Bioresorbable Magnesium-Based Alloys as Novel Biomaterials in Oral Bone Regeneration: General Review and Clinical Perspectives
Abstract
1. Introduction
1.1. Introduction to Magnesium
1.2. First Clinical Implantation of Mg for Orthopaedic Surgery
1.3. Clinical Need of Bioresorbable SYNTHETIC Materials in the Field of Oral Bone Regeneration
2. Mg and Its Alloys
2.1. Pure Mg
2.2. Mg-REE Alloy
2.3. Mg-Ca-Zn Alloy
2.4. Material Specificities for Oral Application
3. Clinical Evaluation of Mg-Based Alloys
3.1. Renewed Interest in the Use of Mg in Orthopaedic Surgery during the 20th Century
3.1.1. Mg and Its Alloys Studied in Orthopaedic Surgery
3.1.2. Associated Complications
3.1.3. Screw Removal
3.2. Application of Mg-Based Implants in the Field of Orthognathic Surgery
4. Bioresorbable Synthetic Materials in Oral Bone Regeneration: Limitations and Perspective Concepts
4.1. Use and Limitations of Current Synthetic Bioresorbable Materials in Bone Regeneration
4.2. Advantages of Bioresorbable Mg-Based Alloys
4.3. Perspectives and Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zeng, Z.; Stanford, N.; Davies, C.H.J.; Nie, J.-F.; Birbilis, N. Magnesium Extrusion Alloys: A Review of Developments and Prospects. Int. Mater. Rev. 2019, 64, 27–62. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Wang, L.; Fan, J.; Li, X.; Zhu, L.; Chen, S.; Roven, H.J.; Zhang, S. Microstructure Evolution and Mechanical Properties of AZ31 Magnesium Alloy Sheets Prepared by Low-Speed Extrusion with Different Temperature. Crystals 2020, 10, 644. [Google Scholar] [CrossRef]
- Maguire, M.E.; Cowan, J.A. Magnesium Chemistry and Biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Magnesium in Clinical Medicine. Front. Biosci. 2004, 9, 1278–1293. [Google Scholar] [CrossRef]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An Update on Physiological, Clinical and Analytical Aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- van de Wal-Visscher, E.R.; Kooman, J.P.; van der Sande, F.M. Magnesium in Chronic Kidney Disease: Should We Care? Blood Purif. 2018, 45, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Renal Magnesium Handling: New Insights in Understanding Old Problems. Kidney Int. 1997, 52, 1180–1195. [Google Scholar] [CrossRef] [PubMed]
- Topf, J.M.; Murray, P.T. Hypomagnesemia and Hypermagnesemia. Rev. Endocr. Metab. Disord. 2003, 4, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In Vitro Corrosion and Biocompatibility of Binary Magnesium Alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Mueller, W.-D.; Lucia Nascimento, M.; Lorenzo de Mele, M.F. Critical Discussion of the Results from Different Corrosion Studies of Mg and Mg Alloys for Biomaterial Applications. Acta Biomater. 2010, 6, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Ulrich, H.; Rudert, M.; Willbold, E. Biodegradable Magnesium Scaffolds: Part 1: Appropriate Inflammatory Response. J. Biomed. Mater. Res. 2007, 81A, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of Biodegradation of Biocompatable Magnesium Alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.-L.; Huang, Y.-C.; Wang, T.-B.; Zeng, H. Hydrogen Inhibits the Osteoclastogenesis of Mouse Bone Marrow Mononuclear Cells. Mater. Sci. Eng. C 2020, 110, 110640. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, S.; Fleck, C. Biodegradable Magnesium Alloys as Temporary Orthopaedic Implants: A Review. Biometals 2019, 32, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Mraied, H.; Wang, W.; Cai, W. Influence of Chemical Heterogeneity and Microstructure on the Corrosion Resistance of Biodegradable WE43 Magnesium Alloys. J. Mater. Chem. B 2019, 7, 6399–6411. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, L.; Xu, W.; Zhang, B.; Yang, K. Dynamic Behaviors of a Ca–P Coated AZ31B Magnesium Alloy during in Vitro and in Vivo Degradations. Mater. Sci. Eng. B 2011, 176, 1718–1726. [Google Scholar] [CrossRef]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-Based Biodegradable Alloys: Degradation, Application, and Alloying Elements. Interv. Med. Appl. Sci. 2017, 9, 27–38. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.F.; Staiger, M.P.; Dias, G.J. Magnesium Biomaterials for Orthopedic Application: A Review from a Biological Perspective. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef]
- Ng, W.F.; Chiu, K.Y.; Cheng, F.T. Effect of PH on the in Vitro Corrosion Rate of Magnesium Degradable Implant Material. Mater. Sci. Eng. C 2010, 30, 898–903. [Google Scholar] [CrossRef]
- Payr, E. Zur Verwendung Des Magnesiums Für Resorbirbare Darmknöpfe Und Andere Chirurgisch-Technische Zwecke. Centralblatt Chir 1901, 28, 513–515. [Google Scholar]
- Payr, E. Beiträge Zur Technik Der Blutgefäss- Und Nervennaht Nebst Mittheilungen Über Die Verwendung Eines Resorbirbaren Metalles in Der Chirurgie. Arch. Klin. Chir. 1900, 62, 67–93. [Google Scholar]
- Verbrugge, J. L’utilisation Du Magnésium Dans Le Traitement Chirurgical Des Fractures: Étude Clinique et Expérimentale. Bull. Mém. Soc. Nat. Cir. 1937, 59, 813–823. [Google Scholar]
- Lambotte, A. Technique et Indications de La Prothèse Perdue Dans La Traitement Des Fractures. Presse Med. Belge 1909, 17, 321–323. [Google Scholar]
- Verbrugge, J. Le Matériel Métallique Résorbable En Chirurgie Osseuse. Presse Med. 1934, 23, 460–465. [Google Scholar]
- McBride, E. Magnesium Screw and Nail Transfixion in Fractures. South. Med. J. 1938, 31, 508–515. [Google Scholar] [CrossRef]
- Maier, O. Über Die Verwendbarkeit von Leichtmetallen in Der Chirurgie (Metallisches Magnesium Als Reizmittel Zur Knochenneubildung). Deutsche Zeitschrift für Chirurgie 1940, 253, 552–556. [Google Scholar] [CrossRef]
- Трoицкий, B.; Цитрин, Д.Н. Рассасывающийся Металлический Сплав, Остеoсинтезит“ Как Материал Для Скрепления Кoсти При Перелoмах. Хирургия 1948, 8, 41–44. [Google Scholar]
- Znamenskii, M. Metallic Osteosynthesis by Means of an Apparatus Made of Resorbing Metal. Khirurgiia 1945, 12, 60–63. [Google Scholar]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone Substitutes in Orthopaedic Surgery: From Basic Science to Clinical Practice. J. Mater. Sci. 2014, 17, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N.; American Academy of Orthopaedic Surgeons. The Committee on Biological Implants Bone-Graft Substitutes: Facts, Fictions, and Applications. J. Bone Joint Surg. Am. 2001, 83, 98–103. [Google Scholar] [CrossRef]
- Global Bone Graft And Substitutes Market Report, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/bone-grafts-substitutes-market (accessed on 23 March 2021).
