Serum Calcium Level as a Useful Surrogate for Risk of Elevated Intraocular Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Ophthalmological Examinations
2.3. Covariates
2.4. Statistical Analyses
3. Results
3.1. Demographics of the Participants
3.2. Association between Serum Total Calcium and Intraocular Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quigley, H.A.; Dunkelberger, G.R.; Green, W.R. Retinal Ganglion Cell Atrophy Correlated with Automated Perimetry in Human Eyes with Glaucoma. Am. J. Ophthalmol. 1989, 107, 453–464. [Google Scholar] [CrossRef]
- Hennis, A.; Wu, S.-Y.; Nemesure, B.; Leske, M. Hypertension, diabetes, and longitudinal changes in intraocular pressure. Ophthalmology 2003, 110, 908–914. [Google Scholar] [CrossRef]
- Wu, C.-J.; Fang, W.-H.; Kao, T.-W.; Chen, Y.-J.; Liaw, F.-Y.; Chang, Y.-W.; Wang, G.-C.; Peng, T.-C.; Chen, W.-L. Postprandial Glucose as a Risk Factor for Elevated Intraocular Pressure. PLoS ONE 2016, 11, e0168142. [Google Scholar] [CrossRef]
- Ye, S.; Chang, Y.; Kim, C.-W.; Kwon, M.-J.; Choi, Y.; Ahn, J.; Kim, J.M.; Kim, H.S.; Shin, H.; Ryu, S. Intraocular pressure and coronary artery calcification in asymptomatic men and women. Br. J. Ophthalmol. 2015, 99, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Ando, F.; Nomura, H.; Sato, Y.; Shimokata, H. Relationship between intraocular pressure and obesity in Japan. Int. J. Epidemiol. 2000, 29, 661–666. [Google Scholar] [CrossRef]
- Tamm, E.R. The trabecular meshwork outflow pathways: Structural and functional aspects. Exp. Eye Res. 2009, 88, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Vosler, P.S.; Brennan, C.S.; Chen, J. Calpain-Mediated Signaling Mechanisms in Neuronal Injury and Neurodegeneration. Mol. Neurobiol. 2008, 38, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Chan, S.L. Neuronal and glial calcium signaling in Alzheimer’s disease. Cell Calcium 2003, 34, 385–397. [Google Scholar] [CrossRef]
- He, Y.; Ge, J.; Tombran-Tink, J. Mitochondrial Defects and Dysfunction in Calcium Regulation in Glaucomatous Trabecular Meshwork Cells. Investig. Opthalmol. Vis. Sci. 2008, 49, 4912–4922. [Google Scholar] [CrossRef] [PubMed]
- Ryskamp, D.A.; Frye, A.M.; Phuong, T.T.T.; Yarishkin, O.; Jo, A.O.; Xu, Y.; Lakk, M.; Iuso, A.; Redmon, S.N.; Ambati, B.; et al. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci. Rep. 2016, 6, 30583. [Google Scholar] [CrossRef]
