Cognitive and Adaptive Characterization of Children and Adolescents with KBG Syndrome: An Explorative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Molecular Analysis
2.1.2. Clinical Examination
2.2. Statistical Analysis
3. Results
3.1. Molecular Results
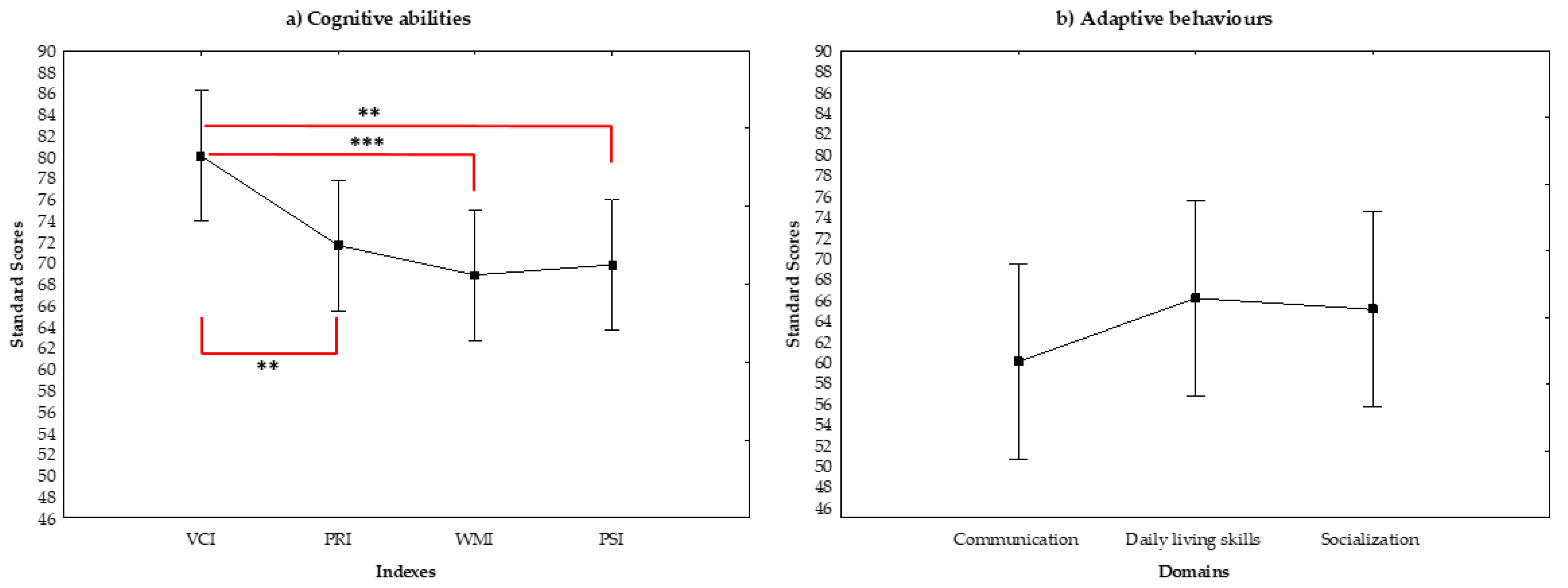
3.2. Cognitive Abilities and Adaptive Behavior
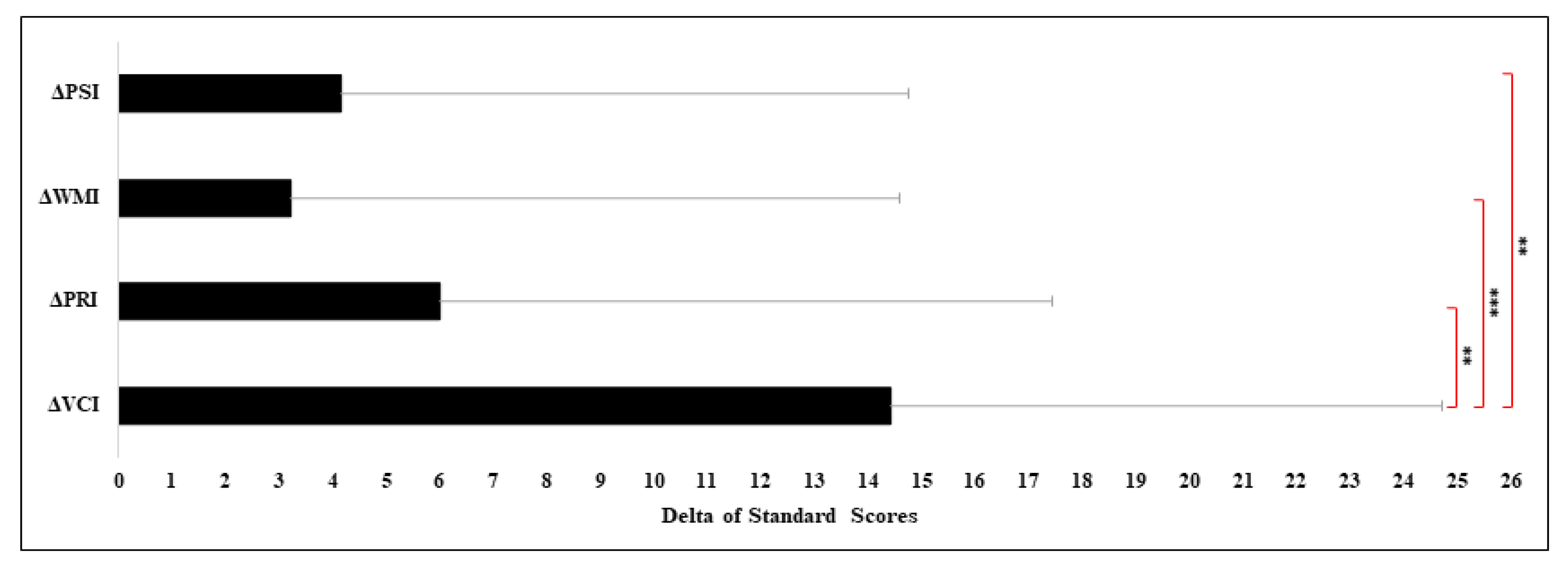
Statistical Differences between VCI and Other Index
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirmaci, A.; Spiliopoulos, M.; Brancati, F.; Powell, E.; Duman, D.; Abrams, A.; Bademci, G.; Agolini, E.; Guo, S.; Konuk, B.; et al. Mutations in ANKRD11 cause KBG Syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am. J. Hum. Genet. 2011, 89, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, M.H.; A Fernandez, B.; A Bacino, C.; Gerkes, E.; De Brouwer, A.P.; Pfundt, R.; Sikkema-Raddatz, B.; Scherer, S.W.; Marshall, C.R.; Potocki, L.; et al. Identification of ANKRD11 and ZNF778 as candidate genes for autism and variable cognitive impairment in the novel 16q24.3 microdeletion syndrome. Eur. J. Hum. Genet. 2009, 18, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Isrie, M.; Hendriks, Y.; Gielissen, N.; A Sistermans, E.; Willemsen, M.H.; Peeters, H.; Vermeesch, J.R.; Kleefstra, T.; Van Esch, H. Haploinsufficiency of ANKRD11 causes mild cognitive impairment, short stature and minor dysmorphisms. Eur. J. Hum. Genet. 2011, 20, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Sacharow, S.; Li, D.; Fan, Y.S.; Tekin, M. Familial 16q24.3 microdeletion involving ANKRD11 causes a KBG-like syndrome. Am. J. Med. Genet. Part A 2012, 158, 547–552. [Google Scholar] [CrossRef]
- Swols, D.M.; FosterII, J.; Tekin, M. KBG syndrome. Orphanet J. Rare Dis. 2017, 12, 183. [Google Scholar] [CrossRef]
- Herrmann, J.; Pallister, P.D.; Tiddy, W.; Opitz, J.M. The KBG syndrome: A syndrome of short stature, characteristic facies, mental retardation, macrodontia and skeletal anomalies. Birth Defects Orig. Artic. Ser. 1975, 11, 7–18. [Google Scholar]
- Smithson, S.F.; Thompson, E.M.; McKinnon, A.G.; Smith, I.S.; Winter, R.M. The KBG syndrome. Clin. Dysmorphol. 2000, 9, 87–91. [Google Scholar] [CrossRef]
- Brancati, F.; Sarkozy, A.; Dallapiccola, B. KBG syndrome. Orphanet J. Rare Dis. 2006, 1, 50. [Google Scholar] [CrossRef]