- Aludden, H.C.; Mordenfeld, A.; Hallman, M.; Dahlin, C.; Jensen, T. Lateral Ridge Augmentation with Bio-Oss Alone or Bio-Oss Mixed with Particulate Autogenous Bone Graft: A Systematic Review. Int. J. Oral Maxillofac. Surg. 2017, 46, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Nkenke, E.; Stelzle, F. Clinical Outcomes of Sinus Floor Augmentation for Implant Placement Using Autogenous Bone or Bone Substitutes: A Systematic Review. Clin. Oral Implant. Res. 2009, 20 (Suppl. 4), 124–133. [Google Scholar] [CrossRef]
- Johansson, B.; Grepe, A.; Wannfors, K.; Hirsch, J.M. A Clinical Study of Changes in the Volume of Bone Grafts in the Atrophic Maxilla. Dentomaxillofacial Radiol. 2001, 30, 157–161. [Google Scholar] [CrossRef]
- von Arx, T.; Buser, D. Horizontal Ridge Augmentation Using Autogenous Block Grafts and the Guided Bone Regeneration Technique with Collagen Membranes: A Clinical Study with 42 Patients. Clin. Oral Implant. Res. 2006, 17, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Younger, E.M.; Chapman, M.W. Morbidity at Bone Graft Donor Sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of Iliac Crest Bone Graft Harvesting. Clin. Orthop. Relat. Res. 1996, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Jupiter, D.C. Bone Graft Substitute: Allograft and Xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft Bone. The Influence of Processing on Safety and Performance. Orthop. Clin. 1999, 30, 571–581. [Google Scholar] [CrossRef]
- Meloni, S.M.; Jovanovic, S.A.; Urban, I.; Baldoni, E.; Pisano, M.; Tallarico, M. Horizontal Ridge Augmentation Using GBR with a Native Collagen Membrane and 1:1 Ratio of Particulate Xenograft and Autologous Bone: A 3-Year after Final Loading Prospective Clinical Study. Clin. Implant. Dent. Relat. Res. 2019, 21, 669–677. [Google Scholar] [CrossRef]
- Catros, S.; Sandgren, R.; Pippenger, B.E.; Fricain, J.C.; Herber, V.; El Chaar, E. A Novel Xenograft Bone Substitute Supports Stable Bone Formation in Circumferential Defects around Dental Implants in Minipigs. Int. J. Oral Maxillofac. Implant. 2020, 35, 1122–1131. [Google Scholar] [CrossRef]
- Gardin, C.; Ricci, S.; Ferroni, L.; Guazzo, R.; Sbricoli, L.; De Benedictis, G.; Finotti, L.; Isola, M.; Bressan, E.; Zavan, B. Decellularization and Delipidation Protocols of Bovine Bone and Pericardium for Bone Grafting and Guided Bone Regeneration Procedures. PLoS ONE 2015, 10, e0132344. [Google Scholar] [CrossRef]
- Güngörmüş, Z.; Güngörmüş, M. Effect of Religious Belief on Selecting of Graft Materials Used in Oral and Maxillofacial Surgery. J. Oral Maxillofac. Surg. 2017, 75, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Kim, S.-G. Membranes for the Guided Bone Regeneration. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent Advances in the Development of GTR/GBR Membranes for Periodontal Regeneration—A Materials Perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Cousin, A.-S.; Bouletreau, P.; Giai, J.; Ibrahim, B.; Louvrier, A.; Sigaux, N. Severity and Long-Term Complications of Surgical Site Infections after Orthognathic Surgery: A Retrospective Study. Sci. Rep. 2020, 10, 12015. [Google Scholar] [CrossRef]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.-T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In Vivo Study of Magnesium Plate and Screw Degradation and Bone Fracture Healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef]
- Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. In Vitro and in Vivo Studies on the Degradation of High-Purity Mg (99.99 wt.%) Screw with Femoral Intracondylar Fractured Rabbit Model. Biomaterials 2015, 64, 57–69. [Google Scholar] [CrossRef]
- Myrissa, A.; Agha, N.A.; Lu, Y.; Martinelli, E.; Eichler, J.; Szakács, G.; Kleinhans, C.; Willumeit-Römer, R.; Schäfer, U.; Weinberg, A.-M. In Vitro and in Vivo Comparison of Binary Mg Alloys and Pure Mg. Mater. Sci. Eng. C 2016, 61, 865–874. [Google Scholar] [CrossRef]
- Marco, I.; Myrissa, A.; Martinelli, E.; Feyerabend, F.; Willumeit-Römer, R.; Weinberg, A.M.; Van der Biest, O. In Vivo and in Vitro Degradation Comparison of Pure Mg, Mg-10Gd and Mg-2Ag: A Short Term Study. Eur. Cell Mater. 2017, 33, 90–104. [Google Scholar] [CrossRef]
- Draxler, J.; Martinelli, E.; Weinberg, A.M.; Zitek, A.; Irrgeher, J.; Meischel, M.; Stanzl-Tschegg, S.E.; Mingler, B.; Prohaska, T. The Potential of Isotopically Enriched Magnesium to Study Bone Implant Degradation in Vivo. Acta Biomater. 2017, 51, 526–536. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Zheng, Y.; Wang, W.; Qiao, W.; Yeung, K.W.K.; Cheung, K.M.C.; Guan, S.; Kulyasova, O.B.; Valiev, R.Z. In Vitro and in Vivo Studies on Ultrafine-Grained Biodegradable Pure Mg, Mg-Ca Alloy and Mg-Sr Alloy Processed by High-Pressure Torsion. Biomater. Sci. 2020, 8, 5071–5087. [Google Scholar] [CrossRef]
- Byun, S.-H.; Lim, H.-K.; Cheon, K.-H.; Lee, S.-M.; Kim, H.-E.; Lee, J.-H. Biodegradable Magnesium Alloy (WE43) in Bone-Fixation Plate and Screw. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2505–2512. [Google Scholar] [CrossRef]
