Rationale, Design, and Feasibility of a Prospective Multicenter Registry Study of Anthracycline-Induced Cardiotoxicity (AIC Registry)
Abstract
1. Introduction
2. Materials and Methods
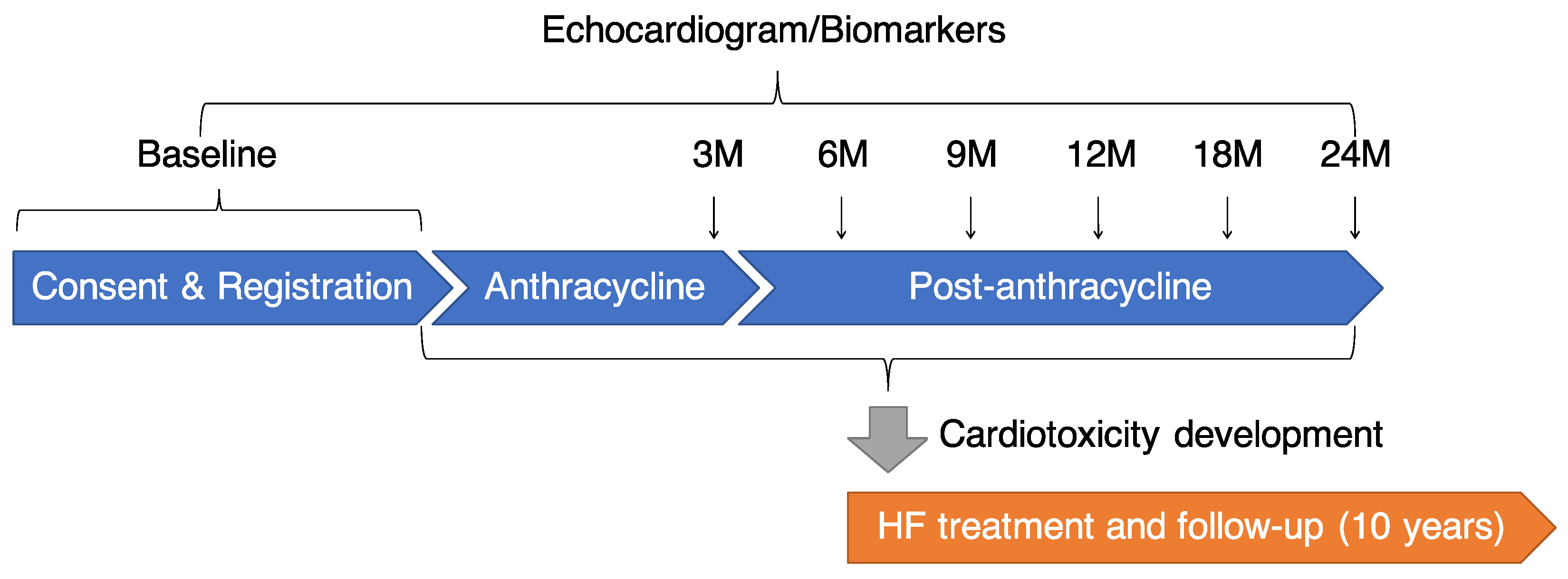
2.1. Design
2.2. Patient Eligibility
2.3. Definition of Cardiotoxicity
2.4. Study Protocol
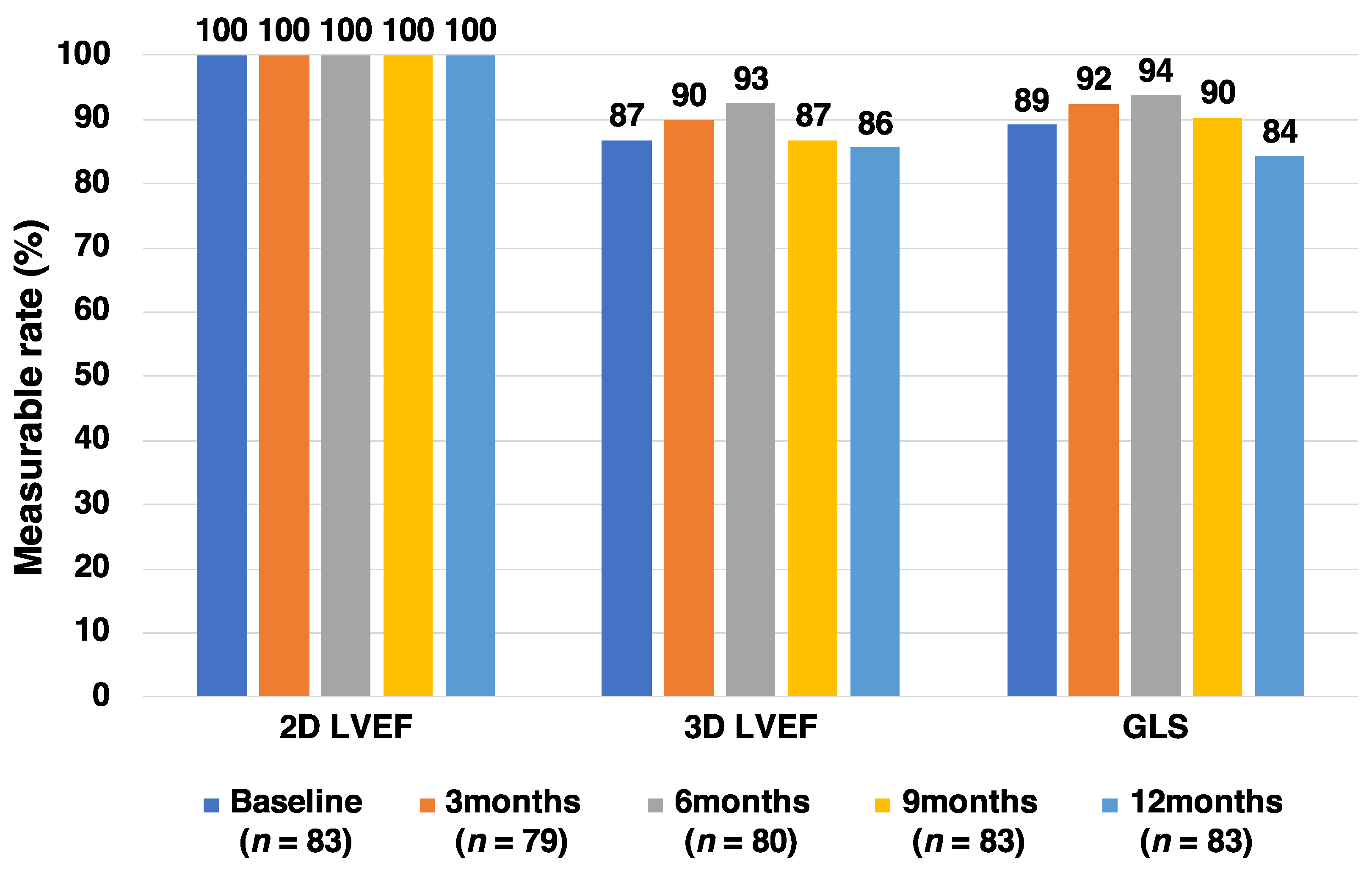
2.5. Echocardiogram
2.6. Biomarkers
2.7. Statistics
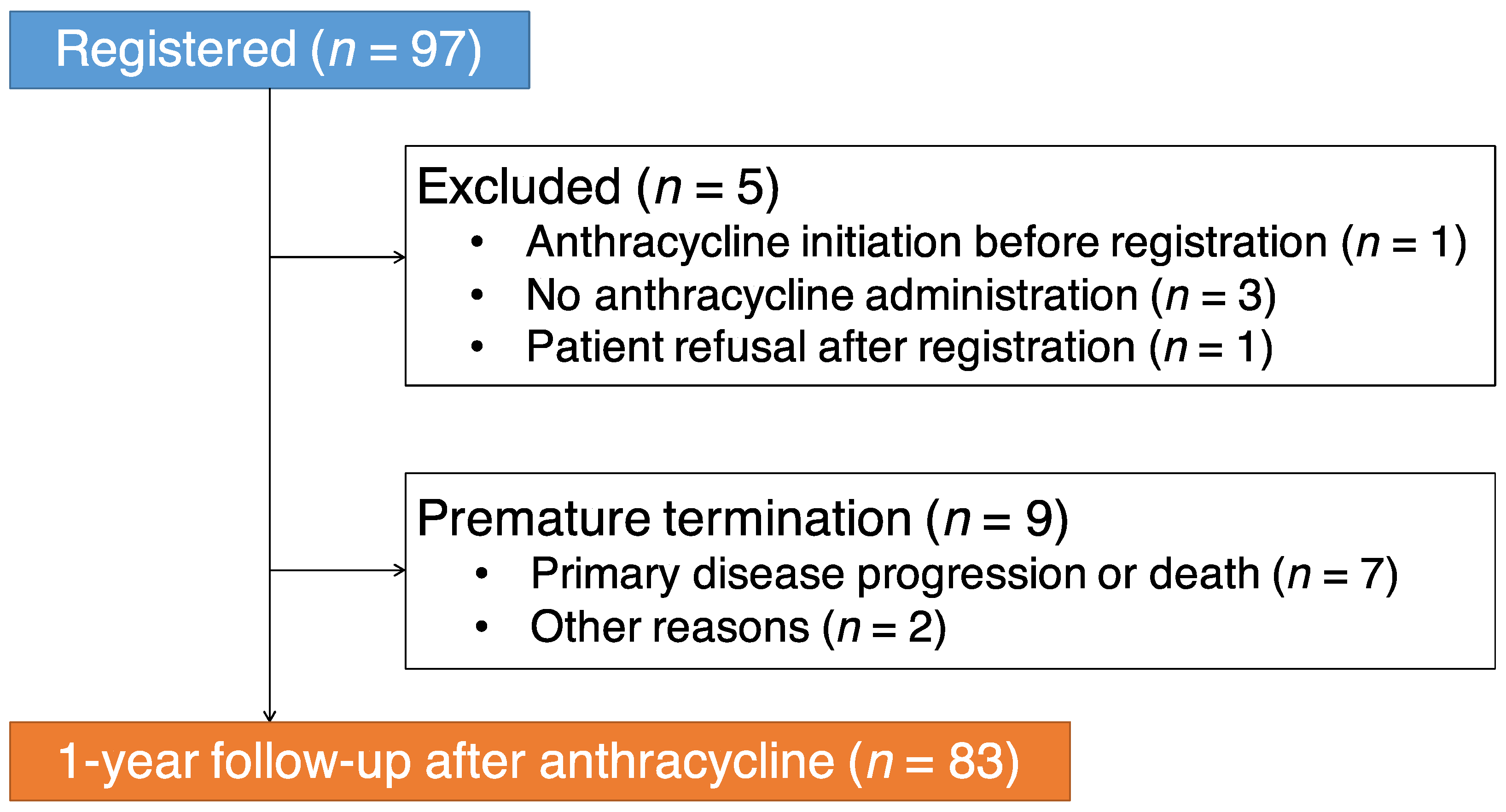
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, L.A.; Cornelius, V.R.; Plummer, C.J.; Levitt, G.; Verrill, M.; Canney, P.; Jones, A. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.R.; Shapiro, C.L.; Ng, A.; Jacobs, L.; Schwartz, C.; Virgo, K.S.; Hagerty, K.L.; Somerfield, M.R.; Vaughn, D.J. American society of clinical oncology clinical evidence review on the ongoing care of adult cancer survivors: Cardiac and pulmonary late effects. J. Clin. Oncol. 2007, 25, 3991–4008. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Ewer, S.M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 2015, 12, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xu, X.; Cheng, L.; Li, L.; Sun, M.; Chen, H.; Pan, C.; Shu, X. