | 0.000 |
| Time Up and Go Test | 19.22 ± 7.70 | 15.92 ± 2.97 | 22.29 ± 9.32 | 0.000 |
| Actual fall * | ||||
| No fall:fall | 189:14 | 98:0 | 91:14 | 0.000 |
| Single:multiple | 10:4 | 0 | 10:4 | 0.000 |
| Postoperative 1 year (unit: seconds) | ||||
| Alternate-Step Test | 14.05 ± 3.35 | 13.69 ± 3.08 | 14.65 ± 3.71 | NS |
| Six-Meter-Walk Test | 6.02 ± 1.98 | 5.42 ± 1.23 | 7.02 ± 2.53 | 0.000 |
| Sit-to-Stand test | 12.57 ± 5.03 | 10.87 ± 1.95 | 15.42 ± 7.00 | 0.000 |
| Time Up and Go Test | 17.23 ± 4.77 | 16.21 ± 5.06 | 19.27 ± 3.33 | 0.000 |
| Actual fall * | ||||
| No fall:fall | 189:14 | 96:2 | 93:12 | 0.000 |
| Single:multiple | 12:2 | 1:1 | 11:1 | 0.000 |
| All | Males | Females | p Value | |
|---|---|---|---|---|
| Preoperative | ||||
| Modified JOA score | 9.51 ± 3.04 | 10.92 ± 3.00 | 8.20 ± 2.41 | 0.000 |
| Modified JOA grade * | ||||
| Grade 0:1:2:3 | 7:49:98:49 | 7:35:42:14 | 0:14:58:35 | 0.000 |
| Neck Disability Index | 17.31 ± 7.77 | 14.85 ± 6.67 | 19.60 ± 8.05 | 0.000 |
| Euro-QoL Visual Analog Scale | 48.62 ± 23.79 | 50.71 ± 18.40 | 46.66 ± 27.86 | NS |
| Postoperative 3 months | ||||
| Modified JOA score | 11.58 ± 3.14 | 12.35 ± 3.1 | 10.86 ± 3.02 | 0.001 |
| Modified JOA grade * | ||||
| Grade 0:1:2:3 | 28:77:63:35 | 21:35:35:7 | 7:42:28:28 | 0.001 |
| Neck Disability Index | 14.82 ± 5.73 | 14.21 ± 7.13 | 15.40 ± 3.96 | NS |
| Euro-QoL Visual Analog Scale | 58.55 ± 22.48 | 62.71 ± 25.68 | 54.66 ± 18.29 | 0.011 |
| Postoperative 1 year | ||||
| Modified JOA score | 12.61 ± 3.50 | 12.444 ± 2.38 | 12.71 ± 3.61 | NS |
| Modified JOA grade * | ||||
| Grade 0:1:2:3 | 30:79:64:30 | 22:34:35:7 | 8:45:29:23 | 0.001 |
| Neck Disability Index | 12.68 ± 9.06 | 12.66 ± 10.80 | 12.71 ± 6.26 | NS |
| Euro-QoL Visual Analog Scale | 60.31 ± 17.88 | 62.22 ± 16.13 | 57.85 ± 19.81 | NS |
| (Unit: lbs.) | All | Males | Females | p Value |
|---|---|---|---|---|
| Preoperative | ||||
| HGS Lt | 53.90 ± 23.83 | 70.30 ± 19.46 | 38.59 ± 16.11 | 0.000 |
| HGS Rt | 57.51 ± 22.43 | 72.35 ± 21.96 | 43.66 ± 11.26 | 0.000 |
| Postoperative 3 months | ||||
| HGS Lt | 56.78 ± 24.47 | 74.79 ± 21.39 | 39.97 ± 12.07 | 0.000 |
| HGS Rt | 56.97 ± 21.42 | 71.01 ± 20.48 | 43.86 ± 11.88 | 0.000 |
| Postoperative 1 year | ||||
| HGS Lt | 65.72 ± 21.50 | 79.60 ± 16.93 | 47.86 ± 10.95 | 0.000 |
| HGS Rt | 67.23 ± 23.21 | 81.32 ± 18.75 | 49.12 ± 14.02 | 0.000 |
| Males | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | AST | SMT | STS | TUGT | ||||