- Levorova, J.; Duskova, J.; Drahos, M.; Vrbova, R.; Vojtech, D.; Kubasek, J.; Bartos, M.; Dugova, L.; Ulmann, D.; Foltan, R. In Vivo Study on Biodegradable Magnesium Alloys: Bone Healing around WE43 Screws. J. Biomater. Appl. 2018, 32, 886–895. [Google Scholar] [CrossRef]
- Oshibe, N.; Marukawa, E.; Yoda, T.; Harada, H. Degradation and Interaction with Bone of Magnesium Alloy WE43 Implants: A Long-Term Follow-up in Vivo Rat Tibia Study. J. Biomater. Appl. 2019, 33, 1157–1167. [Google Scholar] [CrossRef]
- Torroni, A.; Witek, L.; Fahliogullari, H.P.; Bortoli, J.P.; Ibrahim, A.; Hacquebord, J.; Gupta, N.; Coelho, P. WE43 and WE43-T5 Mg Alloys Screws Tested in-Vitro Cellular Adhesion and Differentiation Assay and in-Vivo Histomorphologic Analysis in an Ovine Model. J. Biomater. Appl. 2021, 35, 901–911. [Google Scholar] [CrossRef]
- Waizy, H.; Diekmann, J.; Weizbauer, A.; Reifenrath, J.; Bartsch, I.; Neubert, V.; Schavan, R.; Windhagen, H. In Vivo Study of a Biodegradable Orthopedic Screw (MgYREZr-Alloy) in a Rabbit Model for up to 12 Months. J. Biomater. Appl. 2014, 28, 667–675. [Google Scholar] [CrossRef]
- Guan, X.; Xiong, M.; Zeng, F.; Xu, B.; Yang, L.; Guo, H.; Niu, J.; Zhang, J.; Chen, C.; Pei, J.; et al. Enhancement of Osteogenesis and Biodegradation Control by Brushite Coating on Mg-Nd-Zn-Zr Alloy for Mandibular Bone Repair. ACS Appl. Mater. Interfaces 2014, 6, 21525–21533. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; He, Y.; Jiang, Y.; Zhang, J.; Niu, J.; Mao, L.; Yuan, G. In Vivo Degradation Behavior and Biocompatibility of Mg-Nd-Zn-Zr Alloy at Early Stage. Int. J. Mol. Med. 2012, 29, 178–184. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg-Zn Alloy as a Degradable Biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Grün, N.G.; Holweg, P.; Tangl, S.; Eichler, J.; Berger, L.; van den Beucken, J.J.J.P.; Löffler, J.F.; Klestil, T.; Weinberg, A.M. Comparison of a Resorbable Magnesium Implant in Small and Large Growing-Animal Models. Acta Biomater. 2018, 78, 378–386. [Google Scholar] [CrossRef]
- Holweg, P.; Berger, L.; Cihova, M.; Donohue, N.; Clement, B.; Schwarze, U.; Sommer, N.G.; Hohenberger, G.; van den Beucken, J.J.J.P.; Seibert, F.; et al. A Lean Magnesium–Zinc–Calcium Alloy ZX00 Used for Bone Fracture Stabilization in a Large Growing-Animal Model. Acta Biomater. 2020, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Gao, J.-C.; Qiao, L.-Y.; Xin, R.-L. Corrosion and Bone Response of Magnesium Implants after Surface Modification by Heat-Self-Assembled Monolayer. Front. Mater. Sci. China 2010, 4, 120–125. [Google Scholar] [CrossRef]
- Cheng, P.; Han, P.; Zhao, C.; Zhang, S.; Wu, H.; Ni, J.; Hou, P.; Zhang, Y.; Liu, J.; Xu, H.; et al. High-Purity Magnesium Interference Screws Promote Fibrocartilaginous Entheses Regeneration in the Anterior Cruciate Ligament Reconstruction Rabbit Model via Accumulation of BMP-2 and VEGF. Biomaterials 2016, 81, 14–26. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Y.; Zhang, L.; Bi, Y.; Li, J.; Liu, J.; Yu, Y.; Guo, H.; Li, Y. In Vitro and in Vivo Corrosion and Histocompatibility of Pure Mg and a Mg-6Zn Alloy as Urinary Implants in Rat Model. Mater. Sci. Eng. C 2016, 68, 414–422. [Google Scholar] [CrossRef]
- Chen, K.; Lu, Y.; Tang, H.; Gao, Y.; Zhao, F.; Gu, X.; Fan, Y. Effect of Strain on Degradation Behaviors of WE43, Fe and Zn Wires. Acta Biomater. 2020, 113, 627–645. [Google Scholar] [CrossRef]
- Ezechieli, M.; Meyer, H.; Lucas, A.; Helmecke, P.; Becher, C.; Calliess, T.; Windhagen, H.; Ettinger, M. Biomechanical Properties of a Novel Biodegradable Magnesium-Based Interference Screw. Orthop. Rev. 2016, 8, 6445. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Fu, P.; Ding, W. Effects of Extrusion and Heat Treatment on the Mechanical Properties and Biocorrosion Behaviors of a Mg-Nd-Zn-Zr Alloy. J. Mech. Behav. Biomed. Mater. 2012, 7, 77–86. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Niu, J.; Zhang, J.; Ding, W.; Wu, Y.; Fan, R.; Yuan, G. Nanophasic Biodegradation Enhances the Durability and Biocompatibility of Magnesium Alloys for the Next-Generation Vascular Stents. Nanoscale 2013, 5, 9517–9522. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Chen, J.; Wu, Y.; Kwak, M.; Lu, Y.; Xue, Q.; Pei, J.; Zhang, L.; Yuan, G.; et al. Enhanced Bioactivity of Mg-Nd-Zn-Zr Alloy Achieved with Nanoscale MgF2 Surface for Vascular Stent Application. ACS Appl. Mater. Interfaces 2015, 7, 5320–5330. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Hort, N. Evaluation of Short-Term Effects of Rare Earth and Other Elements Used in Magnesium Alloys on Primary Cells and Cell Lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dai, Y.; Yuan, Z.; Li, J. Effects of Rare Earth Elements on Telomerase Activity and Apoptosis of Human Peripheral Blood Mononuclear Cells. Biol. Trace Elem. Res. 2007, 116, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, L.; Yu, X.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical Properties of Magnesium Alloys for Medical Application: A Review. J. Mech. Behav. Biomed. Mater. 2018, 87, 68–79. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.A.; Huang, C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Wang, X.; Wang, Y.; Geng, L. Mechanical Properties, Degradation Performance and Cytotoxicity of Mg–Zn–Ca Biomedical Alloys with Different Compositions. Mater. Sci. Eng. C 2011, 31, 1667–1673. [Google Scholar] [CrossRef]
