Abstract
Acute kidney injury (AKI) requiring renal replacement therapy (RRT) increases the mortality of acute respiratory distress syndrome (ARDS) patients. The aim of this study was to investigate the outcomes and predictors of RRT in patients with influenza pneumonia-related ARDS. This retrospective cohort study includes patients from eight tertiary referral centers in Taiwan between January and March 2016, and all 282 patients with influenza pneumonia-related ARDS were enrolled. Thirty-four patients suffered from AKI requiring RRT, while 16 patients had underlying end stage renal disease (ESRD). The 30- and 60-day mortality rates were significantly higher in patients with AKI requiring RRT compared with those not requiring RRT (50.0% vs. 19.8%, p value < 0.001; 58.8% vs. 27.2%, p value = 0.001, respectively), but the patients with ESRD had no significant difference in mortality (12.5% vs. 19.8%, p value = 0.744; 31.3% vs. 27.2%, p value = 0.773, respectively). The predictors for AKI requiring RRT included underlying chronic liver disease and C-reactive protein. The mortality predictors for patients with AKI requiring RRT included the pneumonia severity index, tidal volume, and continuous renal replacement therapy. In this study, patients with influenza pneumonia-related ARDS with AKI requiring RRT had significantly higher mortality compared with other patients.
1. Introduction
Acute kidney injury (AKI), defined by the Acute Kidney Injury Network [], happened in 36–67% critically ill patients, and 5–6% of them need renal replacement therapy (RRT) []. For the critically ill patients who suffer from AKI and need RRT, the mortality rate is as high as 60% []. For patients with acute respiratory distress syndrome (ARDS), inadequate mechanical ventilator settings might contribute to adverse renal effects [,].A previous study showed that the incidence of AKI in patients with ARDS was 44.3% and the mortality rate was up to 42.3% []. Another study which investigated patients with prolonged mechanical ventilation revealed that the weaning and survival rates were lower in patients with RRT which started in the ICU compared with those with end stage renal disease (ESRD) [].
Severe influenza infection is one of the most common etiologies of ARDS [,]. In the H1N1 influenza pandemic in 2009, 49–72% of influenza pneumonia patients who were admitted to the ICU had ARDS [,]. In the first quarter of 2016, 1,735 patients were admitted to the ICU with complicated influenza infections in Taiwan [].
Previous studies have evaluated the outcomes of influenza induced ARDS patients with AKI [,], but the studies for the influence of RRT is scanty. We conducted a multicenter, retrospective study of patients admitted to ICUs due to influenza with ARDS.
2. Materials and Methods
2.1. Study Patients and Data Collection
This retrospective cohort study was conducted by the Taiwan Severe Influenza Research Consortium which includes eight tertiary referral centers in Taiwan. All patients who were admitted to the ICU at these 8 centers with virology-proven influenza between January and March in 2016 were enrolled in the current study. The patient’s demographic data, laboratory data, ventilator parameters, therapy process, and outcomes were obtained from their electronic medical records using a standard case report form. ARDS was diagnosed according to the Berlin criteria [].
Chronic liver disease was considered as liver cirrhosis or chronic viral hepatitis, and chronic kidney disease was defined as a baseline estimated glomerular filtration rate of <60. The dynamic driving pressure was defined as peak inspiratory pressure minus positive end expiratory pressure, and compliance was defined as the tidal volume divided by the dynamic driving pressure. The patient’s laboratory data, arterial blood gas, ventilator settings, and severity scores—including the Pneumonia Severity Index (PSI) [], Acute Physiology and Chronic Health Evaluation II (APACHE II) [], CURB-65 pneumonia severity score [], and Sequential Organ Failure Assessment (SOFA) score []—were collected on the ICU admission day. The local Institutional Review Boards for Human Research at all of the involved hospitals approved the current study. Due to the retrospective nature of the study, the need for informed consent was waived.
2.2. Diagnosis of Influenza
All of the patients were diagnosed with influenza by at least 1 of the following laboratory tests: nasopharynx swab or throat swab influenza rapid antigen test; nucleic acid reverse-transcriptase polymerase chain reaction, viral culture from a nasopharynx swab, throat swab, sputum or bronchoalveolar lavage, or serum antibody serologic test (antibody titers decreased >4 times from the acute to convalescent stage).
