Low Hypoperfusion Intensity Ratio Is Associated with a Favorable Outcome Even in Large Ischemic Core and Delayed Recanalization Time
Abstract
1. Introduction
2. Materials and Methods
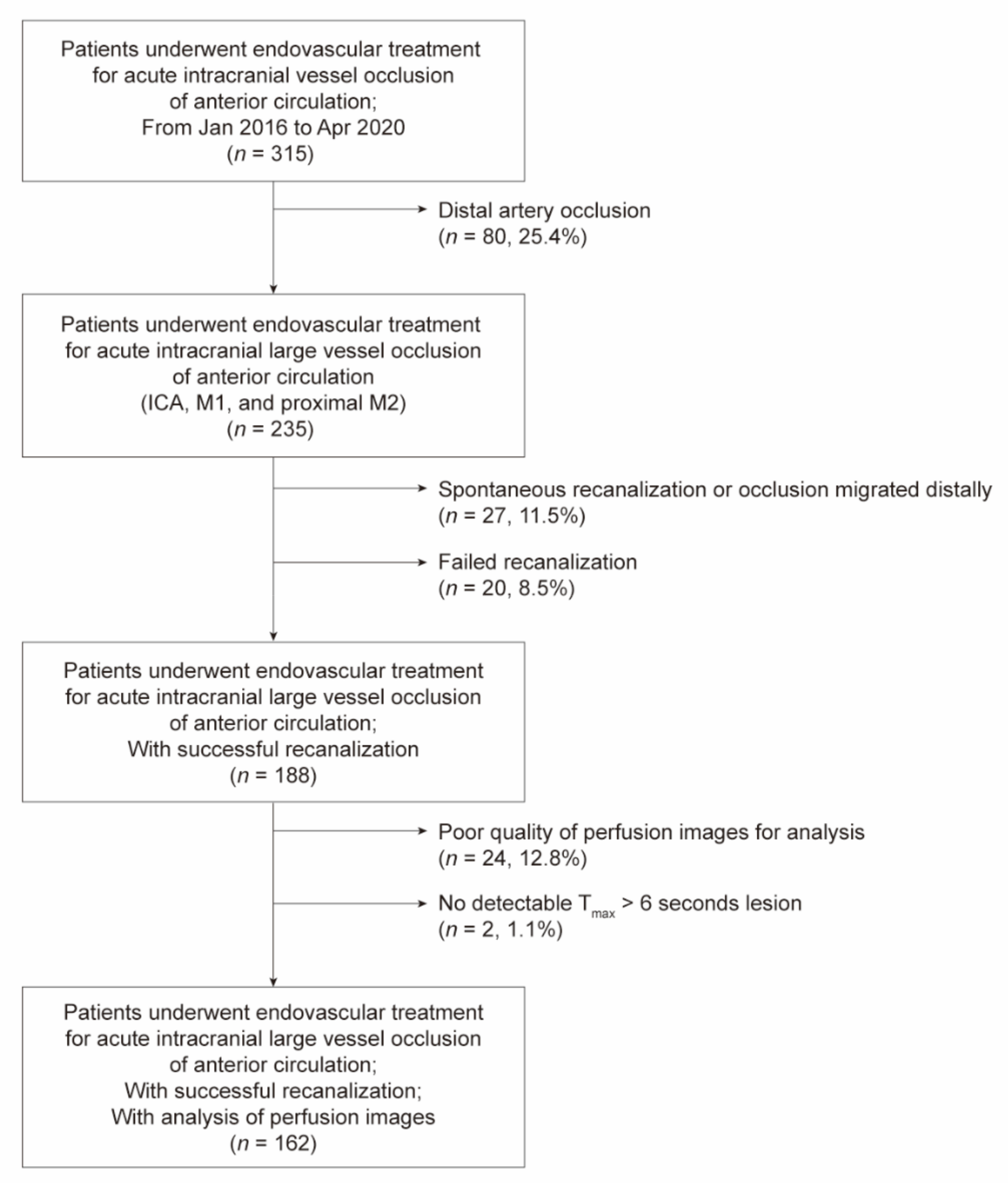
2.1. Participants
2.2. Imaging and Clinical Data
2.3. Statistical Analysis
3. Results
3.1. Association between the HIR and Clinical Outcomes
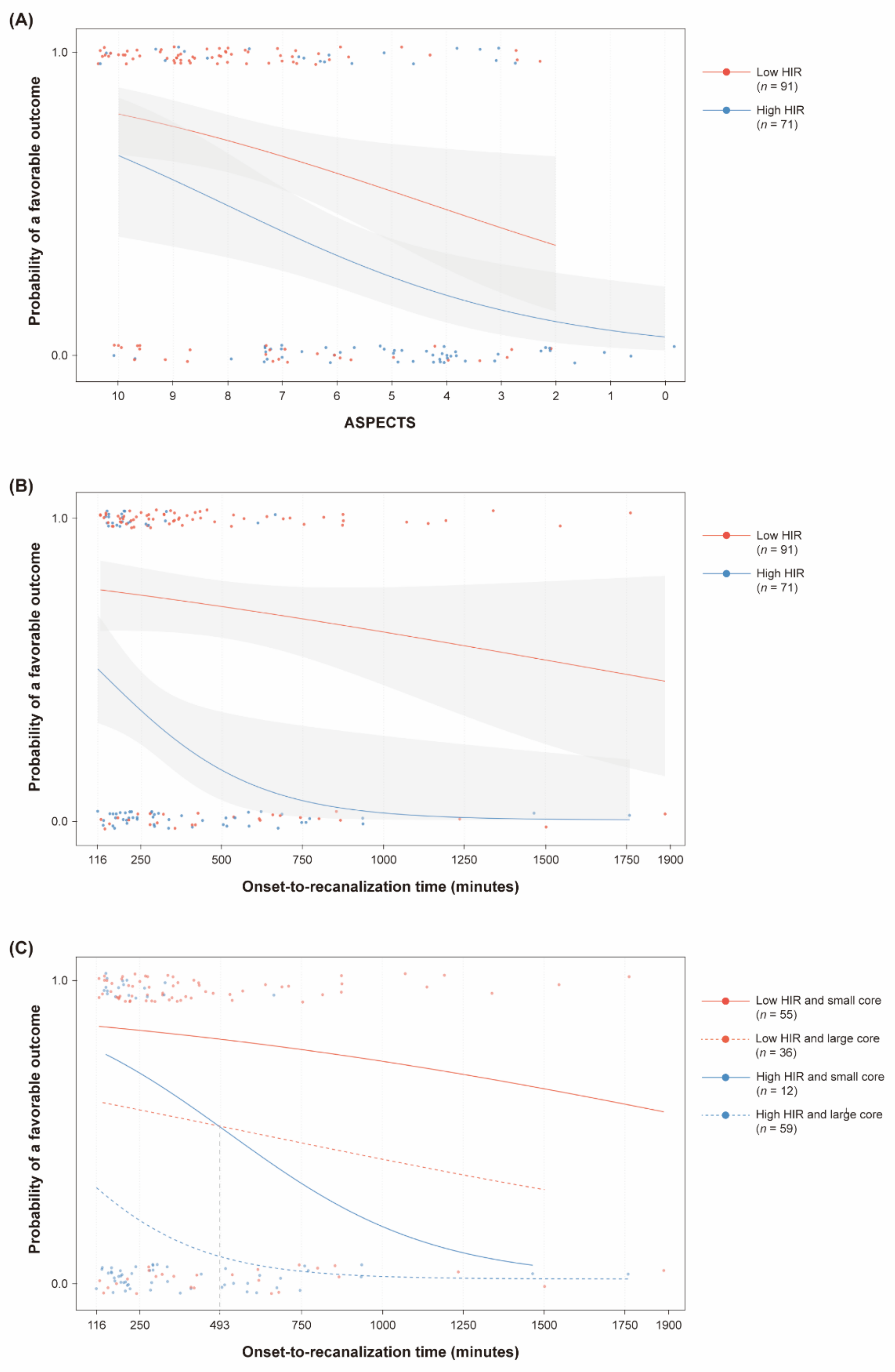
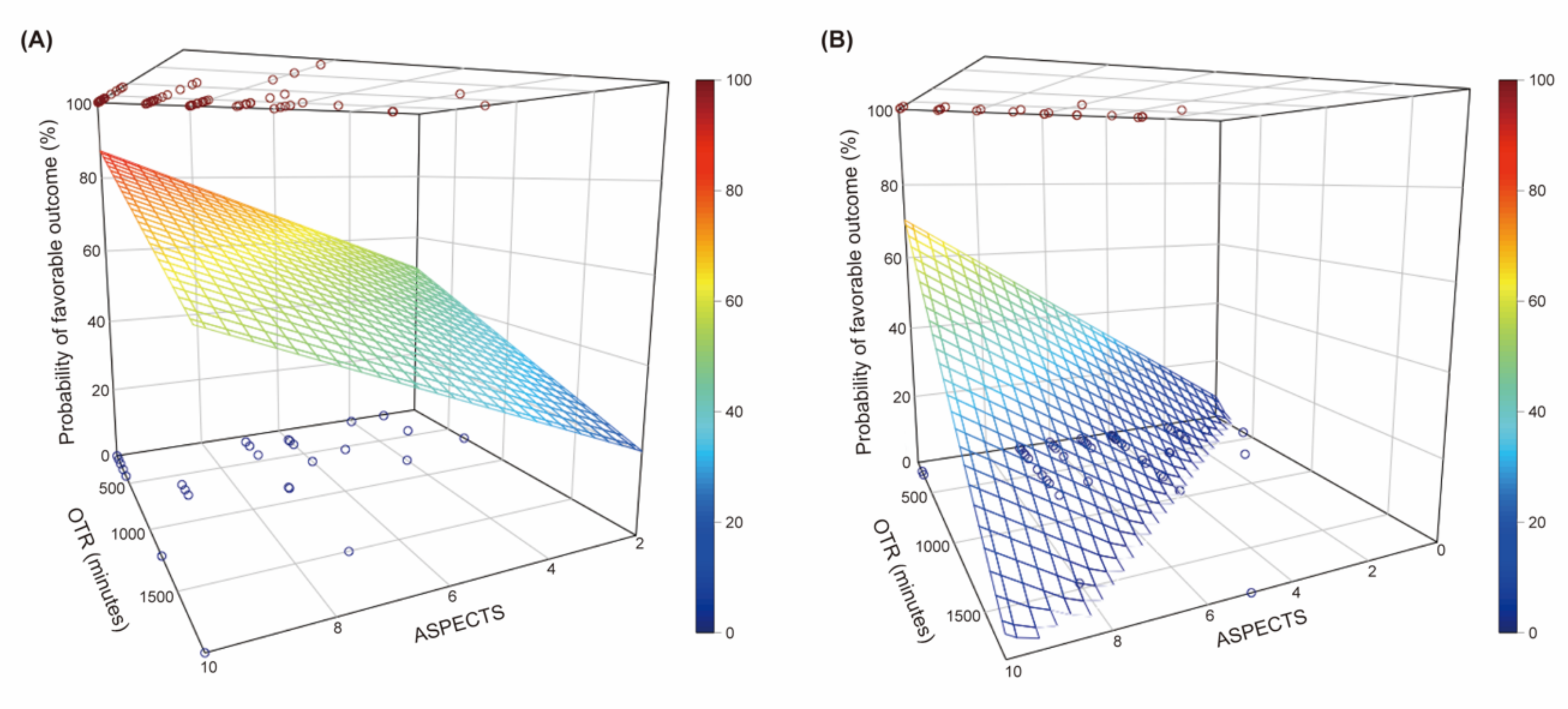
3.2. Association between the ASPECTS, Onset-To-Recanalization Time, and Clinical Outcomes According to the HIR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berkhemer, O.A.; Jansen, I.G.H.; Beumer, D.; Fransen, P.S.S.; Berg, L.A.V.D.; Yoo, A.J.; Lingsma, H.F.; Sprengers, M.E.S.; Jenniskens, S.F.M.; Nijeholt, G.J.L.À.; et al. Collateral Status on Baseline Computed Tomographic Angiography and Intra-Arterial Treatment Effect in Patients With Proximal Anterior Circulation Stroke. Stroke 2016, 47, 768–776. [Google Scholar] [CrossRef] [PubMed]
- De Havenon, A.; Mlynash, M.; Kim-Tenser, M.A.; Lansberg, M.G.; Leslie-Mazwi, T.; Christensen, S.; McTaggart, R.A.; Alexander, M.; Albers, G.; Broderick, J.; et al. Results from DEFUSE 3: Good Collaterals are Associated with Reduced Ischemic Core Growth but not Neurologic Outcome. Stroke 2019, 50, 632–638. [Google Scholar] [CrossRef]
- Kim, B.J.; Chung, J.-W.; Park, H.-K.; Kim, J.Y.; Yang, M.-H.; Han, M.-K.; Jeong, C.; Hwang, G.; Kwon, O.-K.; Bae, H.-J. CT Angiography of Collateral Vessels and Outcomes in Endovascular-Treated Acute Ischemic Stroke Patients. J. Clin. Neurol. 