Combination of Static Echocardiographic Indices for the Prediction of Fluid Responsiveness in Patients Undergoing Coronary Surgery: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anesthetic Management
2.3. Study Protocol
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marik, P.E.; Baram, M.; Vahid, B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008, 134, 172–178. [Google Scholar] [CrossRef]
- Boyd, J.H.; Forbes, J.; Nakada, T.A.; Walley, K.R.; Russell, J.A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit. Care Med. 2011, 39, 259–265. [Google Scholar] [CrossRef]
- Marik, P.E.; Cavallazzi, R.; Vasu, T.; Hirani, A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit. Care Med. 2009, 37, 2642–2647. [Google Scholar] [CrossRef] [PubMed]
- Michard, F. Changes in arterial pressure during mechanical ventilation. Anesthesiology 2005, 103, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wyler von Ballmoos, M.; Takala, J.; Roeck, M.; Porta, F.; Tueller, D.; Ganter, C.C.; Schroder, R.; Bracht, H.; Baenziger, B.; Jakob, S.M. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: A clinical study. Crit. Care 2010, 14, R111. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, Y.; Shim, J.K.; Kwak, Y.L. Effect of pulse pressure on the predictability of stroke volume variation for fluid responsiveness in patients with coronary disease. J. Crit. Care 2013, 28, 318.e1–318.e7. [Google Scholar] [CrossRef] [PubMed]
- Osman, D.; Ridel, C.; Ray, P.; Monnet, X.; Anguel, N.; Richard, C.; Teboul, J.L. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit. Care Med. 2007, 35, 64–68. [Google Scholar] [CrossRef]
- Ommen, S.R.; Nishimura, R.A.; Appleton, C.P.; Miller, F.A.; Oh, J.K.; Redfield, M.M.; Tajik, A.J. Clinical utility of doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous doppler-catheterization study. Circulation 2000, 102, 1788–1794. [Google Scholar] [CrossRef]
- Zile, M.R.; Brutsaert, D.L. New concepts in diastolic dysfunction and diastolic heart failure: Part I: Diagnosis, prognosis, and measurements of diastolic function. Circulation 2002, 105, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Mogelvang, R.; Sogaard, P.; Pedersen, S.A.; Olsen, N.T.; Marott, J.L.; Schnohr, P.; Goetze, J.P.; Jensen, J.S. Cardiac dysfunction assessed by echocardiographic tissue doppler imaging is an independent predictor of mortality in the general population. Circulation 2009, 119, 2679–2685. [Google Scholar] [CrossRef]
- Jacob, R.; Dierberger, B.; Kissling, G. Functional significance of the frank-starling mechanism under physiological and pathophysiological conditions. Eur. Heart J. 1992, 13, 7–14. [Google Scholar] [CrossRef]
