Rota-Lithotripsy—A Novel Bail-Out Strategy for Calcified Coronary Lesions in Acute Coronary Syndrome. The First-in-Man Experience
Abstract
1. Introduction
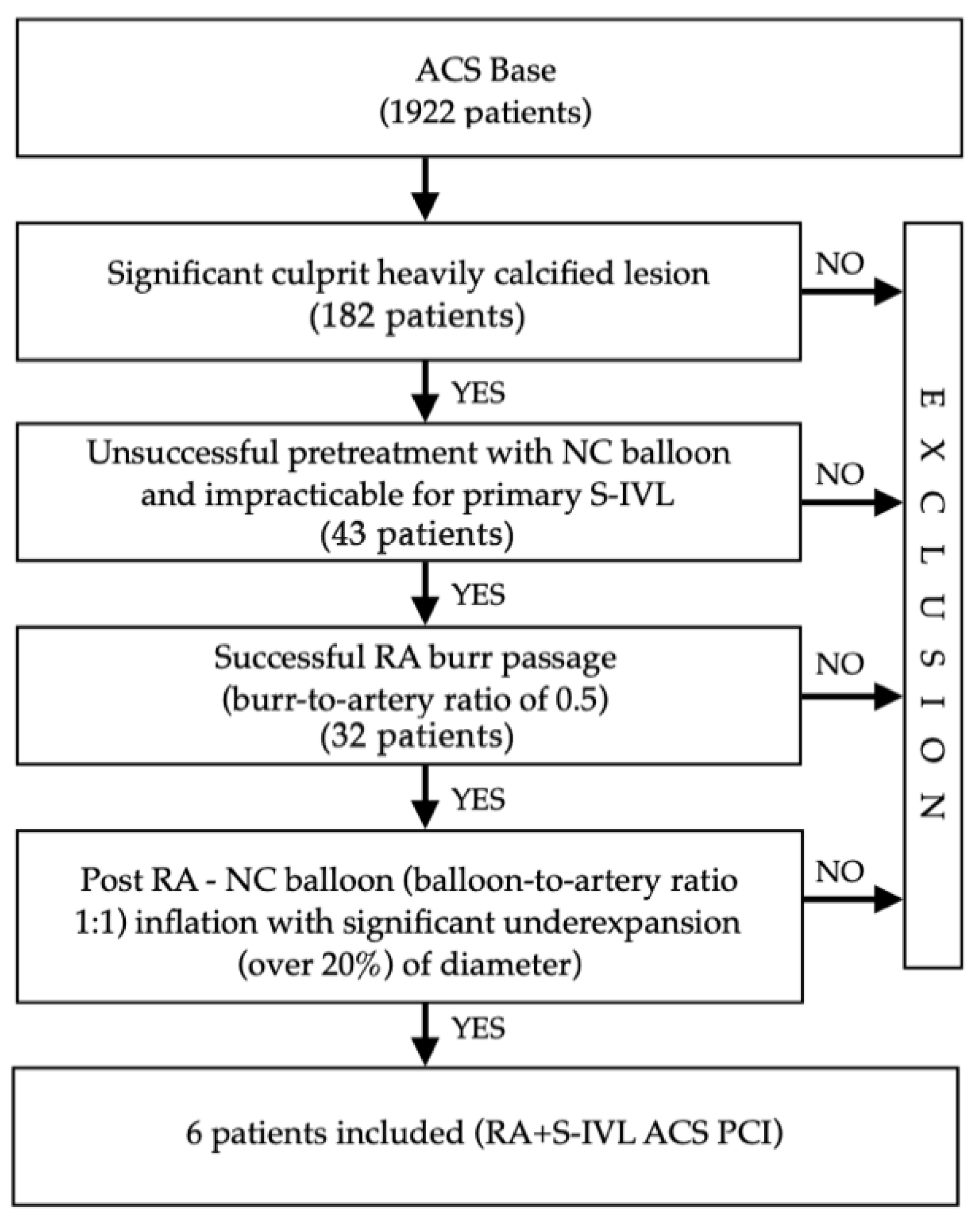
2. Materials and Methods
3. Cases
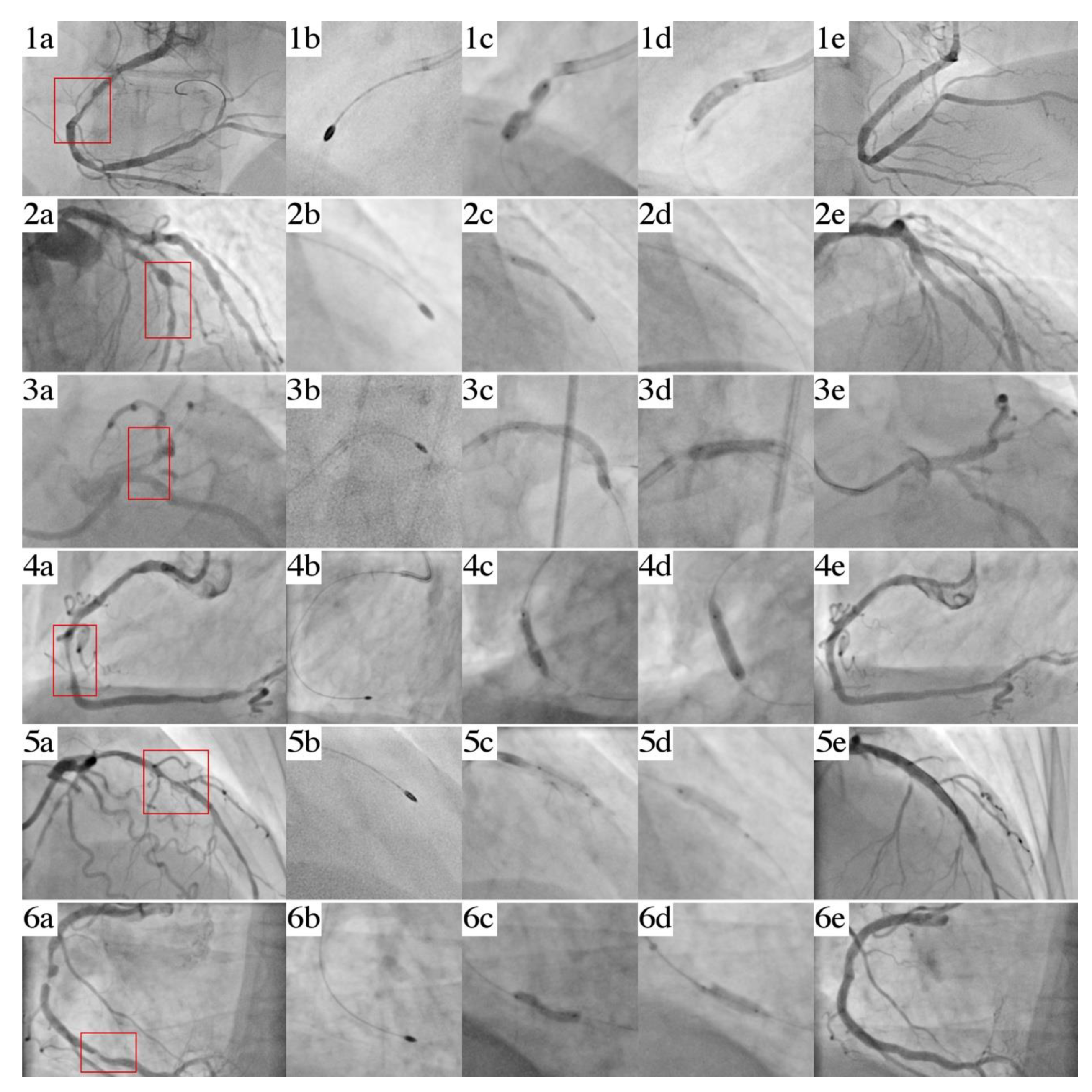
3.1. Case 1
3.2. Case 2
3.3. Case 3
3.4. Case 4
3.5. Case 5
3.6. Case 6
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sugiyama, T.; Yamamoto, E.; Fracassi, F.; Lee, H.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; et al. Calcified plaques in patients with acute coronary syndromes. Cardiovasc. Interv. 2019, 12, 531–540. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bolduan, R.W.; Patel, M.R.; Martinsen, B.J.; Azemi, T.; Giugliano, G.; Resar, J.R.; Mehran, R.; Cohen, D.J.; Popma, J.J.; et al. Impact of calcification on percutaneous coronary intervention: MACE-Trial 1-year results. Catheter. Cardiovasc. Interv. 2019, 94, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Young, R.; Burke, G.; Jeffrey Carr, J.; Detrano, R.C.; Folsom, A.R.; Kronmal, R.; Lima, J.A.C.; Liu, K.J.; McClelland, R.L.; et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: The multi-ethnic study of atherosclerosis (MESA). Eur. Heart J. 2018, 39, 2401–2408. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Neumann, F.J.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardio Thorac. Surg. 2019, 55, 4–90. [Google Scholar] [CrossRef]
- Onuma, Y.; Tanimoto, S.; Ruygrok, P.; Neuzner, J.; Piek, J.J.; Seth, A.; Schofer, J.J.; Richardt, G.; Wiemer, M.; Carrié, D.; et al. Efficacy of everolimus eluting stent implantation in patients with calcified coronary culprit lesions: Two-year angiographic and three-year clinical results from the SPIRIT II study. Catheter. Cardiovasc. Interv. 2010, 76, 634–642. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, C.; Jackson, M.W.P.; Brilakis, E.S.; Egred, M. Uncrossable and undilatable lesions-A practical approach to optimizing outcomes in PCI. Catheter. Cardiovasc. Interv. 2021, 97, 121–126. [Google Scholar] [CrossRef]