2017, 13, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Elijovich, L.; Goyal, N.; Mainali, S.; Hoit, D.; Arthur, A.S.; Whitehead, M.; Choudhri, A.F. CTA Collateral Score Predicts Infarct Volume and Clinical Outcome after Endovascular Therapy for Acute Ischemic Stroke: A Retrospective Chart Review. J. Neuro Interv. Surg. 2016, 8, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Baek, J.-H.; Heo, J.H.; Nam, H.S.; Kim, Y.D.; Yoo, J.; Kim, D.J.; Jeon, P.; Baik, S.K.; Suh, S.H.; et al. Collateral Status Affects the Onset-to-Reperfusion Time Window for Good Outcome. J. Neurol. Neurosurg. Psychiatry 2018, 89, 903–909. [Google Scholar] [CrossRef]
- Bang, O.Y.; Saver, J.L.; Alger, J.R.; Starkman, S.; Ovbiagele, B.; Liebeskind, D.S. Determinants of the Distribution and Severity of Hypoperfusion in Patients with Ischemic Stroke. Neurology 2008, 71, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Olivot, J.M.; Mlynash, M.; Inoue, M.; Marks, M.P.; Wheeler, H.M.; Kemp, S.; Straka, M.; Zaharchuk, G.; Bammer, R.; Lansberg, M.G.; et al. Hypoperfusion Intensity Ratio Predicts Infarct Progression and Functional Outcome in the DEFUSE 2 Cohort. Stroke 2014, 45, 1018–1023. [Google Scholar] [CrossRef]
- Guenego, A.; Mlynash, M.; Christensen, S.; Bs, S.K.; Heit, J.J.; Lansberg, M.G.; Albers, G.W. Hypoperfusion Ratio Predicts Infarct Growth During Transfer for Thrombectomy. Ann. Neurol. 2018, 84, 616–620. [Google Scholar] [CrossRef]
- Guenego, A.; Fahed, R.; Albers, G.W.; Kuraitis, G.; Sussman, E.S.; Martin, B.W.; Marcellus, D.G.; Olivot, J.; Marks, M.P.; Lansberg, M.G.; et al. Hypoperfusion Intensity Ratio Correlates with Angiographic Collaterals in Acute Ischaemic Stroke with M1 Occlusion. Eur. J. Neurol. 2020, 27, 864–870. [Google Scholar] [CrossRef]