- Skjei, K.; Martín, M.; Slavotinek, A. KBG syndrome: Report of twins, neurological characteristics, and delineation of diagnostic criteria. Am. J. Med. Genet. Part A 2007, 143A, 292–300. [Google Scholar] [CrossRef]
- Youngs, E.L.; Hellings, J.A.; Butler, M.G. ANKRD11 gene deletion in a 17-year-old male. Clin. Dysmorphol. 2011, 20, 170–171. [Google Scholar] [CrossRef]
- Low, K.; Ashraf, T.; Canham, N.; Clayton-Smith, J.; Deshpande, C.; Donaldson, A.; Fisher, R.; Flinter, F.; Foulds, N.; Fryer, A.; et al. Clinical and genetic aspects of KBG syndrome. Am. J. Med. Genet. Part A 2016, 170, 2835–2846. [Google Scholar] [CrossRef]
- Gnazzo, M.; Lepri, F.R.; Dentici, M.L.; Capolino, R.; Pisaneschi, E.; Agolini, E.; Rinelli, M.; Alesi, V.; Versacci, P.; Genovese, S.; et al. KBG syndrome: Common and uncommon clinical features based on 31 new patients. Am. J. Med. Genet. Part A 2020, 182, 1073–1083. [Google Scholar] [CrossRef]
- Lo-Castro, A.; Brancati, F.; Digilio, M.C.; Garaci, F.G.; Bollero, P.; Alfieri, P.; Curatolo, P. Neurobehavioral phenotype observed in KBG syndrome caused byANKRD11mutations. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2012, 162, 17–23. [Google Scholar] [CrossRef]
- Van Dongen, L.C.M.; Wingbermühle, E.; Oomens, W.; Bos-Roubos, A.G.; Ockeloen, C.W.; Kleefstra, T.; Egger, J.I.M. Intellectual Profiles in KBG-Syndrome: A Wechsler Based Case-Control Study. Front. Behav. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Oegema, R.; Schot, R.; De Wit, M.C.Y.; Lequin, M.H.; Oostenbrink, R.; De Coo, I.F.; Mancini, G.M. KBG syndrome associated with periventricular nodular heterotopia. Clin. Dysmorphol. 2010, 19, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Walz, K.; Cohen, D.; Neilsen, P.M.; Foster, J.; Brancati, F.; Demir, K.; Fisher, R.; Moffat, M.; Verbeek, N.E.; Bjørgo, K.; et al. Characterization of ANKRD11 mutations in humans and mice related to KBG syndrome. Qual. Life Res. 2014, 134, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.; Riccardi, F.; Tessier, A.; Pfundt, R.; Busa, T.; Cacciagli, P.; Capri, Y.; Coutton, C.; Delahaye-Duriez, A.; Frebourg, T.; et al. Clinical and molecular findings in 39 patients with KBG syndrome caused by deletion or mutation ofANKRD11. Am. J. Med. Genet. Part A 2016, 170, 2847–2859. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, L.C.; Wingbermühle, E.; Van Der Veld, W.M.; Vermeulen, K.; Bos-Roubos, A.G.; Ockeloen, C.W.; Kleefstra, T.; Egger, J.I. Exploring the behavioral and cognitive phenotype of KBG syndrome. Genes Brain Behav. 2018, 18, e12553. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Rapley, M. The Social Construction of Intellectual Disability; University Press: Cambridge, UK, 2004; p. 213. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale, 4th ed.; NCS Pearson: San Antonio, TX, USA, 2008. [Google Scholar]
- Balboni, G.; Belacchi, C.; Bonichini, S.; Coscarelli, A. Vineland-II, Vineland Adaptive Behavior Scales-II–Second Edition–Survey Interview Form; Giunti OS Organizzazioni Speciali: Firenze, Italy, 2016. [Google Scholar]
- Ferri, R.; Orsini, A.; Rea, L. ABAS II Adaptive Behavior Assessment System, 2nd ed.; Giunti Psychometrics: Firenze, Italy, 2014. [Google Scholar]
- Alfieri, P.; DeMaria, F.; Licchelli, S.; Santonastaso, O.; Caciolo, C.; Digilio, M.C.; Sinibaldi, L.; Leoni, C.; Gnazzo, M.; Tartaglia, M.; et al. Obsessive Compulsive Symptoms and Psychopathological Profile in Children and Adolescents with KBG Syndrome. Brain Sci. 2019, 9, 313. [Google Scholar] [CrossRef]
- Ockeloen, C.W.; Willemsen, M.H.; De Munnik, S.; Van Bon, B.W.; De Leeuw, N.; Verrips, A.; Kant, S.G.; A Jones, E.; Brunner, H.G.; Le Van Loon, R.; et al. Further delineation of the KBG syndrome phenotype caused by ANKRD11 aberrations. Eur. J. Hum. Genet. 2014, 23, 1176–1185. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, E.; Park, J.B.; Im, W.Y.; Kim, H.J. A Korean family with KBG syndrome identified by ANKRD11 mutation, and phenotypic comparison of ANKRD11 mutation and 16q24.3 microdeletion. Eur. J. Med. Genet. 2015, 58, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Sells, S.B.; Cattell, R.B. Personality and Motivation Structure and Measurement. Am. J. Psychol. 1958, 71, 620. [Google Scholar] [CrossRef]
- Cattell, R.B. Abilities: Their structure, Growth and Action; Houghton Mifflin: Boston, MA, USA, 1971. [Google Scholar]
- Carroll, J.B. The three-stratum theory of cognitive abilities. In Contemporary Intellectual Assessment: Theories, Tests, and Issues; Flanagan, D.P., Genshaft, J.L., Harrison, P.L., Eds.; Guilford Press: New York, NY, USA, 1997; pp. 122–130. [Google Scholar]