| Beta ± S.E | p Value | Beta ± S.E | p Value | Beta ± S.E | p Value | Beta ± S.E | p Value | |
| Preoperative | ||||||||
| NDI | 0.330 ± 0.016 | 0.000 | 0.325 ± 0.001 | 0.000 | 0.460 ± 0.000 | 0.000 | 0.545 ± 0.014 | 0.000 |
| AGE | 0.168 ± 0.010 | 0.000 | −0.084 ± 0.001 | 0.000 | 0.023 ± 0.000 | 0.000 | ||
| mFI | 1.988 ± 0.145 | 0.000 | 1.605 ± 0.008 | 0.000 | −0.386 ± 0.003 | 0.000 | 2.370 ± 0.143 | 0.000 |
| BMI | 0.681 ± 0.063 | 0.000 | 0.989 ± 0.003 | 0.000 | 1.299 ± 0.001 | 0.000 | 2.029 ± 0.048 | 0.000 |
| Operation length | 0.955 ± 0.095 | 0.000 | 1.153 ± 0.006 | 0.000 | 4.326 ± 0.002 | 0.000 | 3.998 ± 0.128 | 0.000 |
| * Modified JOA grade | −0.711 ± 0.015 | 0.000 | 4.467 ± 0.006 | 0.000 | 3098 ± 0.278 | 0.000 | ||
| Postoperative 3 months | ||||||||
| NDI | −0.387 ± 0.024 | 0.000 | 0.190 ± 0.016 | 0.000 | −0.127 ± 0.007 | 0.000 | ||
| AGE | 0.326 ± 0.007 | 0.000 | 0.224 ± 0.002 | 0.000 | 0.227 ± 0.004 | 0.000 | ||
| mFI | 0.438 ± 0.173 | 0.013 | −2.639 ± 0.024 | 0.000 | 1.164 ± 0.055 | 0.000 | ||
| Smoking | 1.199 ± 0.115 | 0.000 | 1.647 ± 0.068 | 0.000 | 4.264 ± 0.023 | 0.000 | −4.956 ± 0.041 | 0.000 |
| BMI | 2.280 ± 0.051 | 0.000 | 0.053 ± 0.005 | 0.000 | 2.003 ± 0.021 | 0.000 | ||
| Operation length | 3.169 ± 0.152 | 0.000 | 0.672 ± 0.008 | 0.000 | 1.452 ± 0.036 | 0.000 | ||
| * Modified JOA grade | 6.866 ± 0.256 | 0.000 | 1.309 ± 0.209 | 0.000 | 9.980 ± 0.045 | 0.000 | ||
| Postoperative 1 year | ||||||||
| mFI | 3.040 ± 0.004 | 0.000 | 2.156 ± 0.022 | 0.000 | ||||
| Smoking | 0.275 ± 0.009 | 0.000 | −0.079 ± 0.000 | 0.000 | 1.331 ± 0.031 | 0.000 | ||
| HGS | −0.051 ± 0.000 | 0.000 | −0.129 ± 0.001 | 0.000 | −0.024 ± 0.001 | 0.000 | ||
| Symptom duration | 0.063 ± 0.000 | 0.000 | 0.006 ± 0.000 | 0.000 | 0.042 ± 0.000 | 0.000 | 0.074 ± 0.00 | 0.000 |
| * Modified JOA grade | 1.836 ± 0.000 | 0.000 | . | 7.679 ± 0.055 | 0.000 | |||
| Females | ||||||||
| Variables | AST | SMT | STS | TUGT | ||||
| Beta ± S.E | p Value | Beta ± S.E | p Value | Beta ± S.E | p Value | Beta ± S.E | p Value | |
| Preoperative | ||||||||
| NDI | 0.539 ± 0.018 | 0.000 | 0.791 ± 0.010 | 0.000 | 0.890 ± 0.004 | 0.000 | −0.275 ± 0.026 | 0.000 |
| AGE | 0.812 ± 0.034 | 0.000 | 1.162 ± 0.018 | 0.000 | 0.899 ± 0.005 | 0.000 | −0.620 ± 0.044 | 0.000 |
| WC | 0.271 ± 0.018 | 0.000 | 0.387 ± 0.010 | 0.000 | 0.000 | |||
| Cord signal change | 2.674 ± 0.251 | 0.000 | −2.885 ± 0.136 | 0.000 | 5.966 ± 0.042 | 6.574 ± 0.184 | 0.000 | |
| * Modified JOA score | −2.500 ± 0.051 | 0.000 | −2.578 ± 0.028 | 0.000 | −1.309 ± 0.011 | 0.000 | 0.498 ± 0.086 | 0.000 |