- Song, Y.; Kwak, Y.L.; Song, J.W.; Kim, Y.J.; Shim, J.K. Respirophasic carotid artery peak velocity variation as a predictor of fluid responsiveness in mechanically ventilated patients with coronary artery disease. Br. J. Anaesth. 2014, 113, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Denault, A.; Canty, D.; Azzam, M.; Amir, A.; Gebhard, C.E. Whole body ultrasound in the operating room and intensive care unit. Korean J. Anesthesiol. 2019, 72, 413–428. [Google Scholar] [CrossRef]
- Boyd, J.H.; Sirounis, D.; Maizel, J.; Slama, M. Echocardiography as a guide for fluid management. Crit. Care 2016, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Schneck, E.; Habicher, M. Management of perioperative volume therapy-monitoring and pitfalls. Korean J. Anesthesiol. 2020, 73, 103–113. [Google Scholar] [CrossRef]
- Levine, H.J. Compliance of the left ventricle. Circulation 1972, 46, 423–426. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the american society of echocardiography and the european association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the japanese society of echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef]
- Rivas-Gotz, C.; Manolios, M.; Thohan, V.; Nagueh, S.F. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue doppler and flow propagation velocity. Am. J. Cardiol. 2003, 91, 780–784. [Google Scholar] [CrossRef]
- Kasner, M.; Westermann, D.; Steendijk, P.; Gaub, R.; Wilkenshoff, U.; Weitmann, K.; Hoffmann, W.; Poller, W.; Schultheiss, H.P.; Pauschinger, M.; et al. Utility of doppler echocardiography and tissue doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: A comparative doppler-conductance catheterization study. Circulation 2007, 116, 637–647. [Google Scholar] [CrossRef]
- Yip, G.W.; Zhang, Y.; Tan, P.Y.; Wang, M.; Ho, P.Y.; Brodin, L.A.; Sanderson, J.E. Left ventricular long-axis changes in early diastole and systole: Impact of systolic function on diastole. Clin. Sci. 2002, 102, 515–522. [Google Scholar] [CrossRef]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, B.W.; Cramer, M.J.; Oh, J.K.; van der Aa, R.P.; Jaarsma, W. Spectral pulsed tissue doppler imaging in diastole: A tool to increase our insight in and assessment of diastolic relaxation of the left ventricle. Am. Heart J. 2003, 146, 411–419. [Google Scholar] [CrossRef]
- Ohte, N.; Narita, H.; Hashimoto, T.; Akita, S.; Kurokawa, K.; Fujinami, T. Evaluation of left ventricular early diastolic performance by color tissue doppler imaging of the mitral annulus. Am. J. Cardiol. 1998, 82, 1414–1417. [Google Scholar] [CrossRef]