- Mervis, C.B.; Klein-Tasman, B.P. Williams syndrome: Cognition, personality, and adaptive behavior. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 148–158. [Google Scholar] [CrossRef]
- Silverman, W. Down syndrome: Cognitive phenotype. Ment. Retard. Dev. Disabil. Res. Rev. 2007, 13, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.S.; Hesketh, L.J. Behavioral phenotype of individuals with Down syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 84–95. [Google Scholar] [CrossRef] [PubMed]
| N | Age | Gender | Wechsler Scale | Adaptive Scale | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Full-IQ | VCI | PRI | WMI | PSI | Full-Adaptive Scale | COM | DLS | SOC | |||
| 1 | 15.83 | M | 69 | 78 | 87 | 73 | 65 | 23 | 20 | 69 | 20 |
| 2 | 9.56 | F | 63 | 94 | 63 | 61 | 65 | 46 | 51 | 49 | 57 |
| 3 | 10.40 | M | 80 | 88 | 76 | 85 | 91 | 95 | 95 | 94 | 97 |
| 4 | 15.30 | M | 49 | 72 | 50 | 58 | 68 | 56 | 61 | 42 | 78 |
| 5 | 15.12 | F | 47 | 70 | 61 | 58 | 47 | 20 | 20 | 22 | 21 |
| 6 | 23.91 | M | 42 | 51 | 63 | 52 | 42 | 20 | 20 | 47 | 20 |
| 7 | 9.51 | M | 89 | 98 | 91 | 67 | 103 | 50 | 61 | 45 | 66 |
| 8 | 11.65 | F | 82 | 84 | 100 | 76 | 82 | 70 | 72 | 73 | 74 |
| 9 | 11.54 | F | 81 | 96 | 65 | 73 | 85 | 85 | 86 | 89 | 84 |
| 10 | 12.27 | M | 68 | 70 | 76 | 79 | 82 | 67 | 72 | 75 | 66 |
| 11 | 8.99 | F | 57 | 86 | 52 | 70 | 59 | 73 | 83 | 61 | 82 |
| 12 | 14.0 | F | 48 | 64 | 52 | 64 | 65 | 27 | 45 | 55 | 20 |
| 13 | 7.24 | M | 71 | 76 | 71 | 46 | 82 | 52 | 49 | 65 | 60 |
| 14 | 13.58 | M | 76 | 92 | 74 | 79 | 82 | 81 | 83 | 85 | 87 |
| 15 | 13.75 | M | 43 | 58 | 71 | 46 | 47 | 35 | 36 | 47 | 56 |
| 16 | 10.83 | F | 84 | 124 | 74 | 88 | 56 | 74 | 93 | 58 | 72 |
| 17 | 8.0 | M | 77 | 88 | 102 | 67 | 67 | 67 | 77 | 98 | 81 |
| 18 | 10.42 | F | 66 | 76 | 58 | 88 | 79 | 67 | 54 | 41 | 60 |
| 19 | 6.1 | F | 90 | 112 | 87 | 85 | 79 | 70 | 74 | 84 | 90 |
| 20 | 17.33 | M | 58 | 69 | 65 | 63 | 75 | 72 | 36 | 98 | 97 |
| 21 | 9.0 | M | 58 | 68 | 74 | 62 | 58 | 66 | 63 | 84 | 67 |
| 22 | 12.4 | M | 57 | 78 | 65 | 64 | 59 | 67 | 64 | 83 | 68 |
| 23 | 14.0 | F | 42 | 58 | 56 | 58 | 53 | 59 | 66 | 58 | 74 |