- Wouters, A.; Dupont, P.; Christensen, S.; Norrving, B.; Laage, R.; Thomalla, G.; Albers, G.; Thijs, V.; Lemmens, R. Association Between Time From Stroke Onset and Fluid-Attenuated Inversion Recovery Lesion Intensity Is Modified by Status of Collateral Circulation. Stroke 2016, 47, 1018–1022. [Google Scholar] [CrossRef]
- Christensen, S.; Mlynash, M.; Kemp, S.; Yennu, A.; Heit, J.J.; Marks, M.P.; Lansberg, M.G.; Albers, G.W. Persistent Target Mismatch Profile >24 Hours After Stroke Onset in DEFUSE 3. Stroke 2019, 50, 754–757. [Google Scholar] [CrossRef]
- Baek, J.-H.; Kim, B.M.; Kang, D.-H.; Heo, J.H.; Nam, H.S.; Kim, Y.D.; Hwang, Y.-H.; Kim, Y.-W.; Kim, D.J.; Kwak, H.S.; et al. Balloon Guide Catheter Is Beneficial in Endovascular Treatment Regardless of Mechanical Recanalization Modality. Stroke 2019, 50, 1490–1496. [Google Scholar] [CrossRef]
- Yaghi, S.; Willey, J.Z.; Cucchiara, B.; Goldstein, J.N.; Gonzales, N.R.; Khatri, P.; Kim, L.J.; Mayer, S.A.; Sheth, K.N.; Schwamm, L.H. Treatment and Outcome of Hemorrhagic Transformation After Intravenous Alteplase in Acute Ischemic Stroke: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e343–e361. [Google Scholar] [CrossRef]
- Barber, P.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and Reliability of a Quantitative Computed Tomography Score in Predicting Outcome of Hyperacute Stroke Before Thrombolytic Therapy. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef]