- Karimi Galougahi, K.; Patel, S.; Shlofmitz, R.A.; Maehara, A.; Kereiakes, D.J.; Hill, J.M.; Stone, G.W.; Ali, Z.A. Calcific plaque modification by acoustic shockwaves: Intravascular lithotripsy in coronary interventions. Circ. Cardiovasc. Interv. 2021, 14, e009354. [Google Scholar] [CrossRef]
- Wong, B.; El-Jack, S.; Newcombe, R.; Glenie, T.; Armstrong, G.; Khan, A. Shockwave intravascular lithotripsy for calcified coronary lesions: First real-world experience. Heart Lung Circ. 2019, 28, 7–8. [Google Scholar] [CrossRef]
- Wong, B.; El-Jack, S.; Newcombe, R.; Glenie, T.; Armstrong, G.; Cicovic, A.; Khan, A. Shockwave Intravascular Lithotripsy of Calcified Coronary Lesions in ST-Elevation Myocardial Infarction: First-in-Man Experience. J. Invasive Cardiol. 2019, 31, E73–E75. [Google Scholar] [CrossRef]
- Baudinet, T.; Seguy, B.; Cetran, L.; Luttoo, M.K.; Coste, P.; Gerbaud, E. Bail-out therapy in ST segment elevation myocardial infarction due to calcified lesion causing stent underexpansion: Intravascular lithotripsy is in the lead. J. Cardiol. Cases 2021. [Google Scholar] [CrossRef]
- Iannaccone, M.; Piazza, F.; Boccuzzi, G.G.; D’Ascenzo, F.; Latib, A.; Pennacchi, M.; Rossi, M.L.; Ugo, F.; Meliga, E.; Kawamoto, H.; et al. ROTational AThErectomy in acute coronary syndrome: Early and midterm outcomes from a multicentre registry. EuroIntervention 2016, 12, 1457–1464. [Google Scholar] [CrossRef]
- Shahin, M.; Candreva, A.; Siegrist, P.T. Rotational Atherectomy in Acute STEMI with Heavily Calcified Culprit Lesion is a Rule Breaking Solution. Curr. Cardiol. Rev. 2018, 14, 213–221. [Google Scholar] [CrossRef]