- Wong, C.-C.; Wong, P.-C.; Tsai, P.-H.; Jang, J.S.-C.; Cheng, C.-K.; Chen, H.-H.; Chen, C.-H. Biocompatibility and Osteogenic Capacity of Mg-Zn-Ca Bulk Metallic Glass for Rabbit Tendon-Bone Interference Fixation. Int. J. Mol. Sci. 2019, 20, 2191. [Google Scholar] [CrossRef]
- Hofstetter, J.; Becker, M.; Martinelli, E.; Weinberg, A.M.; Mingler, B.; Kilian, H.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. High-Strength Low-Alloy (HSLA) Mg–Zn–Ca Alloys with Excellent Biodegradation Performance. JOM 2014, 66, 566–572. [Google Scholar] [CrossRef]
- de Almeida, P.D.V.; Grégio, A.M.T.; Machado, M.A.N.; de Lima, A.A.S.; Azevedo, L.R. Saliva Composition and Functions: A Comprehensive Review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar]
- Modabber, A.; Zander, D.; Zumdick, N.; Schick, D.; Kniha, K.; Möhlhenrich, S.C.; Hölzle, F.; Goloborodko, E. Impact of Wound Closure on the Corrosion Rate of Biodegradable Mg-Ca-Zn Alloys in the Oral Environment. Materials 2020, 13, 4226. [Google Scholar] [CrossRef]
- Kitamura, E.; Stegaroiu, R.; Nomura, S.; Miyakawa, O. Biomechanical Aspects of Marginal Bone Resorption around Osseointegrated Implants: Considerations Based on a Three-Dimensional Finite Element Analysis. Clin. Oral Implant. Res. 2004, 15, 401–412. [Google Scholar] [CrossRef]
- Swiatkowska, I.; Martin, N.; Hart, A.J. Blood Titanium Level as a Biomarker of Orthopaedic Implant Wear. J. Trace Elem. Med. Biol. 2019, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable Magnesium-Based Screw Clinically Equivalent to Titanium Screw in Hallux Valgus Surgery: Short Term Results of the First Prospective, Randomized, Controlled Clinical Pilot Study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, D.; Huang, S.; Wang, B.; Zhang, X.; Wang, W.; Wei, X. Biodegradable Magnesium Screws and Vascularized Iliac Grafting for Displaced Femoral Neck Fracture in Young Adults. BMC Musculoskelet Disord. 2015, 16, 329. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Han, H.-S.; Han, K.-J.; Park, J.; Jeon, H.; Ok, M.-R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H.; et al. Long-Term Clinical Study and Multiscale Analysis of in Vivo Biodegradation Mechanism of Mg Alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef]
- Plaass, C.; Ettinger, S.; Sonnow, L.; Koenneker, S.; Noll, Y.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Daniilidis, K.; Stukenborg-Colsman, C.; et al. Early Results Using a Biodegradable Magnesium Screw for Modified Chevron Osteotomies. J. Orthop. Res. 2016, 34, 2207–2214. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized Bone Grafting Fixed by Biodegradable Magnesium Screw for Treating Osteonecrosis of the Femoral Head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef]
- Wichelhaus, A.; Emmerich, J.; Mittlmeier, T. A Case of Implant Failure in Partial Wrist Fusion Applying Magnesium-Based Headless Bone Screws. Case Rep. Orthop. 2016, 2016, 7049130. [Google Scholar] [CrossRef]
- Biber, R.; Pauser, J.; Geßlein, M.; Bail, H.J. Magnesium-Based Absorbable Metal Screws for Intra-Articular Fracture Fixation. Case Rep. Orthop. 2016, 2016, 9673174. [Google Scholar] [CrossRef]
- Biber, R.; Pauser, J.; Brem, M.; Bail, H.J. Bioabsorbable Metal Screws in Traumatology: A Promising Innovation. Trauma Case Rep. 2017, 8, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Panzica, M. First results with a resorbable MgYREZr compression screw in unstable scaphoid fractures show extensive bone cysts. Handchir. Mikrochir. Plast Chir. 2017, 49, 37–41. [Google Scholar] [CrossRef]
- Plaass, C.; von Falck, C.; Ettinger, S.; Sonnow, L.; Calderone, F.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Waizy, H.; Daniilidis, K.; et al. Bioabsorbable Magnesium versus Standard Titanium Compression Screws for Fixation of Distal Metatarsal Osteotomies—3 Year Results of a Randomized Clinical Trial. J. Orthop. Sci. 2018, 23, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Kose, O.; Turan, A.; Unal, M.; Acar, B.; Guler, F. Fixation of Medial Malleolar Fractures with Magnesium Bioabsorbable Headless Compression Screws: Short-Term Clinical and Radiological Outcomes in Eleven Patients. Arch. Orthop. Trauma Surg. 2018, 138, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Acar, B.; Kose, O.; Turan, A.; Unal, M.; Kati, Y.A.; Guler, F. Comparison of Bioabsorbable Magnesium versus Titanium Screw Fixation for Modified Distal Chevron Osteotomy in Hallux Valgus. Biomed. Res. Int. 2018, 2018, 5242806. [Google Scholar] [CrossRef]