- Hotter, B.; Ostwaldt, A.-C.; Levichev-Connolly, A.; Rozanski, M.; Audebert, H.J.; Fiebach, J.B. Natural Course of Total Mismatch and Predictors for Tissue Infarction. Neurology 2015, 85, 770–775. [Google Scholar] [CrossRef]
- Jeong, H.G.; Kim, B.J.; Kim, H.; Cheolkyu, J.; Moon-Ku, H.; David, S.; Liebeskind, H.-J.B. Blood Pressure Drop and Penumbral Tissue Loss in Nonrecanalized Emergent Large Vessel Oc-Clusion. Stroke 2019, 50, 2677–2684. [Google Scholar] [CrossRef]
- Rao, V.L.; Mlynash, M.; Christensen, S.; Yennu, A.; Kemp, S.; Zaharchuk, G.; Heit, J.J.; Marks, M.P.; Lansberg, M.G.; Albers, G.W. Collateral Status Contributes to Differences Between Observed and Predicted 24-h Infarct Volumes in DEFUSE 3. Br. J. Pharmacol. 2020, 40, 1966–1974. [Google Scholar] [CrossRef]
- Guenego, A.; Marcellus, D.G.; Martin, B.W.; Soren, C.; Gregory, W.A.; Maarten, G.L.; Michael, P.M.; Max, W.; Jeremy, J.H. Hypoperfusion Intensity Ratio is Correlated with Patient Eligibility for Thrombectomy. Stroke 2019, 50, 917–922. [Google Scholar] [CrossRef]
- Mohammaden, M.H.; Haussen, D.C.; Pisani, L.; Al-Bayati, A.R.; Da Camara, C.P.; Bhatt, N.; Belagaje, S.R.; Liberato, B.B.; Bianchi, N.; Anderson, A.M.; et al. Baseline ASPECTS and Hypoperfusion Intensity Ratio Influence the Impact of First Pass Reperfusion on Functional Outcomes. J. Neuro Interv. Surg. 2021, 13, 124–129. [Google Scholar] [CrossRef]
- Mahdjoub, E.; Turc, G.; Legrand, L.J.; Benzakoun, M.; Edjlali, P.; Seners, S.; Charron, W.; Ben, H.O.; Naggara, J.-F.; Meder, J.-L.; et al. Baron Do Fluid-Attenuated Inversion Recovery Vascular Hyperintensities Represent Good Col-Laterals Before Reperfusion Therapy? AJNR Am. J. Neuroradiol. 2018, 39, 77–83. [Google Scholar] [CrossRef]