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur. J. Heart Fail. 2014, 16, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Arpino, G.; Esposito, R.; Lembo, M.; Paciolla, I.; Cardalesi, C.; De Simone, G.; Trimarco, B.; De Placido, S.; Galderisi, M. 2D and 3D strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients: A balance with feasibility. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Cohen, V.; Gosavi, S.; Carver, J.R.; Wiegers, S.E.; Martin, R.P.; et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 2011, 107, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J.; Keefe, D. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002, 20, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Isobe, M.; Ito, H.; Okumura, K.; Ono, M.; Kitakaze, M.; Kinugawa, K.; Kihara, Y.; Goto, Y.; Komuro, I.; et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure–digest version. Circ. J. 2019, 83, 2084–2184. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
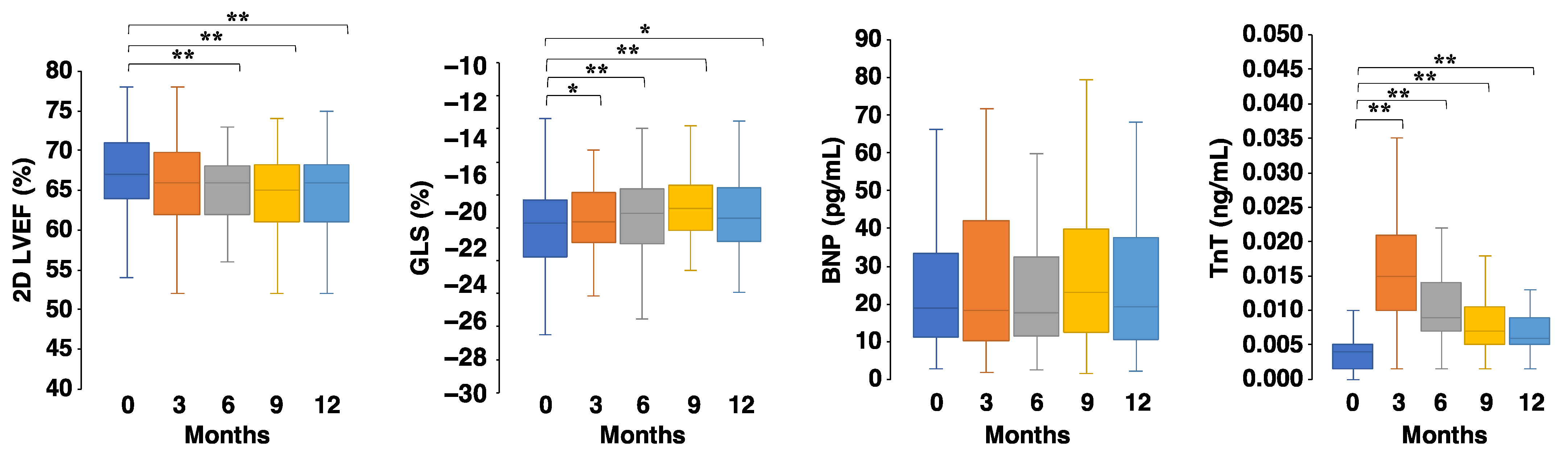
| Inclusion Criteria |
|---|
| 1. Scheduled to receive anthracyclines |
| 2. Age ≥ 20 years |
| 3. Oncologic life expectancy of >1 year |
| 4. Able and willing to provide written informed consent to participate in the study |
| Exclusion criteria |
| 1. Ejection fraction at baseline echo < 55% |