- Chou, C.-W.; Fang, W.-H.; Chen, Y.-Y.; Wang, C.-C.; Kao, T.-W.; Wu, C.-J.; Chen, W.-L. Association between Serum Calcium and Risk of Cardiometabolic Disease among Community-dwelling Adults in Taiwan. Sci. Rep. 2020, 10, 3192. [Google Scholar] [CrossRef]
- Cho, G.J.; Shin, J.-H.; Yi, K.W.; Park, H.T.; Kim, T.; Hur, J.-Y.; Kim, S.H. Serum calcium level is associated with metabolic syndrome in elderly women. Maturitas 2011, 68, 382–386. [Google Scholar] [CrossRef]
- Oh, S.W.; Lee, S.; Park, C.; Kim, D.J. Elevated intraocular pressure is associated with insulin resistance and metabolic syndrome. Diabetes Metab. Res. Rev. 2005, 21, 434–440. [Google Scholar] [CrossRef]
- Williams, A.L.; Gatla, S.; Leiby, B.E.; Fahmy, I.; Biswas, A.; De Barros, D.M.; Ramakrishnan, R.; Bhardwaj, S.; Wright, C.; Dubey, S.; et al. The Value of Intraocular Pressure Asymmetry in Diagnosing Glaucoma. J. Glaucoma 2013, 22, 215–218. [Google Scholar] [CrossRef]
- Kuo, R.N.; Yang, C.-C.; Yen, A.M.-F.; Liu, T.-Y.; Lin, M.-W.; Chen, S.L.-S. Gender Difference in Intraocular Pressure and Incidence of Metabolic Syndrome: A Community-Based Cohort Study in Matsu, Taiwan. Metab. Syndr. Relat. Disord. 2019, 17, 334–340. [Google Scholar] [CrossRef]
- Imai, K.; Hamaguchi, M.; Mori, K.; Takeda, N.; Fukui, M.; Kato, T.; Kawahito, Y.; Kinoshita, S.; Kojima, T. Metabolic syndrome as a risk factor for high-ocular tension. Int. J. Obes. 2010, 34, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Åström, S.; Stenlund, H.; Lindén, C. Intraocular pressure changes over 21 years—A longitudinal age-cohort study in northern Sweden. Acta Ophthalmol. 2013, 92, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Niu, Y.; Guo, X.; Hu, Y.; Yan, W.; He, M. Age-Related Changes of Intraocular Pressure in Elderly People in Southern China: Lingtou Eye Cohort Study. PLoS ONE 2016, 11, e0151766. [Google Scholar] [CrossRef]
- Lee, I.-T.; Wang, J.-S.; Fu, C.-P.; Chang, C.-J.; Lee, W.-J.; Lin, S.-Y.; Sheu, W.H.-H. The synergistic effect of inflammation and metabolic syndrome on intraocular pressure. Medicine 2017, 96, e7851. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, F.; Ying-Lai, M.; Azen, S.P.; Varma, R. Associations with Intraocular Pressure in Latinos: The Los Angeles Latino Eye Study. Am. J. Ophthalmol. 2008, 146, 69–76. [Google Scholar] [CrossRef]
- Liu, J.H.; Dacus, A.C.; Bartels, S.P. Thyrotropin releasing hormone increases intraocular pressure. Mechanism of action. Investig. Ophthalmol. Vis. Sci. 1989, 30, 2200–2208. [Google Scholar]