- Kufner, A.; Galinovic, I.; Ambrosi, V.; Nolte, C.H.; Endres, M.; Fiebach, J.B.; Ebinger, M. Hyperintense Vessels on FLAIR, Hemodynamic Correlates and Response to Throm-Bolysis. AJNR Am. J. Neuroradiol. 2015, 36, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, B.M.; Baek, J.-H.; Kim, J.-H.; Heo, J.H.; Kim, D.J.; Nam, H.S.; Kim, Y.D. Predictors of Good Outcomes in Patients with Failed Endovascular Thrombectomy. Korean J. Radiol. 2020, 21, 582–587. [Google Scholar] [CrossRef] [PubMed]


| Variables | All Patients (n = 162) | Favorable Outcome (n = 85) | Unfavorable Outcome (n = 77) | p-Value |
|---|---|---|---|---|
| Age | 70.7 (± 12.8) | 67.6 (± 12.9) | 74.1 (± 11.8) | 0.001 |
| Sex, male | 84 (51.9) | 48 (56.5) | 36 (46.8) | 0.216 |
| Hypertension | 122 (75.3) | 65 (76.5) | 57 (74.0) | 0.719 |
| Diabetes | 75 (46.3) | 31 (36.5) | 44 (57.1) | 0.008 |
| Hypercholesterolemia | 77 (47.5) | 39 (45.9) | 38 (49.4) | 0.659 |
| Smoking | 31 (19.1) | 21 (24.7) | 10 (13.0) | 0.058 |
| Coronary artery disease | 29 (17.9) | 19 (22.4) | 11 (14.3) | 0.187 |
| Atrial fibrillation | 80 (49.4) | 38 (44.7) | 42 (54.5) | 0.211 |
| Occlusion site | 0.406 | |||
| Internal carotid artery | 64 (39.5) | 31 (36.5) | 33 (42.9) | |
| Middle cerebral artery | 98 (60.5) | 54 (63.5) | 44 (57.1) | |
| Initial NIHSS score | 15.0 [11.0; 19.8] | 12.0 [7.0; 16.0] | 19.0 [15.0; 20.0] | <0.001 |
| Use of intravenous tPA | 78 (48.1) | 50 (58.8) | 28 (36.4) | 0.004 |