| 2. Valvular stenosis or regurgitation of >moderate severity, congenital heart disease, or cardiomyopathy |
| 3. Pregnant or lactating |
| 4. Existing mental disorder which may affect the ability or willingness to provide informed consent |
| 5. Considered unfit by physicians to participate in the study |
| Variable | Value |
|---|---|
| Age at administration (years) | 54 ± 13 |
| Female sex | 75 (90.4) |
| BMI (kg/m2) | 23.5 ± 4.3 |
| Prior cardiovascular disease * | 4 (4.8) |
| Diabetes mellitus | 7 (8.4) |
| Dyslipidemia | 18 (21.7) |
| Hypertension | 16 (19.3) |
| Anthracycline Agents | |
| Epirubicin | 58 (69.9) |
| Doxorubicin | 19 (22.9) |
| Liposomal doxorubicin | 5 (6.0) |
| Daunorubicin | 2 (2.4) |
| Idarubicin | 2 (2.4) |
| Mitoxantrone | 1 (1.2) |
| Pirarubicin | 1 (1.2) |
| Total cumulative dose of anthracyclines (mg/m2) # | 198 ± 57 |
| History of anthracyclines use | 4 (4.8) |
| Cancer Diagnosis | |
| Breast cancer | 58 (69.9) |
| Malignant lymphoma | 17 (20.5) |
| Ovarian cancer | 4 (4.8) |
| Leukemia | 2 (2.4) |
| Endometrial cancer | 2 (2.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, K.; Iida, N.; Tajiri, K.; Bando, H.; Chiba, S.; Tasaka, N.; Nagashio, K.; Sasamura, R.; Naito, H.; Murata, M.; et al. Rationale, Design, and Feasibility of a Prospective Multicenter Registry Study of Anthracycline-Induced Cardiotoxicity (AIC Registry). J. Clin. Med. 2021, 10, 1370. https://doi.org/10.3390/jcm10071370