2.3. Renal Replacement Therapy
Indications for RRT included oliguria with fluid overload, refractory metabolic acidosis or refractory hyperkalemia. The nephrologists were consulted before the RRT was started to evaluate the indication and any contraindications, and the final decision of whether to start RRT was made by the intensive care doctors and the nephrologists together. Continuous renal replacement therapy (CRRT) was used for patients with severe shock that could not tolerate intermittent hemodialysis. CRRT could revert to intermittent hemodialysis if the patient’s blood pressure stabilized.
Every patient had a Foley catheter to monitor the urine output and avoid post-renal etiology of renal failure. Nephrotoxic antibiotics, such as aminoglycoside or colistin, were not the first-line antibiotics in our ICUs.
2.4. Statistical Analyses
Statistical analyses and database management were performed using SPSS version 22.0.0 (SPSS Inc., Chicago, IL, USA). We used number (percentages) for nominal variables and the mean ± standard deviation for continuous variables. Pearson’s chi-squared test was used to compare nominal variables. An independent Student’s t-test was used to compare two groups of continuous variables, and one-way analysis of variance was used to compare multiple groups of continuous variables. We did the post hoc test by Tukey’s honestly significant difference test. We also calculated the statistical power. Univariate and multivariate binary logistic regression were used to analyze the predictive factors for AKI requiring hemodialysis. Univariate and multivariate Cox regression were used to analyze the predictive factors of survival. Variables with p value less than 0.05 in univariate analysis were included for multivariate analysis. In the current study, two-tailed tests were used and statistical significance was defined as a p value < 0.05.
3. Results
3.1. General Data
A total of 336 patients were diagnosed with influenza and admitted to the ICU between January and March 2016, and 282 of these patients met the criteria for ARDS (Figure 1). There were 16 patients who had underlying ESRD disease on regular hemodialysis, and then received RRT during the ARDS course. There were 34 patients who suffered from AKI and received RRT which started in the ICU, and 10 of these patients had previously chronic kidney disease without hemodialysis before their hospital admission. On average, these patients started hemodialysis 3.58 ± 3.53 days after their respiratory failure began, and 16 patients received CRRT.
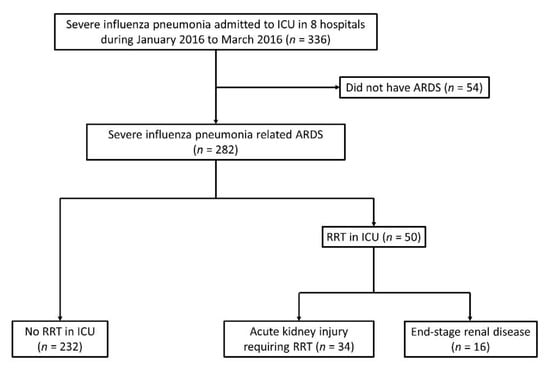
Figure 1.
Flow chart of patients in this study; ARDS: acute respiratory distress syndrome; ICU: intensive care unit; RRT: renal replacement therapy.
The patients’ demographic data, laboratory data, and ventilator parameters are shown in Table 1. The patients with AKI requiring RRT were younger than the no RRT patients and the ESRD patients, but these differences were not statistically significant. The patients with AKI requiring RRT had a higher ratio of underlying chronic liver disease or chronic kidney disease compared with the other patients, whereas the ESRD patients had a higher ratio of underlying diabetes mellitus and hypertension. The AKI patients requiring RRT and ESRD patients had significantly more severe conditions according to their PSI (AKI requiring RRT vs. no RRT: p value = 0.005, ESRD vs. no RRT: p value = 0.150), APACHE II (AKI requiring RRT vs. no RRT: p value =< 0.001, ESRD vs. no RRT: p value = 0.002), and SOFA scores (AKI requiring RRT vs. no RRT: p value =< 0.001, ESRD vs. no RRT: p value = 0.001), and they had significantly higher lactate (AKI requiring RRT vs. no RRT: p value = 0.003) and total bilirubin levels (AKI requiring RRT vs. no RRT: p value = 0.023) than no RRT patients. More patients with AKI requiring RRT needed vasopressor agents (AKI requiring RRT vs. no RRT: p value = 0.008, ESRD vs. no RRT: p value = 0.129). The platelet level was lower in patients who received RRT, but no significant difference in post hoc analysis (AKI requiring RRT vs. no RRT: p value = 0.150, ESRD vs. no RRT: p value = 0.097). More severe metabolic acidosis was noted in the AKI patients requiring RRT, but this difference was not statistically significant. Moreover, the patients with AKI requiring RRT had a poorer PaO2/FiO2 ratio (AKI requiring RRT vs. no RRT: p value = 0.028), and they needed higher peak airway pressure (AKI requiring RRT vs. no RRT: p value = 0.020).

