Exaggerated Cardiac Contractile Response to Hypoxia in Adults Born Preterm
Abstract
1. Introduction
2. Materials and Methods
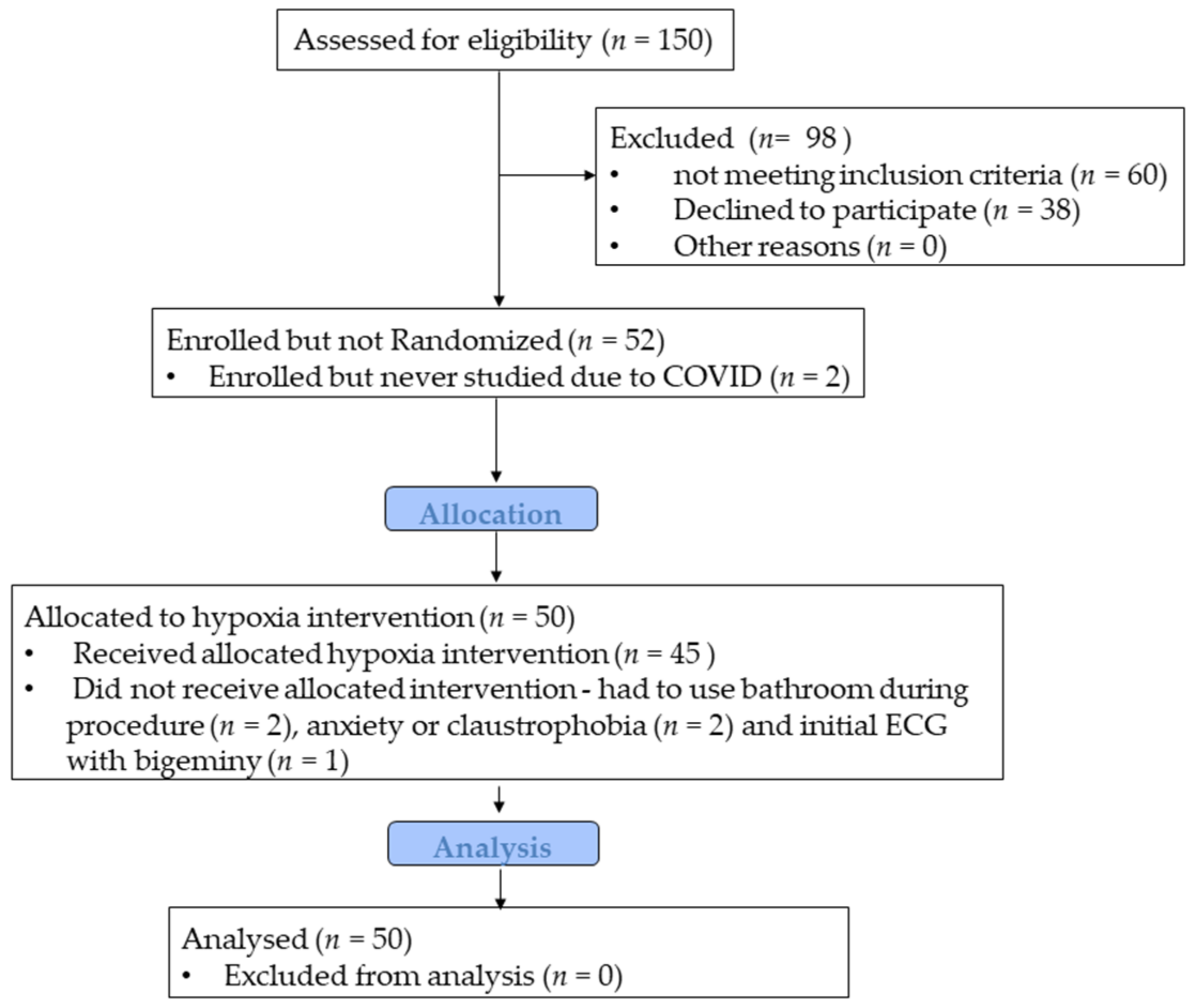
2.1. Participants and Study Design
2.2. Cardiovascular Magnetic Resonance Imaging (MRI) Acquisition
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Cardiovascular Responses to Normoxia
3.3. Cardiovascular Responses to Hypoxia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, J.P.; Chawanpaiboon, S.; Watananirun, K.; Lumbiganon, P.; Petzold, M.; Moller, A.B.; Thinkhamrop, J.; Laopaiboon, M.; Seuc, A.H.; Hogan, D.; et al. Global, regional and national levels and trends of preterm birth rates for 1990 to 2014: Protocol for development of World Health Organization estimates. Reprod. Health 2016, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.N.K.; Buist, A.S.; Blaisdell, C.J.; Moxey-Mims, M.; Saigal, S. Adults born preterm: A review of general health and system-specific outcomes. Acta Paediatr. 2017, 106, 1409–1437. [Google Scholar] [CrossRef]
- Raju, T.N.K.; Pemberton, V.L.; Saigal, S.; Blaisdell, C.J.; Moxey-Mims, M.; Buist, S. Long-Term Healthcare Outcomes of Preterm Birth: An Executive Summary of a Conference Sponsored by the National Institutes of Health. J. Pediatr. 2017, 181, 309–318.e1. [Google Scholar] [CrossRef]
- Crump, C.; Friberg, D.; Li, X.; Sundquist, J.; Sundquist, K. Preterm birth and risk of sleep-disordered breathing from childhood into mid-adulthood. Int. J. Epidemiol. 2019, 48, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Goss, K.N.; Haraldsdottir, K.; Beshish, A.G.; Barton, G.P.; Watson, A.M.; Palta, M.; Chesler, N.C.; Francois, C.J.; Wieben, O.; Eldridge, M.W. Association Between Preterm Birth and Arrested Cardiac Growth in Adolescents and Young Adults. JAMA Cardiol. 2020, 5, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Augustine, D.; Lamata, P.; Davis, E.F.; Lazdam, M.; Francis, J.; McCormick, K.; Wilkinson, A.R.; Singhal, A.; Lucas, A.; et al. Preterm heart in adult life: Cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013, 127, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Bradlow, W.M.; Augustine, D.; Davis, E.F.; Francis, J.; Singhal, A.; Lucas, A.; Neubauer, S.; McCormick, K.; Leeson, P. Right ventricular systolic dysfunction in young adults born preterm. Circulation 2013, 128, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.L.; Bray, H.; Troughton, R.; Elliott, J.; Frampton, C.; Horwood, J.; Darlow, B.A. Cardiovascular Outcomes in Young Adulthood in a Population-Based Very Low Birth Weight Cohort. J. Pediatr. 2020, 225, 74–79.e3. [Google Scholar] [CrossRef]