- Aznaouridis, K.; Bonou, M.; Masoura, C.; Kapelios, C.; Tousoulis, D.; Barbetseas, J. Rotatripsy: A Hybrid “Drill and Disrupt” Approach for Treating Heavily Calcified Coronary Lesions. J. Invasive Cardiol. 2020, 32, E175. [Google Scholar]
- Ielasi, A.; Loffi, M.; De Blasio, G.; Tespili, M. “Rota-Tripsy”: A Successful Combined Approach for the Treatment of a Long and Heavily Calcified Coronary Lesion. Cardiovasc. Revasc. Med. 2020, 21, 152–154. [Google Scholar] [CrossRef]
- Jurado-Román, A.; Gonzálvez, A.; Galeote, G.; Jiménez-Valero, S.; Moreno, R. RotaTripsy: Combination of Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Lesions. JACC Cardiovasc. Interv. 2019, 12, e127–e129. [Google Scholar] [CrossRef]
- Vaquerizo, B.; Serra, A.; Miranda, F.; Triano, J.L.; Sierra, G.; Delgado, G.; Puentes, A.; Mojal, S.; Brugera, J. Aggressive plaque modification with rotational atherectomy and/or cutting balloon before drug-eluting stent implantation for the treatment of calcified coronary lesions. J. Interv. Cardiol. 2010, 23, 240–248. [Google Scholar] [CrossRef]
- Januszek, R.; Siudak, Z.; Dziewierz, A.; Dudek, D.; Bartuś, S. Predictors of in-hospital effectiveness and complications of rotational atherectomy (from the ORPKI Polish National Registry 2014-2016). Catheter. Cardiovasc. Interv. 2018, 92, E278–E287. [Google Scholar] [CrossRef]
- Tomey, M.I.; Kini, A.S.; Sharma, S.K. Current status of rotational atherectomy. JACC Cardiovasc. Interv. 2014, 7, 345–353. [Google Scholar] [CrossRef]
- Ogden, J.A.; Tóth-Kischkat, A.; Schultheiss, R. Principles of shock wave therapy. Clin. Orthop. Relat. 2001, 387, 8–17. [Google Scholar] [CrossRef]