| 24 | 7.0 | M | 79 | 72 | 87 | 91 | 85 | n.a. | n.a. | n.a. | n.a. |
| Subject | Genomic Coordinate | Nucleotide Position | Protein Position | Exon | dbSNP | gnomAD | Mutation Type | ClinVar ID | Segregation | ACMG Classification | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | chr16:89350973 | c.1977C > G | p.Tyr659 * | 9 | rs749201074 | - | nonsense | 489328 | de novo | Pathogenic | [12] |
| 2 | chr16:89350772 | c.2175_2178delCAAA | p.Asn725Lysfs * 23 | 9 | rs886039734 | 0.000003993 | frameshift | 265689 | de novo | Pathogenic | [12] |
| 3 | chr16:89347745 | c.5205delC | p.Val1736Cysfs * 227 | 9 | - | - | frameshift | - | de novo | Pathogenic | [12] |
| 4 | chr16:89345758 | c.7192C > T | p.Gln2398 * | 9 | rs1265287370 | - | nonsense | - | de novo | Pathogenic | [12] |
| 5 | chr16:89350538 | c.2412delA | p.Glu805Lysfs * 58 | 9 | rs886039902 | - | frameshift | - | maternal (affected mother) | Pathogenic | [12] |
| 6 | chr16:89346866 | c.6071_6084del14 | p.Pro2024Argfs * 3 | 9 | - | - | frameshift | - | de novo | Pathogenic | This study |
| 7 | chr16:89345534 | c.7416C > G | p.Tyr2472 * | 9 | - | - | nonsense | - | de novo | Pathogenic | [12] |
| 8 | chr16:89349179 | c.3770_3771delAA | p.Lys1257Argfs * 25 | 9 | rs886039477 | - | frameshift | 265324 | de novo | Pathogenic | [12] |
| 9 | chr16:89348560 | c.4389_4390delGA | p.Lys1464Thrfs * 89 | 9 | rs1597451815 | - | frameshift | 817640 | de novo | Pathogenic | [26] |
| 10 | chr16:89283689 | chr16:89283689_89572450 deletion | entire gene | - | - | microdeletion | - | de novo | Pathogenic | [12] | |
| 11 | chr16:89351043 | c.1903_1907delAAACA | p.Lys635Glnfs * 26 | 9 | rs886041125 | - | frameshift | 279678 | de novo | Pathogenic | [26] |
| 12 | chr16:89351664 | c.1285_1286delTC | p.Ser429Glyfs * 8 | 9 | rs1597465419 | - | frameshift | 633543 | de novo | Pathogenic | [12] |
| 13 | chr16:89351043 | c.1903_1907delAAACA | p.Lys635Glnfs * 26 | 9 | rs886041125 | - | frameshift | 279678 | de novo | Pathogenic | [26] |
| 14 | chr16:89350772 | c.2175_2178delCAAA | p.Asn725Lysfs * 23 | 9 | rs886039734 | 0.000003993 | frameshift | 265689 | de novo | Pathogenic | [12] |
| 15 | chr16:89350549 | c.2398_2401delGAAA | p.Glu800Asnfs * 62 | 9 | rs797045027 | - | frameshift | 209131 | de novo | Pathogenic | [27] |