| HGS | −0.725 ± 0.014 | 0.000 | −0.035 ± 0.003 | 0.000 | −0.300 ± 0.018 | 0.000 | ||
| Operation length | 4.793 ± 0.075 | 0.000 | 2.934 ± 0.041 | 0.000 | 3.301 ± 0.013 | 0.000 | −0.825 ± 0.149 | 0.000 |
| Postoperative 3 months | ||||||||
| NDI | −1.414 ± 0.080 | 0.000 | −0.079 ± 0.034 | 0.022 | 0.412 ± 0.038 | 0.000 | 0.831 ± 0.278 | 0.004 |
| AGE | 0.036 ± 0.013 | 0.006 | 0.132 ± 0.015 | 0.000 | 0.141 ± 0.006 | 0.000 | 0.654 ± 0.055 | 0.000 |
| WC | 0.425 ± 0.031 | 0.000 | 0.177 ± 0.028 | 0.000 | 0.612 ± 0.017 | 0.000 | 1.132 ± 0.100 | 0.000 |
| * Modified JOA score | −1.528 ± 0.118 | −0.725 ± 0.163 | 0.000 | −2.569 ± 0.070 | 0.000 | −2.606 ± 0.553 | 0.000 | |
| HGS | −0.335 ± 0.052 | 0.000 | −0.168 ± 0.012 | 0.000 | ||||
| BMI | 0.000 | −0.066 ± 0.063 | 0.000 | −0.989 ± 0.029 | 0.000 | −1.954 ± 0.227 | 0.000 | |
| Postoperative 1 year | ||||||||
| * Modified JOA score | −1.474 ± 0.055 | 0.000 | −1.616 ± 0.000 | 0.000 | −3.734 ± 0.050 | 0.000 | 0.837 ± 0.008 | 0.000 |
| HGS | −0.269 ± 0.016 | 0.000 | 0.010 ± 0.000 | 0.000 | ||||
| Operation length | −0.974 ± 0.001 | 0.000 | −5.774 ± 0.170 | 0.000 | −1.758 ± 0.017 | 0.000 | ||
| * Modified JOA grade | −6.145 ± 0.232 | 0.000 | −6.533 ± 0.002 | 0.000 | −13.403 ± 0.277 | 0.000 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, K.A.; Kwon, J.-W.; Yoon, J.; Lee, H.-M.; Moon, S.-H.; Suk, K.-S.; Kim, H.-S.; Lee, B.H. Handgrip Strength Correlated with Falling Risk in Patients with Degenerative Cervical Myelopathy. J. Clin. Med. 2021, 10, 1980. https://doi.org/10.3390/jcm10091980
Jimenez KA, Kwon J-W, Yoon J, Lee H-M, Moon S-H, Suk K-S, Kim H-S, Lee BH. Handgrip Strength Correlated with Falling Risk in Patients with Degenerative Cervical Myelopathy. Journal of Clinical Medicine. 2021; 10(9):1980. https://doi.org/10.3390/jcm10091980
Chicago/Turabian StyleJimenez, Kathryn Anne, Ji-Won Kwon, Jayeong Yoon, Hwan-Mo Lee, Seong-Hwan Moon, Kyung-Soo Suk, Hak-Sun Kim, and Byung Ho Lee. 2021. "Handgrip Strength Correlated with Falling Risk in Patients with Degenerative Cervical Myelopathy" Journal of Clinical Medicine 10, no. 9: 1980. https://doi.org/10.3390/jcm10091980
APA StyleJimenez, K. A., Kwon, J.-W., Yoon, J., Lee, H.-M., Moon, S.-H., Suk, K.-S., Kim, H.-S., & Lee, B. H. (2021). Handgrip Strength Correlated with Falling Risk in Patients with Degenerative Cervical Myelopathy. Journal of Clinical Medicine, 10(9), 1980. https://doi.org/10.3390/jcm10091980