Table 1.
Demography data.
Seventy-six patients were met the diagnosis of AKI, but they did not need RRT during the ICU course. The comparison of the AKI patients requiring RRT and not requiring RRT are shown in Table 2. The patients with AKI requiring RRT, comparing with the patients with AKI not requiring RRT, had significant higher PSI (142.0 ± 46.2 vs. 120.6 ± 45.4, p value = 0.025), APACHE II score (29.5 ± 8.1 vs. 23.4 ± 7.4, p value < 0.001), SOFA score (13.9 ± 3.5 vs. 10.3 ± 3.8, p value < 0.001), C-reactive protein (19.4 ± 10.2 vs. 14.9 ± 10.3, p value = 0.041), peak airway pressure (31.6 ± 5.1 vs. 29.1 ± 4.7, p value = 0.028), and significant poorer PaO2/FiO2 ratio (82.4 ± 46.7 vs. 105.7 ± 62.0, p value = 0.037). More patients with AKI requiring RRT needed vasopressor agents than not requiring RRT (73.5% vs. 40.8%, p value = 0.002).

Table 2.
Comparison of the AKI patients requiring RRT and not requiring RRT.
3.2. Clinical Outcomes
The clinical outcomes are showen in Table 3. The patients with AKI requiring RRT had significantly higher 30- and 60-day mortality rates compared with the no RRT patients (30 days: 50.0 vs. 19.8%, p value < 0.001; 60 days: 58.8 vs. 27.2%, p value = 0.001). The statistical power for 30- and 60-day mortality were 0.977 and 0.967, respectively. However, patients with ESRD who received RRT did not have a significantly different 30-day or 60-day mortality compared with the no RRT patients (30 days: 12.5 vs. 19.8%, p value = 0.744; 60 days: 31.3 vs. 27.2%, p value = 0.773). The Kaplan–Meier curve is shown in Figure 2. The patients who had AKI without RRT during their ICU course had significantly lower 60 day mortality rate than those patients who had AKI requiring RRT (30.3% vs. 58.8%, p value = 0.005). Compared with the no hemodialysis survival patients, the survival patients with AKI requiring RRT had a longer ICU stay (29.9 ± 23.3 vs. 19.8 ± 18.5 days, p value = 0.059) and hospital stay (58.8 ± 42.9 vs. 35.3 ± 25.9, p value = 0.002), whereas the survival patients with ESRD receiving RRT did not have a significantly different ICU stay (25.6 ± 22.0 vs. 19.8 ± 18.5 days, p value = 0.331) or hospital stay (46.8 ± 35.1 vs. 35.3 ± 25.9 days, p value = 0.184). Patients with AKI requiring RRT also had a significantly reduced number of ventilator free days in 30- or 60-days compared with the no RRT patients or the ESRD on RRT patients. Among the patients with AKI requiring RRT, only one patient progressed to ESRD and needed long term hemodialysis after discharge from the hospital. The average duration of RRT in the other patients was 33.7 ± 20.6 days (maximum: 60 days, minimum: 10 days). The predictors of 60-day survival in all patients are shown in Table 4. In the multivariate Cox regression analysis, the AKI requiring RRT (hazard ratio: 3.548, p value = 0.003) and tidal volume/predicted body weight (hazard ratio: 1.317, p value = 0.003) were two independent risk factors.

Table 3.
Clinical outcome.
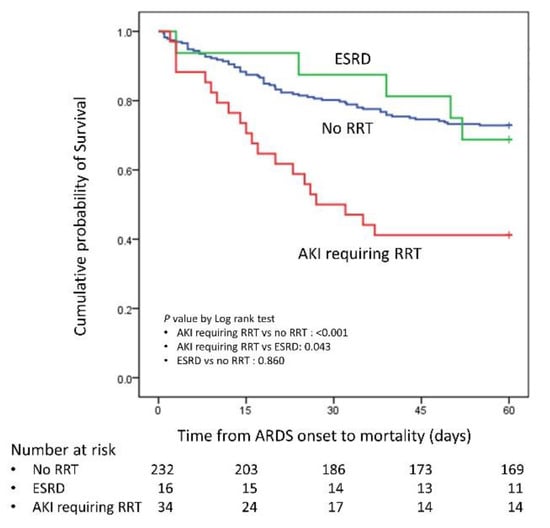
Figure 2.