| 16 | chr16:89347238 | c.5712_5713insT | p.Gly1905Trpfs * 45 | 9 | - | - | frameshift | - | parents not tested | Pathogenic | This study |
| 17 | chr16:89283689 | chr16:89283689_89559189 deletion | entire gene | - | - | microdeletion | - | de novo | Pathogenic | This study | |
| 18 | chr16:89347806 | c.5144dupA | p.Tyr1715 * | 9 | - | - | nonsense | - | de novo | Pathogenic | This study |
| 19 | chr16:89349641 | c.3309dupA | p.Asp1104Argfs * 2 | 9 | rs772267579 | 0.000007970 | frameshift | 812782 | de novo | Pathogenic | [12] |
| 20 | chr16:89348452 | c.4498C > T | p.Gln1500 * | 9 | - | - | nonsense | - | parents not tested | Pathogenic | [12] |
| 21 | chr16:89351566 | c.1381_1384delGAAA | p.Glu461Glnfs * 48 | 9 | rs1597464953 | - | frameshift | 633578 | parents not tested | Pathogenic | [17] |
| 22 | chr16:89349356 | c.3591_3594delAAAA | p.Lys1198Argfs * 119 | 9 | - | - | frameshift | - | de novo | Pathogenic | This study |
| 23 | chr16:89351566 | c.1381_1384delGAAA | p.Glu461Glnfs * 49 | 9 | rs1597464953 | - | frameshift | 633578 | parents not tested | Pathogenic | [17] |
| 24 | chr16:89341503 | c.7567C > T | p.Arg2523Trp | 10 | - | - | missense | - | de novo | Likely pathogenic | This study |
| Wechsler Indexes | Mean | Min–Max | SD | CI 95% |
|---|---|---|---|---|
| Full-IQ | 65.67 | 42–90 | 15.44 | 12.00–21.66 |
| Verbal Comprehension Index | 80.08 | 51–124 | 17.14 | 13.32–24.04 |
| Perceptual Reasoning Index | 71.67 | 50–102 | 14.64 | 11.38–20.54 |
| Working Memory Index | 68.88 | 46–91 | 13.05 | 10.14–18.31 |
| Processing Speed Index | 69.83 | 42–103 | 15.52 | 12.07–21.78 |
| Mean | Min–Max | SD | CI 95% | |
|---|---|---|---|---|
| Full-Adaptive Scale | 58.35 | 20–95 | 21.14 | 16.35–29.92 |
| Communication domain | 60.04 | 20–95 | 22.66 | 17.53–32.08 |
| Daily Living Skills domain | 66.17 | 22–98 | 20.96 | 16.21–29.66 |
| Socialization domain | 65.09 | 20–97 | 23.99 | 18.56–33.96 |
| N | VCI vs. PRI | VCI vs. WMI | VCI vs. PSI | |||
|---|---|---|---|---|---|---|
| Delta | p Value | Delta | p Value | Delta | p Value | |
| 1 | −9 | 0.26 | 5 | 0.37 | 13 | 0.24 |
| 2 | 31 | 0.01 ** | 33 | 0.02 * | 29 | 0.06 |
| 3 | 12 | 0.20 | 3 | 0.42 | −3 | 0.44 |
| 4 | 22 | 0.06 | 14 | 0.19 | 4 | 0.41 |
| 5 | 9 | 0.26 | 12 | 0.22 | 23 | 0.10 |