| ASPECTS | 7.0 [5.0; 9.0] | 8.0 [7.0; 9.0] | 6.0 [4.0; 7.0] | <0.001 |
| Onset-to-recanalization, min | 298.5 [197.0; 583.5] | 274.0 [190.0; 434.0] | 336.0 [212.0; 674.0] | 0.045 |
| Symptomatic ICH | 10 (6.2) | 4 (4.7) | 6 (7.8) | 0.520 |
| Volume of rCBF < 30% (mm3) | 33.7 (± 53.6) | 12.0 (± 21.7) | 57.6 (± 66.7) | <0.001 |
| Volume of Tmax > 6 s (mm3) | 160.8 (± 76.7) | 140.4 (± 67.6) | 183.2 (± 80.2) | <0.001 |
| Hypoperfusion intensity ratio | 0.51 [0.29; 0.68] | 0.45 [0.15; 0.54] | 0.60 [0.44; 0.73] | <0.001 |
| Variables | Model 1 | p-Value | Model 2 | p-Value |
|---|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | |||
| Age | 0.97 (0.94–1.01) | 0.153 | 0.98 (0.94–1.01) | 0.171 |
| Diabetes | 0.95 (0.40–2.25) | 0.899 | 0.77 (0.34–1.76) | 0.531 |
| Smoking | 2.35 (0.75–7.37) | 0.142 | 1.74 (0.59–5.14) | 0.314 |
| Initial NIHSS score | 0.81 (0.74–0.89) | <0.001 | 0.82 (0.74–0.90) | <0.001 |
| Use of intravenous tPA | 2.72 (1.06–6.97) | 0.037 | 2.66 (1.07–6.65) | 0.036 |
| ASPECTS | 1.33 (1.10–1.61) | 0.003 | ||
| Onset-to-recanalization time (per 30 min) | 0.97 (0.93–1.00) | 0.086 | 0.96 (0.93–1.00) | 0.069 |
| Hypoperfusion intensity ratio (per 0.1) | 0.84 (0.68–1.03) | 0.094 | 0.76 (0.62–0.92) | 0.006 |
| Variables | Low HIR Group (n = 91) | p-Value | High HIR Group (n = 71) | p-Value |
|---|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | |||
| Age | 0.96 (0.92–1.01) | 0.137 | ||
| Diabetes | 0.26 (0.07–1.01) | 0.051 | ||
| Smoking | 2.70 (0.42–17.6) | 0.298 | ||
| Initial NIHSS score | 0.82 (0.73–0.93) | 0.002 | 0.80 (0.68–0.95) | 0.010 |