Kaplan–Meier curve for AKI requiring RRT, ESRD and no RRT; RRT: renal replacement therapy; AKI: acute kidney injury; ESRD: end stage renal disease.

Table 4.
Predictive factors for 60 days mortality in all patients.
3.3. Predictive Factors for Acute Kidney Injury Requiring Renal Replacement Therapy
The predictive factors for AKI requiring RRT in influenza pneumonia induced ARDS patients are shown in Table 5. Patients with underlying ESRD and RRT were excluded from the analysis. In the univariate binary logistic regression, the PaO2/FiO2 ratio, underlying chronic liver disease, underlying chronic kidney disease, initial hemoglobin, C-reactive protein (CRP), lactate, total bilirubin, first day peak airway pressure and dynamic driving pressure were significant factors. In the multivariate analysis, only underlying chronic liver disease (odds ratio: 5.446, p value = 0.031) and CRP (odds ratio: 1.078, p value = 0.036) were significant predictive factors.

Table 5.
Predictive factors for acute kidney injury requiring RRT.
3.4. Survival Predictors for Patients with Acute Kidney Injury Requiring Renal Replacement Therapy
The predictors of 60-day survival in patients with AKI requiring RRT are shown in Table 6. In the univariate Cox regression test, the PSI (hazard ratio: 1.014, p value = 0.010), and tidal volume/predicted body weight (hazard ratio: 1.218, p value = 0.040) were significant predictors. We also used the factors with a p value < 0.100 to conduct the multivariate Cox regression test, and the results revealed that the PSI (hazard ratio: 1.037, p value = 0.002), tidal volume/predicted body weight (hazard ratio: 1.541, p value = 0.022), and CRRT (hazard ratio: 4.752, p value = 0.045) were independent factors for predicting the patient’s 60 day survival.

Table 6.
Predictive factors for 60 days mortality in AKI requiring RRT patients.
4. Discussion
In the current study, we found that patients with influenza pneumonia induced ARDS with AKI requiring RRT had a significantly higher mortality rate compared with patients who did not require RRT. However, patients with ESRD had a similar mortality rate to patients without RRT. The most important risk factors for patients with AKI requiring RRT included chronic liver disease and high CRP, and the most important mortality predictors in patients with AKI requiring RRT were the PSI, tidal volume and CRRT.
In our study, the patients with AKI requiring RRT had higher mortality, and they also had higher severity in general condition (higher PSI, APACHE II, and SOFA score) and in respiratory condition (poorer PaO2/FiO2 ratio). The ARDS is a systemic disease that the inadequate ventilator setting in ARDS patients with barotrauma, volutrauma, or biotrauma may induce multi-organ failure [], and inadequate PEEP setting may result adverse renal hemodynamic effects []. On the other side, the acute kidney injury may induce acute pulmonary edema, electrolyte imbalance, or metabolic acidosis that may worse the respiratory condition. It seem that the higher mortality in these patients may be due to overall disease status. However, in the multivariate Cox regression analysis, we found the AKI requiring RRT was one of the independent predictors for 60-day mortality (hazard ratio: 3.548, p value = 0.003).
Several previous studies have discussed influenza pneumonia induced ARDS patients with AKI and RRT, but they have reported conflicting results concerning patient mortality. A study with 47 patients revealed that 19.1% of patients needed hemodialysis, and that 66.7% of these patients had a significantly higher mortality than the patients without hemodialysis []. Another study with 89 patients showed that the incidence of AKI requiring hemodialysis was 13.5%, and that the mortality rate was 50%, which was significantly higher than the mortality rate for patients without hemodialysis []. A Korean study including 221 patients, found that 33 patients (14.9%) had AKI requiring hemodialysis, and that their mortality rate was significantly increased compared with the other patient groups within the study (28.2% vs. 7.9%) []. On the other hand, two studies showed that hemodialysis patients did not have a significantly higher mortality rate. In one study 24.0% of patients needed hemodialysis and their mortality rate was 32.1% []; in the other study 23.8% of patients received hemodialysis and their mortality rate was 72% []. In our study, 12.1% of patients had AKI which needed RRT, which was similar to that of previous studies. The 60 day mortality rate for patients with AKI requiring hemodialysis was 58.8%; this was in the middle of the reported mortality rates from previous studies. When our outcome was compared with those studies that reported a higher mortality rate, it was clear that our study included the largest number of patients.