- Chiotoroiu, S.M.; De Popa, D.P.; I Ştefăniu, G.; A Secureanu, F.; Purcărea, V.L. The importance of alcohol abuse and smoking in the evolution of glaucoma disease. J. Med. Life 2013, 6, 226–229. [Google Scholar]
- Tanito, M.; Itai, N.; Dong, J.; Ohira, A.; Chihara, E. Correlation between intraocular pressure level and optic disc changes in high-tension glaucoma suspects. Ophthalmology 2003, 110, 915–921. [Google Scholar] [CrossRef]
- Wiederholt, M.; Thieme, H.; Stumpff, F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog. Retin. Eye Res. 2000, 19, 271–295. [Google Scholar] [CrossRef]
- Chintalapudi, S.R.; Maria, D.; Di Wang, X.; Bailey, J.N.C.; Allingham, R.; Aung, T.; Hysi, P.G.; Wiggs, J.L.; Williams, R.W.; Jablonski, M.M. Systems genetics identifies a role for Cacna2d1 regulation in elevated intraocular pressure and glaucoma susceptibility. Nat. Commun. 2017, 8, 1755. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.H.; Oum, B.S.; Chung, J.S.; Cho, B.M.; Hong, J.W. Relationship between intraocular pressure and systemic health parameters in a Korean population. Clin. Exp. Ophthalmol. 2002, 30, 237–241. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xu, L.; Zhang, X.H.; You, Q.S.; Zhao, L.; Jonas, J.B. Five-Year Change in Intraocular Pressure Associated with Changes in Arterial Blood Pressure and Body Mass Index. The Beijing Eye Study. PLoS ONE 2013, 8, e77180. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, L.G.M.; Gracitelli, C.P.B.; Da Silva, L.S.C.; Souza, A.K.S.; Prata, T.S. Association between Glucose Levels and Intraocular Pressure: Pre-and Postprandial Analysis in Diabetic and Nondiabetic Patients. J. Ophthalmol. 2015, 2015, 832058. [Google Scholar] [CrossRef]
- Nongpiur, M.E.; Wong, T.Y.; Sabanayagam, C.; Lim, S.-C.; Tai, E.-S.; Aung, T. Chronic Kidney Disease and Intraocular Pressure. Ophthalmology 2010, 117, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.; Klein, R.; Linton, K.L. Intraocular pressure in an American community. The Beaver Dam Eye Study. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2224–2228. [Google Scholar]
- Wu, S.Y.; Leske, M.C. Associations with intraocular pressure in the Barbados Eye Study. Arch. Ophthalmol. 1997, 115, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Bulpitt, C.J.; Hodes, C.; Everitt, M.G. Intraocular pressure and systemic blood pressure in the elderly. Br. J. Ophthalmol. 1975, 59, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Doughty, M.J.; Zaman, M.L. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv. Ophthalmol. 2000, 44, 367–408. [Google Scholar] [CrossRef]