- Choo, J.T.; Lai, S.H.S.; Tang, C.Q.Y.; Thevendran, G. Magnesium-Based Bioabsorbable Screw Fixation for Hallux Valgus Surgery—A Suitable Alternative to Metallic Implants. Foot Ankle Surg. 2019, 25, 727–732. [Google Scholar] [CrossRef]
- Klauser, H. Internal Fixation of Three-Dimensional Distal Metatarsal I Osteotomies in the Treatment of Hallux Valgus Deformities Using Biodegradable Magnesium Screws in Comparison to Titanium Screws. Foot Ankle Surg. 2019, 25, 398–405. [Google Scholar] [CrossRef]
- Gigante, A.; Setaro, N.; Rotini, M.; Finzi, S.S.; Marinelli, M. Intercondylar Eminence Fracture Treated by Resorbable Magnesium Screws Osteosynthesis: A Case Series. Injury 2018, 49, S48–S53. [Google Scholar] [CrossRef]
- Acar, B.; Unal, M.; Turan, A.; Kose, O. Isolated Lateral Malleolar Fracture Treated with a Bioabsorbable Magnesium Compression Screw. Cureus 2018, 10, e2539. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, K.-B.; Kim, S.-Y.; Bode, K.; Jang, Y.-S.; Kwon, T.-Y.; Jeon, M.H.; Lee, M.-H. Gas Formation and Biological Effects of Biodegradable Magnesium in a Preclinical and Clinical Observation. Sci. Technol. Adv. Mater. 2018, 19, 324–335. [Google Scholar] [CrossRef]
- Aktan, C.; Ertan, M.B.; Turan, A.; Kose, O. Fixation of Small Osteochondral Fragments in a Comminuted Distal Humerus Fracture with Magnesium Bioabsorbable Screws: A Case Report. Cureus 2018, 10, e3752. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.D.; Khan, S.; Lashgari, Y.; Ziegler, A. Hallux Valgus Correction Utilising a Modified Short Scarf Osteotomy with a Magnesium Biodegradable or Titanium Compression Screws—A Comparative Study of Clinical Outcomes. BMC Musculoskelet Disord. 2019, 20, 334. [Google Scholar] [CrossRef] [PubMed]
- Acar, B.; Kose, O.; Unal, M.; Turan, A.; Kati, Y.A.; Guler, F. Comparison of Magnesium versus Titanium Screw Fixation for Biplane Chevron Medial Malleolar Osteotomy in the Treatment of Osteochondral Lesions of the Talus. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Turan, A.; Kati, Y.A.; Acar, B.; Kose, O. Magnesium Bioabsorbable Screw Fixation of Radial Styloid Fractures: Case Report. J. Wrist Surg. 2020, 9, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lin, Z.; Wang, M.; Huang, W.; Ke, J.; Zhao, D.; Yin, Q.; Zhang, Y. Treatment of Trauma-Induced Femoral Head Necrosis with Biodegradable Pure Mg Screw-Fixed Pedicle Iliac Bone Flap. J. Orthop. Transl. 2019, 17, 133–137. [Google Scholar] [CrossRef] [PubMed]
- May, H.; Alper Kati, Y.; Gumussuyu, G.; Yunus Emre, T.; Unal, M.; Kose, O. Bioabsorbable Magnesium Screw versus Conventional Titanium Screw Fixation for Medial Malleolar Fractures. J. Orthop. Traumatol. 2020, 21, 9. [Google Scholar] [CrossRef]
- Holweg, P.; Herber, V.; Ornig, M.; Hohenberger, G.; Donohue, N.; Puchwein, P.; Leithner, A.; Seibert, F. A Lean Bioabsorbable Magnesium-Zinc-Calcium Alloy ZX00 Used for Operative Treatment of Medial Malleolus Fractures: Early Clinical Results of a Prospective Non-Randomized First in Man Study. Bone Joint Res. 2020, 9, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, L.; Guo, Y.; Zhao, S.; Yang, Y.; Dong, D.; Ding, W.; Dai, K.; Gong, W.; Yuan, G.; et al. Effectiveness and Safety of Biodegradable Mg-Nd-Zn-Zr Alloy Screws for the Treatment of Medial Malleolar Fractures. J. Orthop. Transl. 2021, 27, 96–100. [Google Scholar] [CrossRef]
- Jungesblut, O.D.; Moritz, M.; Spiro, A.S.; Stuecker, R.; Rupprecht, M. Fixation of Unstable Osteochondritis Dissecans Lesions and Displaced Osteochondral Fragments Using New Biodegradable Magnesium Pins in Adolescents. Cartilage 2020. [Google Scholar] [CrossRef]
- Leonhardt, H.; Ziegler, A.; Lauer, G.; Franke, A. Osteosynthesis of the Mandibular Condyle with Magnesium-Based Biodegradable Headless Compression Screws Show Good Clinical Results During a 1-Year Follow-Up Period. J. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Sukotjo, C.; Lima-Neto, T.J.; Santiago Júnior, J.F.; Faverani, L.P.; Miloro, M. Is There a Role for Absorbable Metals in Surgery? A Systematic Review and Meta-Analysis of Mg/Mg Alloy Based Implants. Materials 2020, 13, 3914. [Google Scholar] [CrossRef]
- Viehe, R.; Haupt, D.J.; Heaslet, M.W.; Walston, S. Complications of Screw-Fixated Chevron Osteotomies for the Correction of Hallux Abducto Valgus. J. Am. Podiatr. Med. Assoc. 2003, 93, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Prediger, B.; Mathes, T.; Probst, C.; Pieper, D. Elective Removal vs. Retaining of Hardware after Osteosynthesis in Asymptomatic Patients-a Scoping Review. Syst. Rev. 2020, 9, 225. [Google Scholar] [CrossRef]
- Vollstationäre Patientinnen und Patienten der Krankenhäuser. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/Tabellen/diagnose-kapitel-geschlecht.html (accessed on 4 January 2021).