Inoue K, Iida N, Tajiri K, Bando H, Chiba S, Tasaka N, Nagashio K, Sasamura R, Naito H, Murata M, et al. Rationale, Design, and Feasibility of a Prospective Multicenter Registry Study of Anthracycline-Induced Cardiotoxicity (AIC Registry). Journal of Clinical Medicine. 2021; 10(7):1370. https://doi.org/10.3390/jcm10071370
Chicago/Turabian StyleInoue, Keiko, Noriko Iida, Kazuko Tajiri, Hiroko Bando, Shigeru Chiba, Nobutaka Tasaka, Kenji Nagashio, Rumi Sasamura, Hiroyuki Naito, Momoko Murata, and et al. 2021. "Rationale, Design, and Feasibility of a Prospective Multicenter Registry Study of Anthracycline-Induced Cardiotoxicity (AIC Registry)" Journal of Clinical Medicine 10, no. 7: 1370. https://doi.org/10.3390/jcm10071370
APA StyleInoue, K., Iida, N., Tajiri, K., Bando, H., Chiba, S., Tasaka, N., Nagashio, K., Sasamura, R., Naito, H., Murata, M., Li, S., Ishizu, T., Nakazawa, Y., Sekine, I., & Ieda, M. (2021). Rationale, Design, and Feasibility of a Prospective Multicenter Registry Study of Anthracycline-Induced Cardiotoxicity (AIC Registry). Journal of Clinical Medicine, 10(7), 1370. https://doi.org/10.3390/jcm10071370