- Mansoori, T.; Balakrishna, N. Effect of central corneal thickness on intraocular pressure and comparison of Topcon CT-80 non-contact tonometry with Goldmann applanation tonometry. Clin. Exp. Optom. 2018, 101, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Su, D.H.; Wong, T.Y.; Foster, P.J.; Tay, W.-T.; Saw, S.-M.; Aung, T. Central Corneal Thickness and its Associations with Ocular and Systemic Factors: The Singapore Malay Eye Study. Am. J. Ophthalmol. 2009, 147, 709–716.e1. [Google Scholar] [CrossRef]

| Variables | Male (n = 7712) | Female (n = 6325) | p-Value |
|---|---|---|---|
| Continuous Variables, Mean (SD) | |||
| Age (years) | 46.88 (13.00) | 47.00 (12.61) | 0.577 |
| IOP (mmHg) | 14.80 (3.10) | 14.54 (3.09) | <0.001 |
| Percentage body fat (%) | 25.00 (6.33) | 31.94 (6.67) | <0.001 |
| Total cholesterol (mg/dL) | 189.31 (36.09) | 191.29 (36.66) | 0.001 |
| Uric acid (mg/dL) | 6.49 (1.33) | 4.76 (1.10) | <0.001 |
| Aspartate aminotransferase (U/L) | 23.01 (13.52) | 19.63 (9.31) | <0.001 |
| Creatinine (mg/dL) | 0.97 (0.34) | 0.68 (0.17) | <0.001 |
| highly sensitive C-reactive protein (mg/dL) | 0.25 (0.56) | 0.21 (0.42) | <0.001 |
| Thyroid-stimulating hormone (IU/mL) | 2.15 (1.54) | 2.47 (1.96) | <0.001 |
| Serum total calcium (mg/dL) | 9.28 (0.40) | 9.21 (0.41) | 0.310 |
| Category Variables, (n, %) | |||
| Smoking | 3448 (44.7) | 515 (8.1) | <0.001 |
| Drinking | 4242 (55.0) | 1544 (24.1) | <0.001 |
| Variable | Model a 1 β (95% CI) | p Value | Model a 2 β (95% CI) | p Value | Model a 3 β (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Total | 0.045 (0.033–0.058) | <0.001 | 0.039 (0.026–0.053) | <0.001 | 0.040 (0.027–0.053) | <0.001 |
| Male | 0.037 (0.020–0.055) | <0.001 | 0.024 (0.007–0.042) | 0.007 | 0.025 (0.007–0.043) | 0.006 |
| Female | 0.051 (0.032–0.070) | <0.001 | 0.049 (0.030–0.069) | <0.001 | 0.050 (0.030–0.069) | <0.001 |
| Variables | Tertiles | Model a 1 β (95% CI) | p Value | Model a 2 β (95% CI) | p Value | Model a 3 β (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| Total | T2 b vs. T1 b | 0.023 (0.011–0.036) | <0.001 | 0.023 (0.010–0.035) | <0.001 | 0.023 (0.011–0.035) | <0.001 |
| T3 b vs. T1 b | 0.039 (0.026–0.052) | <0.001 | 0.035 (0.022–0.048) | <0.001 | 0.035 (0.022–0.048) | <0.001 | |
| Male | T2 b vs. T1 b | 0.021 (0.005–0.038) | 0.012 | 0.020 (0.004–0.037) | 0.016 | 0.021 (0.004–0.037) | 0.013 |