- Solomonica, A.; Lavi, S.; Cui, J.; Garg, P.; Gao, R.; Levi, Y.; Choudhury, T.; Israeli, Z.; Alraies, M.C.; Mamas, M.A.; et al. Radial versus femoral approach for rotational atherectomy. Coron. Artery Dis. 2020, 31, 393–395. [Google Scholar] [CrossRef]
- Khan, A.A.; Panchal, H.B.; Zaidi, S.I.; Papireddy, M.R.; Mukherjee, D.; Cohen, M.G.; Banerjee, S.; Rao, S.V.; Pancholy, S.; Paul, T.K. Safety and efficacy of radial versus femoral access for rotational Atherectomy: A systematic review and meta-analysis. Cardiovasc. Revascularization Med. 2019, 20, 241–247. [Google Scholar] [CrossRef]
- Allali, A.; Abdelghani, M.; Mankerious, N.; Abdel-Wahab, M.; Richardt, G.; Toelg, R. Feasibility and clinical outcome of rotational atherectomy in patients presenting with an acute coronary syndrome. Catheter. Cardiovasc. Interv. 2019, 93, 382–389. [Google Scholar] [CrossRef]
- Tokarek, T.; Dziewierz, A.; Plens, K.; Rakowski, T.; Zabojszcz, M.; Dudek, D.; Siudak, Z. Radial Approach Expertise and Clinical Outcomes of Percutanous Coronary Interventions Performed Using Femoral Approach. J. Clin. Med. 2019, 8, 1484. [Google Scholar] [CrossRef]
| Clinical Data | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Age | 71 | 75 | 60 | 66 | 81 | 62 |
| Hypertension | No | Yes | Yes | Yes | Yes | Yes |
| Type 2 Diabetes Mellitus | No | Yes | No | No | No | No |
| Hyperlipidemia | Yes | Yes | Yes | Yes | Yes | Yes |
| Atrial Fibrillation | No | No | No | Yes | Yes | No |
| Post PCI status | No | Yes | Yes | Yes | No | Yes |
| Primary Diagnosis | STEMI | STEMI | NSTEMI | NSTEMI | NSTEMI | NSTEMI |
| Treated Vessel | RCA | LAD | LM/LAD | RCA | LAD | RCA |
| Initial LVEF | 60% | 44% | 50% | 50% | 55% | 35% |
| Access | 7F RAD 1 | 6F RAD 1 | 6F RAD 1 | 7F RAD 1 | 7F RAD 1 | 7F FEM 2 |
| Syntax Score | 18 | 25 | 35 | 28 | 22 | 36.5 |