- Leonhardt, H.; Franke, A.; McLeod, N.M.H.; Lauer, G.; Nowak, A. Fixation of Fractures of the Condylar Head of the Mandible with a New Magnesium-Alloy Biodegradable Cannulated Headless Bone Screw. Br. J. Oral Maxillofac. Surg. 2017, 55, 623–625. [Google Scholar] [CrossRef]
- Bengisu, M. Borate Glasses for Scientific and Industrial Applications: A Review. J. Mater. Sci. 2016, 51, 2199–2242. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic Bone Graft Substitutes: SYNTHETIC BONE GRAFT SUBSTITUTES. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Nery, E.B.; LeGeros, R.Z.; Lynch, K.L.; Lee, K. Tissue Response to Biphasic Calcium Phosphate Ceramic with Different Ratios of HA/Beta TCP in Periodontal Osseous Defects. J. Periodontol. 1992, 63, 729–735. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Aguado, E.; Pilet, P.; Daculsi, G. Macroporous Biphasic Calcium Phosphate Ceramics: Influence of Macropore Diameter and Macroporosity Percentage on Bone Ingrowth. Biomaterials 1998, 19, 133–139. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Lange, S.; Esslinger, S.; Latorre, S.H.; Krastev, R.; Gadow, R.; Mayr, H.O.; Bernstein, A. Inversely 3D-Printed β-TCP Scaffolds for Bone Replacement. Materials 2019, 12, 3417. [Google Scholar] [CrossRef]
- Nandi, S.K.; Fielding, G.; Banerjee, D.; Bandyopadhyay, A.; Bose, S. 3D Printed β-TCP Bone Tissue Engineering Scaffolds: Effects of Chemistry on in Vivo Biological Properties in a Rabbit Tibia Model. J. Mater. Res. 2018, 33, 1939–1947. [Google Scholar] [CrossRef]
- Park, J. Bioceramics: Properties, Characterizations, and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Huang, Q.; Wang, L.; Wang, J. Mechanical Properties of Artificial Materials for Bone Repair. J. Shanghai Jiaotong Univ. 2014, 19, 675–680. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.A.; Schlegel, K.A.; Schultze-Mosgau, S.; Kloss, F.R.; Rupprecht, S.; Kessler, P. Degradation Characteristics of Alpha and Beta Tri-Calcium-Phosphate (TCP) in Minipigs. J. Biomed. Mater. Res. 2002, 63, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Rolvien, T.; Barvencik, F.; Klatte, T.O.; Busse, B.; Hahn, M.; Rueger, J.M.; Rupprecht, M. SS-TCP Bone Substitutes in Tibial Plateau Depression Fractures. Knee 2017, 24, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Hydroxyapatite Coatings for Biomedical Applications Deposited by Different Thermal Spray Techniques. Surf. Coat. Technol. 2010, 205, 1157–1164. [CrossRef]
- Ali Akbari Ghavimi, S.; Ebrahimzadeh, M.H.; Solati-Hashjin, M.; Abu Osman, N.A. Polycaprolactone/Starch Composite: Fabrication, Structure, Properties, and Applications. J. Biomed. Mater. Res. A 2015, 103, 2482–2498. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue Engineering for Bone Regeneration and Osseointegration in the Oral Cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Runge, M.B.; Dadsetan, M.; Chen, Q.; Lu, L.; Yaszemski, M.J. Cross-Linking Characteristics and Mechanical Properties of an Injectable Biomaterial Composed of Polypropylene Fumarate and Polycaprolactone Co-Polymer. J. Biomater. Sci. Polym. Ed. 2011, 22, 489–504. [Google Scholar] [CrossRef]
- Dai, W.; Kawazoe, N.; Lin, X.; Dong, J.; Chen, G. The Influence of Structural Design of PLGA/Collagen Hybrid Scaffolds in Cartilage Tissue Engineering. Biomaterials 2010, 31, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, E.L.; Kroese-Deutman, H.C.; Shih, C.K.; Crowther, R.S.; Carney, D.H.; Mikos, A.G.; Jansen, J.A. In Vivo Degradation of Porous Poly(Propylene Fumarate)/Poly(DL-Lactic-Co-Glycolic Acid) Composite Scaffolds. Biomaterials 2005, 26, 4616–4623. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which Is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A High-Specific-Strength and Corrosion-Resistant Magnesium Alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar] [CrossRef]
- Denard, P.J.; Raiss, P.; Gobezie, R.; Edwards, T.B.; Lederman, E. Stress Shielding of the Humerus in Press-Fit Anatomic Shoulder Arthroplasty: Review and Recommendations for Evaluation. J. Shoulder Elbow Surg. 2018, 27, 1139–1147. [Google Scholar] [CrossRef]
- Guo, Y.; Sealy, M.P.; Guo, C. Significant Improvement of Corrosion Resistance of Biodegradable Metallic Implants Processed by Laser Shock Peening. CIRP Ann. 2012, 61, 583–586. [Google Scholar] [CrossRef]
- Sealy, M.P.; Guo, Y.B.; Caslaru, R.C.; Sharkins, J.; Feldman, D. Fatigue Performance of Biodegradable Magnesium–Calcium Alloy Processed by Laser Shock Peening for Orthopedic Implants. Int. J. Fatigue 2016, 82, 428–436. [Google Scholar] [CrossRef]
- Rahman, M.; Dutta, N.K.; Roy Choudhury, N. Magnesium Alloys with Tunable Interfaces as Bone Implant Materials. Front. Bioeng. Biotechnol. 2020, 8, 564. [Google Scholar] [CrossRef]
- Bauser, M.; Sauer, G.; Siegert, K. (Eds.) Extrusion, 2nd ed.; ASM International: Materials Park, OH, USA, 2006; ISBN 978-0-87170-837-3. [Google Scholar]