| T3 b vs. T1 b | 0.031 (0.014–0.047) | <0.001 | 0.022 (0.005–0.038) | 0.012 | 0.022 (0.005–0.039) | 0.010 | |
| Female | T2 b vs. T1 b | 0.024 (0.006–0.043) | 0.011 | 0.023 (0.004–0.042) | 0.017 | 0.024 (0.004–0.043) | 0.016 |
| T3 b vs. T1 b | 0.048 (0.028–0.067) | <0.001 | 0.046 (0.025–0.066) | <0.001 | 0.046 (0.025–0.067) | <0.001 |
| Variables | Tertiles | Model a 1 Odds Ratio (95% CI) | p Value | Model a 2 Odds Ratio (95% CI) | p Value | Model a 3 Odds Ratio (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| Female | T2 b vs. T1 b | 1.379 (1.013–1.877) | 0.041 | 1.310 (0.958–1.792) | 0.091 | 1.323 (0.967–1.810) | 0.080 |
| T3 b vs. T1 b | 1.599 (1.171–2.184) | <0.003 | 1.522 (1.105–2.097) | 0.010 | 1.539 (1.116–2.122) | 0.008 | |
| Male | T2 b vs. T1 b | 1.232 (0.950–1.598) | 0.116 | 1.192 (0.916–1.550) | 0.191 | 1.208 (0.928–1.572) | 0.160 |
| T3 b vs. T1 b | 1.207 (0.931–1.566) | 0.155 | 1.038 (0.792–1.360) | 0.787 | 1.046 (0.799–1.371) | 0.742 |
| Variables | Male | Female | |
|---|---|---|---|
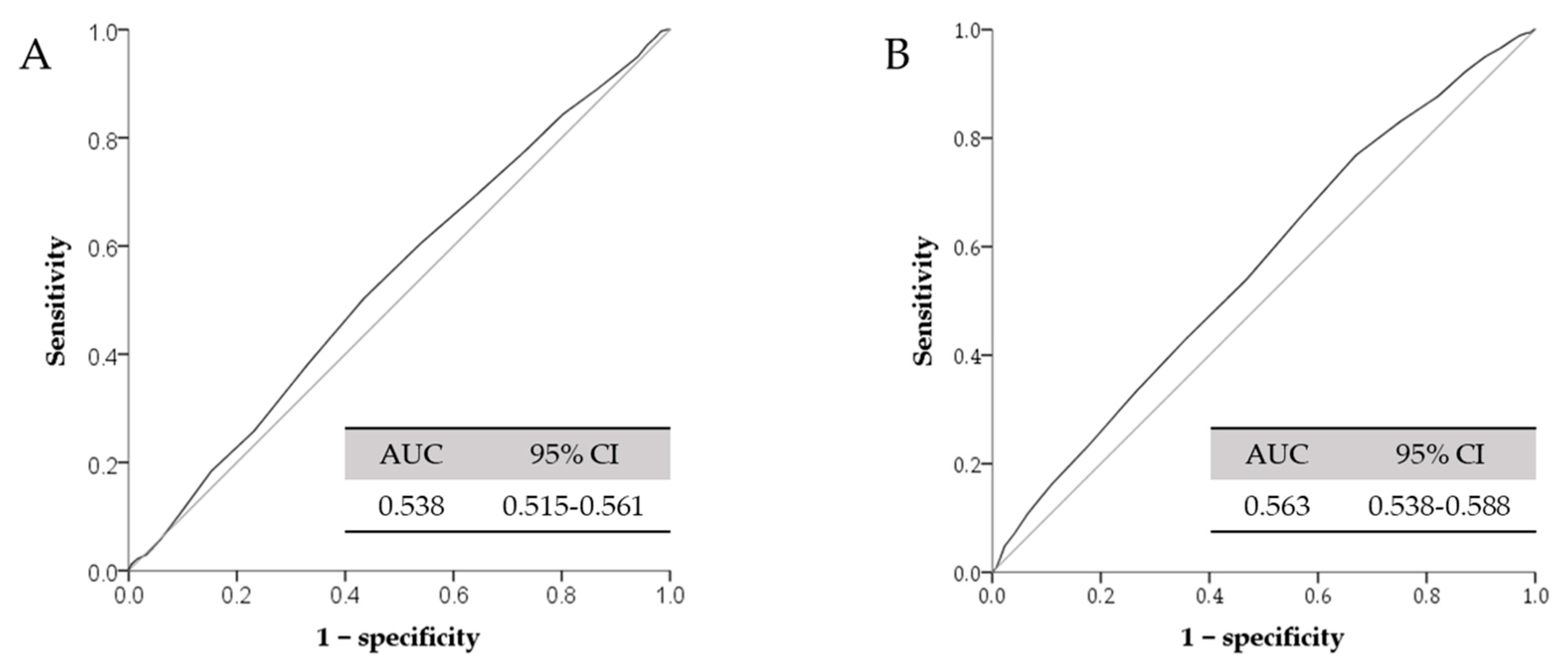
| Cut-off value of serum total calcium (mg/dL) | 9.35 | 9.05 | |
| High IOP(>18 mmHg) | Model a 1 Odds Ratio (95% CI) p Value | 1.258 (1.015–1.559) 0.036 | 1.764 (1.316–2.366) <0.001 |
| Model a 2 Odds Ratio (95% CI) p Value | 1.129 (0.904–1.409) 0.285 | 1.689 (1.254–2.275) 0.001 | |
| Model a 3 Odds Ratio (95% CI) p Value | 1.136 (0.910–1.418) 0.261 | 1.704 (1.264–2.297) <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-M.; Chen, J.-T.; Tai, M.-C.; Chen, W.-L.; Chen, Y.-J. Serum Calcium Level as a Useful Surrogate for Risk of Elevated Intraocular Pressure. J. Clin. Med. 2021, 10, 1839. https://doi.org/10.3390/jcm10091839
Chang Y-M, Chen J-T, Tai M-C, Chen W-L, Chen Y-J. Serum Calcium Level as a Useful Surrogate for Risk of Elevated Intraocular Pressure. Journal of Clinical Medicine. 2021; 10(9):1839. https://doi.org/10.3390/jcm10091839
Chicago/Turabian StyleChang, Yu-Min, Jiann-Torng Chen, Ming-Cheng Tai, Wei-Liang Chen, and Ying-Jen Chen. 2021. "Serum Calcium Level as a Useful Surrogate for Risk of Elevated Intraocular Pressure" Journal of Clinical Medicine 10, no. 9: 1839. https://doi.org/10.3390/jcm10091839
APA StyleChang, Y.-M., Chen, J.-T., Tai, M.-C., Chen, W.-L., & Chen, Y.-J. (2021). Serum Calcium Level as a Useful Surrogate for Risk of Elevated Intraocular Pressure. Journal of Clinical Medicine, 10(9), 1839. https://doi.org/10.3390/jcm10091839