- Mohamed, A.; Lamata, P.; Williamson, W.; Alsharqi, M.; Tan, C.M.J.; Burchert, H.; Huckstep, O.J.; Suriano, K.; Francis, J.M.; Pelado, J.L.; et al. Multimodality Imaging Demonstrates Reduced Right-Ventricular Function Independent of Pulmonary Physiology in Moderately Preterm-Born Adults. JACC Cardiovasc. Imaging 2020, 13, 2046–2048. [Google Scholar] [CrossRef]
- Huckstep, O.J.; Williamson, W.; Telles, F.; Burchert, H.; Bertagnolli, M.; Herdman, C.; Arnold, L.; Smillie, R.; Mohamed, A.; Boardman, H.; et al. Physiological Stress Elicits Impaired Left Ventricular Function in Preterm-Born Adults. J. Am. Coll. Cardiol. 2018, 71, 1347–1356. [Google Scholar] [CrossRef]
- Haraldsdottir, K.; Watson, A.M.; Pegelow, D.F.; Palta, M.; Tetri, L.H.; Levin, T.; Brix, M.D.; Centanni, R.M.; Goss, K.N.; Eldridge, M.M. Blunted cardiac output response to exercise in adolescents born preterm. Eur. J. Appl. Physiol. 2020, 120, 2547–2554. [Google Scholar] [CrossRef] [PubMed]
- Goss, K.N.; Beshish, A.G.; Barton, G.P.; Haraldsdottir, K.; Levin, T.S.; Tetri, L.H.; Battiola, T.J.; Mulchrone, A.M.; Pegelow, D.F.; Palta, M.; et al. Early Pulmonary Vascular Disease in Young Adults Born Preterm. Am. J. Respir. Crit. Care Med. 2018, 198, 1549–1558. [Google Scholar] [CrossRef]
- Mulchrone, A.; Bellofiore, A.; Douwes, J.M.; Duong, N.; Beshish, A.G.; Barton, G.P.; Francois, C.J.; Eldridge, M.W.; Goss, K.N.; Chesler, N.C. Impaired Right Ventricular-Vascular Coupling in Young Adults Born Preterm. Am. J. Respir. Crit. Care Med. 2020, 201, 615–618. [Google Scholar] [CrossRef]
- Alfakih, K.; Plein, S.; Bloomer, T.; Jones, T.; Ridgway, J.; Sivananthan, M. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J. Magn. Reson. Imaging 2003, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F.; Smith, G.C.; Moon, J.C.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Moon, J.C.C.; Lorenz, C.H.; Francis, J.M.; Smith, G.C.; Pennell, D.J. Breath-hold FLASH and FISP Cardiovascular MR Imaging: Left Ventricular Volume Differences and Reproducibility. Radiology 2002, 223, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Palta, M.; Gabbert, D.; Fryback, D.; Widjaja, I.; Peters, M.E.; Farrell, P.; Johnson, J. Development and validation of an index for scoring baseline respiratory disease in the very low birth weight neonate. Severity Index Development and Validation Panels and Newborn Lung Project. Pediatrics 1990, 86, 714–721. [Google Scholar] [PubMed]
- Palta, M.; Gabbert, D.; Weinstein, M.R.; Peters, M.E. Multivariate assessment of traditional risk factors for chronic lung disease in very low birth weight neonates. The Newborn Lung Project. J. Pediatr. 1991, 119, 285–292. [Google Scholar] [CrossRef]