- Marques, N.R.; De Riese, J.; Yelverton, B.C.; McQuitty, C.; Jupiter, D.; Willmann, K.; Salter, M.; Kinsky, M.; Johnston, W.E. Diastolic function and peripheral venous pressure as indices for fluid responsiveness in cardiac surgical patients. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2208–2215. [Google Scholar] [CrossRef]
- Shim, J.K.; Song, J.W.; Song, Y.; Kim, J.H.; Kang, H.M.; Kwak, Y.L. Pulse pressure variation is not a valid predictor of fluid responsiveness in patients with elevated left ventricular filling pressure. J. Crit. Care 2014, 29, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.S.; Shah, S.J.; Thomas, J.D. A test in context: E/A and E/e’ to assess diastolic dysfunction and LV filling pressure. J. Am. Coll. Cardiol. 2017, 69, 1451–1464. [Google Scholar] [CrossRef]

| Responders (n = 40) | Non-Responders (n = 24) | p | |
|---|---|---|---|
| Age (years) | 66 ± 8 | 65 ± 8 | 0.577 |
| Female | 12 (30) | 2 (8) | 0.042 |
| Body mass index (kg/m2) | 25.3 ± 2.6 | 23.8 ± 3.1 | 0.037 |
| Hypertension | 24 (60) | 17 (71) | 0.382 |
| Diabetes mellitus | 12 (30) | 15 (63) | 0.011 |
| Cerebrovascular accident | 7 (18) | 0 (0) | 0.039 |
| Myocardial infarction (<3 months) | 4 (10) | 2 (8) | 1.000 |
| Left main disease (>50% stenosis) | 17 (43) | 10 (42) | 0.948 |
| LVEF (%) | 64 ± 6 | 67 ± 8 | 0.227 |
| Medications | |||
| Statin | 27 (68) | 19 (80) | 0.315 |
| Nitrate | 16 (40) | 10 (42) | 0.895 |
| Beta blocker | 19 (48) | 14 (58) | 0.401 |
| Calcium channel blocker | 20 (50) | 6 (25) | 0.049 |
| Renin-angiotensin system antagonist | 23 (58) | 12 (50) | 0.560 |
| Patients requiring norepinephrine | 35 (88) | 21 (88) | 1.000 |
| Plateau inspiratory pressure (cmH2O) | 15 (14–16) | 15 (14–16) | 0.486 |
| Responders (n = 40) | Non-Responders (n = 24) | |||||
|---|---|---|---|---|---|---|
| Baseline | After Fluid Challenge | p | Baseline | After Fluid Challenge | p | |
| HR (bpm) | 59 ± 9 | 55 ± 6 | <0.001 | 57 ± 5 | 55 ± 5 | <0.001 |
| MAP (mmHg) | 72 ± 8 | 76 ± 9 | <0.001 | 70 ± 8 | 76 ± 6 | <0.001 |
| MPAP (mmHg) | 17 (15–19) | 20 (18–22) | <0.001 | 16 (14–17) | 19 (17–22) | <0.001 |
| CI (L/min/m2) | 2.1 (1.9–2.3) | 2.6 (2.3–2.9) * | <0.001 | 2.3 (2.0–2.6) | 2.1 (1.9–2.4) | <0.001 |
| SVI (mL/beat/m2) | 35 (32–38) * | 46 (42–52) * | <0.001 | 38 (33–48) | 37 (34–44) | <0.001 |
| CVP (mmHg) | 9 (8–11) | 12 (10–13) | <0.001 | 9 (8–10) | 11 (10–13) | <0.001 |
| PAOP (mmHg) | 13 (12–14) | 16 (14–17) | <0.001 | 12 (10–14) | 15 (13–18) | <0.001 |
| PPV (%) | 13 (8–14) * | 4 (3–6) * | <0.001 | 9 (7–11) | 6 (5–7) | <0.001 |
| Baseline | After Fluid Challenge | p1 a | ||
|---|---|---|---|---|
| LVEDA (cm2) | Responder | 21.1 ± 3.8 | 23.9 ± 3.6 | <0.001 |