| Burr Size | 1.75 mm | 1.5 mm | 1.5 mm | 1.5 mm | 1.5 mm | 1.75 mm |
| IVL Diameter | 3.5 mm | 3.0 mm | 3.5 mm | 3.5 mm | 3.5 mm | 3.5 mm |
| Number of Pulses | 40 | 70 | 50 | 50 | 20 | 40 |
| DES Size/Pressure | 4.0 mm × 34 mm 20 atm. | 4.0 mm × 18 mm 20 atm. | 3.5 mm × 40 mm 16 atm. | 3.0 mm × 26 mm 12 atm. | 3.5 mm × 38 mm 16 atm. | 4.0 mm × 38 mm 16 atm. |
| In Hospital MACCE | No | No | No | No | Yes | No |
| 30-Days MACCE | No | No | No | No | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczak, A.; Rola, P.; Barycki, M.; Kulczycki, J.J.; Szudrowicz, M.; Lesiak, M.; Doroszko, A. Rota-Lithotripsy—A Novel Bail-Out Strategy for Calcified Coronary Lesions in Acute Coronary Syndrome. The First-in-Man Experience. J. Clin. Med. 2021, 10, 1872. https://doi.org/10.3390/jcm10091872
Włodarczak A, Rola P, Barycki M, Kulczycki JJ, Szudrowicz M, Lesiak M, Doroszko A. Rota-Lithotripsy—A Novel Bail-Out Strategy for Calcified Coronary Lesions in Acute Coronary Syndrome. The First-in-Man Experience. Journal of Clinical Medicine. 2021; 10(9):1872. https://doi.org/10.3390/jcm10091872
Chicago/Turabian StyleWłodarczak, Adrian, Piotr Rola, Mateusz Barycki, Jan Jakub Kulczycki, Marek Szudrowicz, Maciej Lesiak, and Adrian Doroszko. 2021. "Rota-Lithotripsy—A Novel Bail-Out Strategy for Calcified Coronary Lesions in Acute Coronary Syndrome. The First-in-Man Experience" Journal of Clinical Medicine 10, no. 9: 1872. https://doi.org/10.3390/jcm10091872
APA StyleWłodarczak, A., Rola, P., Barycki, M., Kulczycki, J. J., Szudrowicz, M., Lesiak, M., & Doroszko, A. (2021). Rota-Lithotripsy—A Novel Bail-Out Strategy for Calcified Coronary Lesions in Acute Coronary Syndrome. The First-in-Man Experience. Journal of Clinical Medicine, 10(9), 1872. https://doi.org/10.3390/jcm10091872