| 6 | −12 | 0.20 | −1 | 0.48 | 9 | 0.31 |
| 7 | 7 | 0.32 | 31 | 0.02 * | −5 | 0.39 |
| 8 | −16 | 0.13 | 8 | 0.31 | 2 | 0.40 |
| 9 | 31 | 0.01 ** | 23 | 0.08 | 11 | 0.25 |
| 10 | −6 | 0.34 | −9 | 0.28 | −12 | 0.25 |
| 11 | 34 | 0.01 ** | 16 | 0.15 | 27 | 0.07 |
| 12 | 12 | 0.20 | 0 | 0.50 | −1 | 0.48 |
| 13 | 5 | 0.36 | 30 | 0.03 * | −6 | 0.37 |
| 14 | 18 | 0.10 | 13 | 0.20 | 10 | 0.29 |
| 15 | −13 | 0.18 | 12 | 0.22 | 11 | 0.27 |
| 16 | 50 | 0.00 *** | 36 | 0.01 ** | 68 | 0.01 ** |
| 17 | −14 | 0.16 | 21 | 0.09 | 21 | 0.13 |
| 18 | 18 | 0.10 | −12 | 0.22 | −3 | 0.44 |
| 19 | 25 | 0.04 * | 27 | 0.04 * | 33 | 0.04 * |
| 20 | 4 | 0.39 | 6 | 0.35 | −6 | 0.37 |
| 21 | −6 | 0.34 | 6 | 0.35 | 10 | 0.29 |
| 22 | 13 | 0.18 | 14 | 0.19 | 19 | 0.15 |
| 23 | 2 | 0.44 | 0 | 0.50 | 5 | 0.39 |
| 24 | −15 | 0.14 | −19 | 0.12 | −13 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, P.; Caciolo, C.; Lazzaro, G.; Menghini, D.; Cumbo, F.; Dentici, M.L.; Digilio, M.C.; Gnazzo, M.; Demaria, F.; Pironi, V.; et al. Cognitive and Adaptive Characterization of Children and Adolescents with KBG Syndrome: An Explorative Study. J. Clin. Med. 2021, 10, 1523. https://doi.org/10.3390/jcm10071523
Alfieri P, Caciolo C, Lazzaro G, Menghini D, Cumbo F, Dentici ML, Digilio MC, Gnazzo M, Demaria F, Pironi V, et al. Cognitive and Adaptive Characterization of Children and Adolescents with KBG Syndrome: An Explorative Study. Journal of Clinical Medicine. 2021; 10(7):1523. https://doi.org/10.3390/jcm10071523
Chicago/Turabian StyleAlfieri, Paolo, Cristina Caciolo, Giulia Lazzaro, Deny Menghini, Francesca Cumbo, Maria Lisa Dentici, Maria Cristina Digilio, Maria Gnazzo, Francesco Demaria, Virginia Pironi, and et al. 2021. "Cognitive and Adaptive Characterization of Children and Adolescents with KBG Syndrome: An Explorative Study" Journal of Clinical Medicine 10, no. 7: 1523. https://doi.org/10.3390/jcm10071523
APA StyleAlfieri, P., Caciolo, C., Lazzaro, G., Menghini, D., Cumbo, F., Dentici, M. L., Digilio, M. C., Gnazzo, M., Demaria, F., Pironi, V., Zampino, G., Novelli, A., Tartaglia, M., & Vicari, S. (2021). Cognitive and Adaptive Characterization of Children and Adolescents with KBG Syndrome: An Explorative Study. Journal of Clinical Medicine, 10(7), 1523. https://doi.org/10.3390/jcm10071523