| Use of intravenous tPA | 2.34 (0.67–8.16) | 0.182 | 4.27 (0.76–23.9) | 0.098 |
| ASPECTS | 1.20 (0.92–1.56) | 0.178 | 1.49 (1.09–2.03) | 0.012 |
| Onset-to-recanalization time (per 30 min) | 0.97 (0.94–1.01) | 0.194 | 0.92 (0.79–1.07) | 0.283 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.-H.; Kim, Y.D.; Lee, K.J.; Choi, J.K.; Baik, M.; Kim, B.M.; Kim, D.J.; Heo, J.H.; Nam, H.S. Low Hypoperfusion Intensity Ratio Is Associated with a Favorable Outcome Even in Large Ischemic Core and Delayed Recanalization Time. J. Clin. Med. 2021, 10, 1869. https://doi.org/10.3390/jcm10091869
Baek J-H, Kim YD, Lee KJ, Choi JK, Baik M, Kim BM, Kim DJ, Heo JH, Nam HS. Low Hypoperfusion Intensity Ratio Is Associated with a Favorable Outcome Even in Large Ischemic Core and Delayed Recanalization Time. Journal of Clinical Medicine. 2021; 10(9):1869. https://doi.org/10.3390/jcm10091869
Chicago/Turabian StyleBaek, Jang-Hyun, Young Dae Kim, Ki Jeong Lee, Jin Kyo Choi, Minyoul Baik, Byung Moon Kim, Dong Joon Kim, Ji Hoe Heo, and Hyo Suk Nam. 2021. "Low Hypoperfusion Intensity Ratio Is Associated with a Favorable Outcome Even in Large Ischemic Core and Delayed Recanalization Time" Journal of Clinical Medicine 10, no. 9: 1869. https://doi.org/10.3390/jcm10091869
APA StyleBaek, J.-H., Kim, Y. D., Lee, K. J., Choi, J. K., Baik, M., Kim, B. M., Kim, D. J., Heo, J. H., & Nam, H. S. (2021). Low Hypoperfusion Intensity Ratio Is Associated with a Favorable Outcome Even in Large Ischemic Core and Delayed Recanalization Time. Journal of Clinical Medicine, 10(9), 1869. https://doi.org/10.3390/jcm10091869