In the current study, the ARDS patients with ESRD on regular hemodialysis did not have a significantly different mortality rate compared with the patients without RRT. However, this result was different to previous studies in general ICU patients. A study in 2006 showed that patients with ESRD who were admitted to the ICU had a significantly higher hospital mortality rate compared with patients without ESRD (45.3% vs. 31.2%) []. Another study in 2015 reported that the mortality rate in ESRD patients was significantly higher than in patients without ESRD (34.2% vs. 18.0%) []. A third study in 2017 showed that ICU patients with ESRD had higher ICU, 28-day, and in-hospital mortality rates compared with other patients (21.1% vs. 12.0%) []. In the current study, we focused on patients with ARDS who typically have much more severe conditions than other ICU patients. The mortality rate of ESRD patients was similar to that of previous studies, but the mortality rate of patients without hemodialysis was much higher (27.2%). Perhaps this could indicate that in patients with more severe disease, ESRD is not the reason for the increased mortality rate.
Multivariate analysis revealed that chronic liver disease and CRP were two predictive factors for patients with AKI requiring RRT. Chronic liver disease was associated with AKI development in a previous study that focused on ICU patients [] and another study which focused on ARDS patients []. CRP was also found to be related to AKI in a previous study of influenza patients []. Some previous studies considered that CRP is not only a marker for AKI, but also plays a pathogenic role in AKI [].
The current study also found that the PSI, tidal volume/predicted body weight and CRRT free days were predictive factors for the mortality of AKI patients requiring RRT. The fact that low tidal volume ventilation can improve survival in ARDS patients has been confirmed by a previous study []. In another study by our group, we found that a first day tidal volume/predicted body weight >8 mL/kg was related to an increase in mortality []. Higher tidal volume/predicted body weight being associated with higher mortality was also noted in AKI patients requiring RRT. A previous study showed that CRRT was associated with an increase in morality, but severity of illness was a confounder []. A study of influenza ARDS patients showed that patients requiring CRRT had significantly higher mortality compared with other patients []. In the present study, CRRT usage increased the mortality rate. Determining the predictive factors, enables doctors to know which patients are at risk of suffering from AKI or increased mortality and can allow them to take extra precautions.
There were several limitations to the current study. Firstly, this was a retrospective study, and there are many confounders that could have influenced the results. Secondly, we only focused on patients with influenza related ARDS in this study, so whether these results can be extrapolated to ARDS due to other etiologies needs further evaluation. Thirdly, indications for RRT usage are universal, but there are no clear criteria which are used in Taiwan, especially for CRRT usage. A well-designed prospective study with strict patient selection and a standardized protocol is needed to confirm the results of the present study.
5. Conclusions
In the current study, we found that patients with AKI requiring RRT had a significantly higher mortality rate compared with patients not requiring RRT, but that ESRD patients had a similar mortality rate compared with non RRT patients in influenza pneumonia induced ARDS patients. CRP and chronic liver disease were two independent predictive factors for patients requiring RRT. PSI score, tidal volume, and CRRT usage were independent predictive factors of mortality for AKI patients requiring RRT.