- Aghion, E.; Bronfin, B. Magnesium Alloys Development towards the 21st Century. In Materials Science Forum; Kojima, Y., Aizawa, T., Kamado, S., Eds.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2000; Volume 350–351, pp. 19–30. [Google Scholar]
- Zhang, X.-Y.; Fang, G.; Zhou, J. Additively Manufactured Scaffolds for Bone Tissue Engineering and the Prediction of Their Mechanical Behavior: A Review. Materials 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, R.; Ortgies, S.; Tamayol, A.; Bobaru, F.; Sealy, M.P. Additive Manufacturing of Magnesium Alloys. Bioact. Mater. 2020, 5, 44–54. [Google Scholar] [CrossRef]
- Demishtein, K.; Reifen, R.; Shemesh, M. Antimicrobial Properties of Magnesium Open Opportunities to Develop Healthier Food. Nutrients 2019, 11, 2363. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Zhai, Z.; Liu, L.; Li, H.; Yang, K.; Tan, L.; Wan, P.; Liu, X.; Ouyang, Z.; et al. Antibacterial Properties of Magnesium In Vitro and in an In Vivo Model of Implant-Associated Methicillin-Resistant Staphylococcus Aureus Infection. Antimicrob. Agents Chemother. 2014, 58, 7586–7591. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.A.; Griffith, R.W.; Shechtman, D.; Evans, R.B.; Conzemius, M.G. In Vitro Antibacterial Properties of Magnesium Metal against Escherichia Coli, Pseudomonas Aeruginosa and Staphylococcus Aureus. Acta Biomater. 2010, 6, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Imwinkelried, T.; Beck, S.; Schaller, B. Pre-Clinical Testing of Human Size Magnesium Implants in Miniature Pigs: Implant Degradation and Bone Fracture Healing at Multiple Implantation Sites. Mater. Sci. Eng. C 2020, 108, 110389. [Google Scholar] [CrossRef] [PubMed]
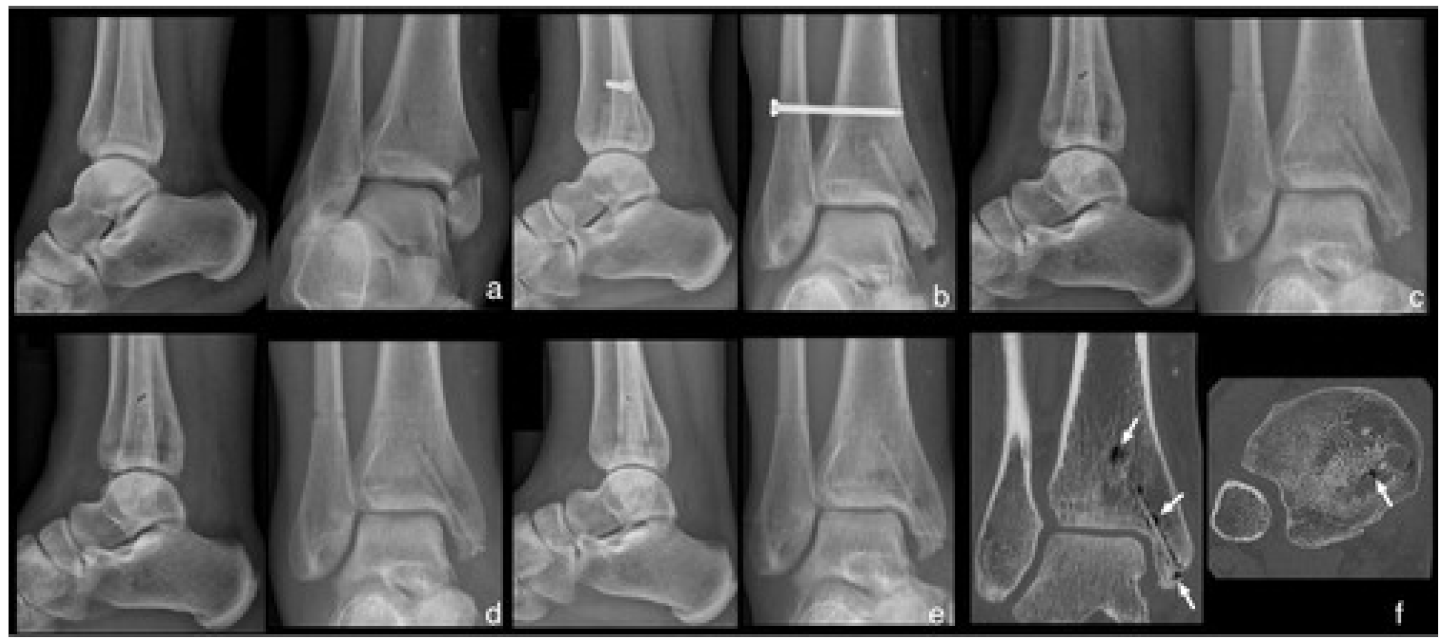
| Type of Material | Designed Material | Animals and Implantation Site | Time Period | Calculated In Vivo Degradation Rate |
|---|---|---|---|---|
| Pure Mg (99.99 wt.%) [49] | plate and screw | Rabbit ulnae | 8 and 16 weeks | 0.40 ± 0.04 mm/y (8 weeks) |
| Pure Mg (99.99 wt.%) [50] | screw | Rabbit femoral bones | 4, 8, 16, 24 weeks | 1.38 ± 0.03 mm/y(4 weeks) 0.57 ± 0.03 mm/y (24 weeks) |
| Pure Mg (99.99 wt.%) [51] | pin | Rat femoral bones | 1, 4 and 12 weeks | 0.2–0.4 mm/y |
| Pure Mg (99.99 wt.%) [52] | pin | Rat femoral bones | 7 days | 0.15 ± 0.03 mm/y |
| 26Mg enriched (>99 wt.%) [53] | pin | Rat femoral bones | 4, 24, 52 weeks | 16 ± 5 µm/y |
| Pure Mg high pressure (99.98 Mg in wt.%) [54] | disk | Rat femoral bones | 24 weeks | 0.41 ± 0.02 mm/y |
| WE43 (>92.0 Mg in wt.%) [55] | plates and screw | Dogs-LeFort I osteotomy | 4, 12, and 24 weeks | NA 1 |
| WE43 (>92.0 Mg in wt.%) [56] | screw | Rabbit tibiae | 4, 8, 12 and 16 weeks | NA 1 |
| WE43 (>92.0 Mg in wt.%) [57] | pin | Rat tibiae | 52 weeks | NA 1 |
| WE43/WE43T5 (>92.0 Mg in wt.%) [58] | screw | Sheep mandibule | 6 and 24 weeks | NA 1 |
| MgYREZr (>92.0 Mg in wt.%) [59] | screw | Rabbit femoral bones | 1, 12, and 52 weeks | NA 1 |
| JDBM (>95.0 Mg in wt.%) [60] | screw | Rabbit mandible bones | 1, 4, and 7 months | 0.161 ± 0.025 mm/y (1 month), 00.218 ± 0.030 mm/y (7 months) |
| NZK (>96.0 Mg in wt.%) [61] | rod | Rabbit femoral bones | 28 and 56 days | 0.66 mm/y (28 days) and 0.48 mm/y (56 days) |
| Mg-Zn (94.0 Mg in wt.%) [62] | rod | Rabbit femoral bones | 14 weeks | 2.32 mm/y |
| ZX00 (>99.0 Mg in wt.%) [63] | pin | Rat femoral bones and sheep tibiae | 6, 12 and 24weeks | 0.08 mm/y (rat) and 0.045 mm/y (sheep) |
| ZX00 (>99.0 Mg in wt.%) [64] | screw | Sheep tibiae | 3, 6 and 12 weeks | NA 1 |
| Authors | Type of Study | Intervention | Type of Alloy | n | Outcomes | Complications |
|---|---|---|---|---|---|---|
| Windhagen et al. [85] | Randomized controlled clinical trial | Hallux valgus | MgYREZr | 26 (13 Mg and 13 Ti) | Fracture union without healing disorders except for 2 patients | Wound healing delayed in 2 patients with Mg screws |
| Yu X et al. [86] | Retrospective observational study | Displaced femoral neck fracture | Pure Magnesium | 19 | 17 patients with fracture union | One patient with non-union fracture |
| Lee et al. [87] | Case series | Radial styloid fracture | Mg-5wt%Ca-1wt%Zn | 53 | Fracture union, fracture healing, retrieved function | None |
| Plaass C et al. [88] | Case series | Hallux valgus | MgYREZr | 45 | Fracture union, fracture healing, retrieved function | One complication (dorsal subluxation) |
| Zhao et al. [89] | Randomized controlled clinical trial | Hip-preserving surgery | Pure Magnesium | 48 | Fracture union, fracture healing, retrieved function | None |
| Wichelhaus A et al. [90] | Case report | Scaphotrapeziotrapezoidal fracture | MgYREZr | 1 | / | Screw breakage, pain, paraesthesia |
| Biber R et al. [91] | Case report | Humeral capitelum fracture | MgYREZr | 1 | Fracture union, fracture healing, retrieved function | None |