| Non-responder | 22.5 ± 3.0 | 23.9 ± 2.7 | <0.001 | |
| p2 b | 0.140 | 0.990 | ||
| E/E’ | Responder | 6.2 (5.6–7.0) | 7.1 (6.1–8.0) | <0.001 |
| Non-responder | 8.2 (6.1–9.2) | 8.9 (7.4–9.8) | 0.031 | |
| p2 | 0.006 | 0.002 | ||
| E’/S’ | Responder | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.004 |
| Non-responder | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.035 | |
| p2 | 0.024 | 0.099 | ||
| E’/A’ | Responder | 1.3 (1.0–1.5) | 1.4 (1.2–1.6) | 0.013 |
| Non-responder | 1.1 (0.9–1.4) | 1.1 (0.9–1.5) | 0.147 | |
| p2 | 0.140 | 0.048 |
| AUROC | 95% CI | p | |
|---|---|---|---|
| Invasive indices | |||
| Central venous pressure | 0.58 | 0.43–0.73 | 0.311 |
| Pulmonary artery occlusion pressure | 0.56 | 0.41–0.71 | 0.442 |
| Pulse pressure variation | 0.70 | 0.57–0.83 | 0.002 |
| Echocardiographic indices | |||
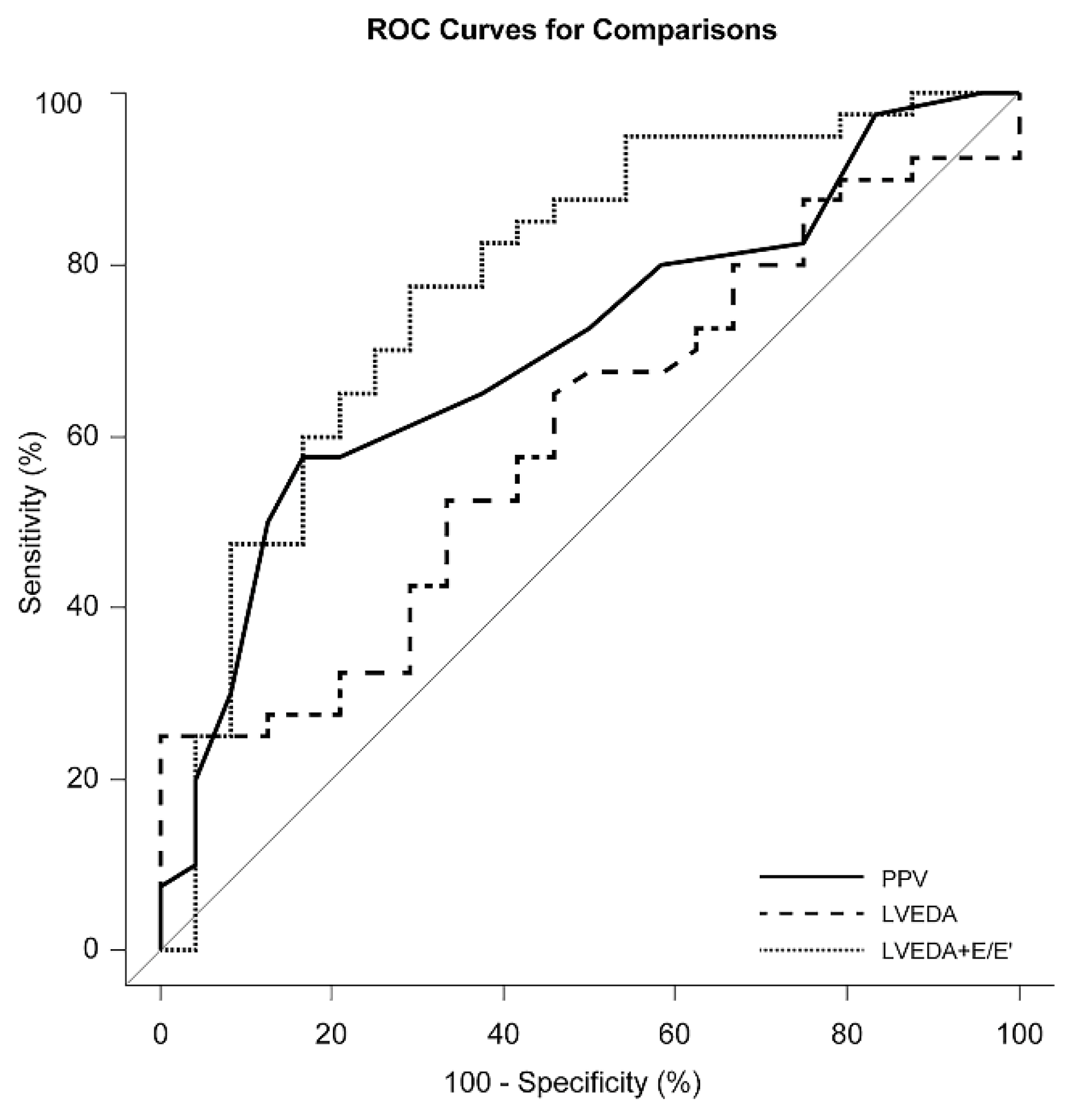
| Left ventricular end-diastolic area | 0.60 | 0.46–0.74 | 0.170 |
| E/E’ | 0.71 | 0.56–0.85 | 0.006 |
| E’/S’ | 0.68 | 0.54–0.82 | 0.017 |
| E’/A’ | 0.61 | 0.47–0.75 | 0.140 |
| Combination of echocardiographic indices | |||
| Left ventricular end-diastolic area with E/E’ | 0.78 | 0.66–0.90 | <0.001 |
| Left ventricular end-diastolic area with E’/S’ | 0.68 | 0.54–0.82 | 0.012 |
| Left ventricular end-diastolic area with E’/A’ | 0.66 | 0.52–0.80 | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-B.; Soh, S.; Song, J.-W.; Kim, M.-Y.; Kwak, Y.-L.; Shim, J.-K. Combination of Static Echocardiographic Indices for the Prediction of Fluid Responsiveness in Patients Undergoing Coronary Surgery: A Pilot Study. J. Clin. Med. 2021, 10, 1886. https://doi.org/10.3390/jcm10091886
Kim H-B, Soh S, Song J-W, Kim M-Y, Kwak Y-L, Shim J-K. Combination of Static Echocardiographic Indices for the Prediction of Fluid Responsiveness in Patients Undergoing Coronary Surgery: A Pilot Study. Journal of Clinical Medicine. 2021; 10(9):1886. https://doi.org/10.3390/jcm10091886
Chicago/Turabian StyleKim, Hye-Bin, Sarah Soh, Jong-Wook Song, Min-Yu Kim, Young-Lan Kwak, and Jae-Kwang Shim. 2021. "Combination of Static Echocardiographic Indices for the Prediction of Fluid Responsiveness in Patients Undergoing Coronary Surgery: A Pilot Study" Journal of Clinical Medicine 10, no. 9: 1886. https://doi.org/10.3390/jcm10091886
APA StyleKim, H.-B., Soh, S., Song, J.-W., Kim, M.-Y., Kwak, Y.-L., & Shim, J.-K. (2021). Combination of Static Echocardiographic Indices for the Prediction of Fluid Responsiveness in Patients Undergoing Coronary Surgery: A Pilot Study. Journal of Clinical Medicine, 10(9), 1886. https://doi.org/10.3390/jcm10091886