Author Contributions
Conceptualization, K.-W.C. and K.-C.K.; Validation, K.-Y.Y., H.-C.H., W.-C.C., W.-F.F., Y.-M.C., C.-C.S. and C.-L.W.; Formal analysis, S.-W.L. (Shaw-Woei Leu), S.-W.L. (Shih-Wei Lin), M.-J.T., S.-J.L., H.-C.W., Y.-C.C. and C.-K.P.; Data curation, K.-W.C., M.-C.C., H.-C.H., W.-C.C., Y.-M.C., M.-J.T. and Y.-C.C.; Writing—original draft preparation, K.-W.C.; Writing—review and editing, K.-C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The local Institutional Review Boards for Human Research at the involved hospitals all approved this study (Linkou Chang-Gung Memorial Hospital IRB no. 201600632B0, Taichung Veterans General Hospital CE16093A, National Taiwan University Hospital 201605036RIND, Taipei Veterans General Hospital 2016-05-020CC, Tri-Service General Hospital 1-105-05-086, China Medical University Hospital 105-REC2-053(FR), Kaohsiung Medical University Hospital KUMHIRB-E(I)-20170097, Kaohsiung Chang-Gung Memorial Hospital 201600988B0).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The Taiwan Severe Influenza Research Consortium (TSIRC) included the following investigators in Taiwan. Taipei: National Taiwan University Hospital–H.-C.W., Y.-C.C., S.-C.K. and J.-Y.C.; Taipei Veterans General Hospital–K.-Y.Y., W.-C.C. and J.-Y.F.; Tri- Service General Hospital–W.-C.P. and C.-K.P.; Taoyuan: Linkou Chang Gung Memorial Hospi-tal–K.-C.K., H.-C.H., L.-C.C. and K.-W.C.; Taichung: Taichung Veterans General Hospital–C.-L.W., M.-C.C., W.-C.C., C.-H.T., Y.-H.H. and Z.-R.Z.; China Medical University Hospital–S.-J.L. and W.-C.C.; Kaohsiung: Kaohsiung Medical University Hospital–C.-C.S., J.-R.T., M.-J.T. and W.-A.C.; Kaohsiung Chang Gung Memorial Hospital–W.-F.F., Y.-M.C., C.-Y.L. and H.-C.K.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Dennen, P.; Douglas, I.S.; Anderson, R. Acute kidney injury in the intensive care unit: An update and primer for the intensivist. Crit. Care Med. 2010, 38, 261–275. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Jacob, L.P.; Chazalet, J.J.; Payen, D.M.; Villiers, S.M.; Boudaoud, S.; Teillac, P.; Pruna, A.S.; Idatte, J.M.; Eurin, B.G. Renal hemodynamic and functional effect of PEEP ventilation in human renal transplantations. Am. J. Respir. Crit. Care Med. 1995, 152, 103–107. [Google Scholar] [CrossRef]
- Darmon, M.; Clec’h, C.; Adrie, C.; Argaud, L.; Allaouchiche, B.; Azoulay, E.; Bouadma, L.; Garrouste-Orgeas, M.; Haouache, H.; Schwebel, C.; et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.C.; Hu, H.C.; Fu, J.Y.; Hsieh, M.J.; Wu, Y.K.; Chen, Y.C.; Chen, Y.H.; Huang, C.C.; Yang, C.T.; Tsai, Y.H. Renal replacement therapy in prolonged mechanical ventilation patients with renal failure in Taiwan. J. Crit. Care 2011, 26, 600–607. [Google Scholar] [CrossRef]
- Ramsey, C.; Kumar, A. H1N1: Viral pneumonia as a cause of acute respiratory distress syndrome. Curr. Opin. Crit. Care 2011, 17, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Kroeze, E.; Fouchier, R.A.M.; Kuiken, T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 2014, 14, 57–69. [Google Scholar] [CrossRef]
- Investigators, A.I.; Webb, S.A.; Pettila, V.; Seppelt, I.; Bellomo, R.; Bailey, M.; Cooper, D.J.; Cretikos, M.; Davies, A.R.; Finfer, S.; et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N. Engl. J. Med. 2009, 361, 1925–1934. [Google Scholar] [CrossRef]
- Kumar, A.; Zarychanski, R.; Pinto, R.; Cook, D.J.; Marshall, J.; Lacroix, J.; Stelfox, T.; Bagshaw, S.; Choong, K.; Lamontagne, F.; et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009, 302, 1872–1879. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Tsao, K.C.; Huang, C.T.; Chang, K.Y.; Huang, Y.C.; Gong, Y.N. Clinical characteristics of patients with laboratory-confirmed influenza A(H1N1)pdm09 during the 2013/2014 and 2015/2016 clade 6B/6B.1/6B.2-predominant outbreaks. Sci. Rep. 2018, 8, 15636. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Sood, M.M.; Long, J.; Fowler, R.A.; Adhikari, N.K.; Canadian Critical Care Trials Group. Acute kidney injury among critically ill patients with pandemic H1N1 influenza A in Canada: Cohort study. BMC Nephrol. 2013, 14, 123. [Google Scholar] [CrossRef]