| Biber R et al. [92] | Case report | Distal fibular fracture | MgYREZr | 1 | Fracture union, fracture healing, retrieved function | None |
| Meyer R and Panzica M [93] | Case series | Scaphoid fracture | MgYREZr | 5 | / | Extensive cyst formation and delayed consolidation |
| Plaass C et al. [94] | Randomized controlled clinical trial | Hallux valgus | MgYREZr | 26 (13 Mg and 13 Ti) | Fracture union, fracture healing, retrieved function | None |
| Kose O et al. [95] | Retrospective observational study | Medial malleolar fracture | MgYREZr | 11 | Fracture union, fracture healing, retrieved function | None |
| Acar B. et al. [96] | Retrospective observational study | Hallux valgus | MgYREZr | 31 (16 Mg and 15 Ti) | Fracture union, fracture healing, retrieved function | One delayed wound healing -Mg screw |
| Choo JT et al. [97] | Randomized controlled clinical trial | Hallux valgus | MgYREZr | 93 (24 Mg and 69 Ti) | Fracture union, fracture healing, retrieved function | 3 superficial cellulitis and one neuropathic operative site pain. |
| Klauser H. [98] | Retrospective observational study | Hallux valgus | MgYREZr | 200 (100 Mg and 100 Ti) | Fracture union, fracture healing, retrieved function | None |
| Gigante A. et al. [99] | Case series | Anterior cruciate ligament avulsion fracture | MgYREZr | 3 | Fracture union, fracture healing | None |
| Acar B. et al. [100] | Case report | Lateral malleolar fracture | MgYREZr | 1 | Fracture union, fracture healing, retrieved function | None |
| Kim, Y.-K et al. [101] | Retrospective observational study | Meta-tarsal or midfoot fractures | Pure Magnesium | 22 | Fracture union, fracture healing | 2 wound dehiscence |
| Aktan C et al. [102] | Case report | Distal humerus intra-articular fracture | MgYREZr | 1 | Fracture union, fracture healing, retrieved function | None |
| Atkinson HD et al. [103] | Randomized controlled clinical trial | Hallux valgus | MgYREZr | 36 (11 Mg and 25 Ti) | Fracture union, fracture healing, retrieved function | None |
| Acar B et al. [104] | Randomized controlled clinical trial | Biplane chevron medial malleolar osteotomy | MgYREZr | 22 | Fracture union, fracture healing, retrieved function | None |
| Turan A. [105] | Case report | Radial styloid fracture | MgYREZr | 2 | Fracture union, fracture healing, retrieved function | None |
| Chen L et al. [106] | Case report | Traumatic femoral head necrosis | Pure magnesium | 1 | Improvement of patient’s hip function | None |
| May H et al. [107] | Retrospective observational study | Medial malleolar fracture | MgYREZr | 48 (25 Mg and 23 Ti) | Fracture union, fracture healing, retrieved function | None in the Mg group |
| Holweg et al. [108] | Case series | Medial malleolar fracture | Mg-Zn0.45-Ca0.45, in wt. (ZX00) | 20 | Fracture union, fracture healing, retrieved function | None |
| Xie K et al. [109] | Case series | Medial malleolar fracture | Mg-Nd3.0-Zn0.2- Zr0.5 in wt. and coating | 9 | Fracture union, fracture healing, retrieved function | None |
| Jungesblut et al. [110] | Retrospective observational study | Osteochondritis dissecans lesion | MgYREZr | 19 | Fracture union, fracture healing | One post-operative implant failure |
| Authors | Type of Study | Intervention | Type of Device | n | Outcomes | Complications |
|---|---|---|---|---|---|---|
| Leonhardt H et al. [116] | Case series | Mandibular fracture | MgYREZr | 5 | Fracture healing, with restored function of the temporomandibular joint | One fracture of a screw |
| Leonhardt H et al. [111] | Retrospective observational study | Mandibular fracture | MgYREZr | 6 | Fracture healing with restored function of the temporomandibular joint | Penetration of one screw tip through the condylar surface without screw removal necessary |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herber, V.; Okutan, B.; Antonoglou, G.; Sommer, N.G.; Payer, M. Bioresorbable Magnesium-Based Alloys as Novel Biomaterials in Oral Bone Regeneration: General Review and Clinical Perspectives. J. Clin. Med. 2021, 10, 1842. https://doi.org/10.3390/jcm10091842
Herber V, Okutan B, Antonoglou G, Sommer NG, Payer M. Bioresorbable Magnesium-Based Alloys as Novel Biomaterials in Oral Bone Regeneration: General Review and Clinical Perspectives. Journal of Clinical Medicine. 2021; 10(9):1842. https://doi.org/10.3390/jcm10091842
Chicago/Turabian StyleHerber, Valentin, Begüm Okutan, Georgios Antonoglou, Nicole G. Sommer, and Michael Payer. 2021. "Bioresorbable Magnesium-Based Alloys as Novel Biomaterials in Oral Bone Regeneration: General Review and Clinical Perspectives" Journal of Clinical Medicine 10, no. 9: 1842. https://doi.org/10.3390/jcm10091842
APA StyleHerber, V., Okutan, B., Antonoglou, G., Sommer, N. G., & Payer, M. (2021). Bioresorbable Magnesium-Based Alloys as Novel Biomaterials in Oral Bone Regeneration: General Review and Clinical Perspectives. Journal of Clinical Medicine, 10(9), 1842. https://doi.org/10.3390/jcm10091842