- Tignanelli, C.J.; Wiktor, A.J.; Vatsaas, C.J.; Sachdev, G.; Heung, M.; Park, P.K.; Raghavendran, K.; Napolitano, L.M. Outcomes of Acute Kidney Injury in Patients With Severe ARDS Due to Influenza A(H1N1) pdm09 Virus. Am. J. Crit. Care 2018, 27, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; van der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; Town, G.I.; Lewis, S.A.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Abdulkader, R.C.; Ho, Y.L.; de Sousa Santos, S.; Caires, R.; Arantes, M.F.; Andrade, L. Characteristics of acute kidney injury in patients infected with the 2009 influenza A (H1N1) virus. Clin. J. Am. Soc. Nephrol. 2010, 5, 1916–1921. [Google Scholar] [CrossRef]
- Demirjian, S.G.; Raina, R.; Bhimraj, A.; Navaneethan, S.D.; Gordon, S.M.; Schreiber, M.J., Jr.; Guzman, J.A. 2009 influenza A infection and acute kidney injury: Incidence, risk factors, and complications. Am. J. Nephrol. 2011, 34, 1–8. [Google Scholar] [CrossRef]
- Jung, J.Y.; Park, B.H.; Hong, S.B.; Koh, Y.; Suh, G.Y.; Jeon, K.; Koh, S.O.; Kim, J.Y.; Cho, J.H.; Choi, H.S.; et al. Acute kidney injury in critically ill patients with pandemic influenza A pneumonia 2009 in Korea: A multicenter study. J. Crit. Care 2011, 26, 577–585. [Google Scholar] [CrossRef]
- Nin, N.; Lorente, J.A.; Soto, L.; Rios, F.; Hurtado, J.; Arancibia, F.; Ugarte, S.; Echevarria, E.; Cardinal, P.; Saldarini, F.; et al. Acute kidney injury in critically ill patients with 2009 influenza A (H1N1) viral pneumonia: An observational study. Intensive Care Med. 2011, 37, 768–774. [Google Scholar] [CrossRef]
- Hutchison, C.A.; Crowe, A.V.; Stevens, P.E.; Harrison, D.A.; Lipkin, G.W. Case mix, outcome and activity for patients admitted to intensive care units requiring chronic renal dialysis: A secondary analysis of the ICNARC Case Mix Programme Database. Crit. Care 2007, 11, R50. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Lai, C.C.; Cheng, K.C.; Weng, S.F.; Liu, W.L.; Shen, H.N. Effect of end-stage renal disease on long-term survival after a first-ever mechanical ventilation: A population-based study. Crit. Care 2015, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, M.; Yasunaga, H.; Matsui, H.; Horiguchi, H.; Fushimi, K.; Noiri, E.; Nangaku, M.; Doi, K. Impact of end-stage renal disease on hospital outcomes among patients admitted to intensive care units: A retrospective matched-pair cohort study. Nephrology 2017, 22, 617–623. [Google Scholar] [CrossRef]
- Panitchote, A.; Mehkri, O.; Hasting, A.; Hanane, T.; Demirjian, S.; Torbic, H.; Mireles-Cabodevila, E.; Krishnan, S.; Duggal, A. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Mak, S.K.; Xu, A.P.; Lan, H.Y. Role of C-reactive protein in the pathogenesis of acute kidney injury. Nephrology 2018, 23 (Suppl. 4), 50–52. [Google Scholar] [CrossRef]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Chao, W.C.; Liang, S.J.; Tseng, C.H.; Wang, H.C.; Chien, Y.C.; Yang, K.Y.; Chen, W.C.; Perng, W.C.; Kao, K.C.; et al. First tidal volume greater than 8 mL/kg is associated with increased mortality in complicated influenza infection with acute respiratory distress syndrome. J. Formos. Med. Assoc. 2019, 118, 378–385. [Google Scholar] [CrossRef]
- Cho, K.C.; Himmelfarb, J.; Paganini, E.; Ikizler, T.A.; Soroko, S.H.; Mehta, R.L.; Chertow, G.M. Survival by dialysis modality in critically ill patients with acute kidney injury. J. Am. Soc. Nephrol. 2006, 17, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).