- Palta, M.; Weinstein, M.R.; McGuinness, G.; Gabbert, D.; Brady, W.; Peters, M.E. A population study. Mortality and morbidity after availability of surfactant therapy. Newborn Lung Project. Arch. Pediatr. Adolesc. Med. 1994, 148, 1295–1301. [Google Scholar] [CrossRef]
- Morais, P.; Marchi, A.; Bogaert, J.A.; Dresselaers, T.; Heyde, B.; D’Hooge, J.; Bogaert, J. Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm: Assessment of variability in a real-life clinical setting. J. Cardiovasc. Magn. Reson. 2017, 19, 24. [Google Scholar] [CrossRef]
- Mohiaddin, R.H.; Firmin, D.N.; Longmore, D.B. Age-related changes of human aortic flow wave velocity measured noninvasively by magnetic resonance imaging. J. Appl. Physiol. 1993, 74, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Barton, G.P.; Goss, K.N. Increased mitochondrial oxygen consumption in adult survivors of preterm birth. Pediatr. Res. 2021. [Google Scholar] [CrossRef]
- Netzer, N.C.; Strohl, K.P.; Högel, J.; Gatterer, H.; Schilz, R. Right ventricle dimensions and function in response to acute hypoxia in healthy human subjects. Acta Physiol. 2017, 219, 478–485. [Google Scholar] [CrossRef]
- Luks, A.M.; Levett, D.; Martin, D.S.; Goss, C.H.; Mitchell, K.; Fernandez, B.O.; Feelisch, M.; Grocott, M.P.; Swenson, E.R. Changes in acute pulmonary vascular responsiveness to hypoxia during a progressive ascent to high altitude (5300 m). Exp. Physiol. 2017, 102, 711–724. [Google Scholar] [CrossRef]
- Dedobbeleer, C.; Hadefi, A.; Naeije, R.; Unger, P. Left ventricular adaptation to acute hypoxia: A speckle-tracking echocardiography study. J. Am. Soc. Echocardiogr. 2013, 26, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.D.; Abman, S.H.; Mourani, P.M. Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Pediatr. Allergy Immunol. Pulmonol. 2014, 27, 8–16. [Google Scholar] [CrossRef]
- Joshi, S.; Wilson, D.G.; Kotecha, S.; Pickerd, N.; Fraser, A.G.; Kotecha, S. Cardiovascular function in children who had chronic lung disease of prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F373–F379. [Google Scholar] [CrossRef] [PubMed]
- Lovering, A.T.; Elliott, J.E.; Laurie, S.S.; Beasley, K.M.; Gust, C.E.; Mangum, T.S.; Gladstone, I.M.; Duke, J.W. Ventilatory and Sensory Responses in Adult Survivors of Preterm Birth and Bronchopulmonary Dysplasia with Reduced Exercise Capacity. Ann. Am. Thorac. Soc. 2014, 11, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Haraldsdottir, K.; Watson, A.M.; Beshish, A.G.; Pegelow, D.F.; Palta, M.; Tetri, L.H.; Brix, M.D.; Centanni, R.M.; Goss, K.N.; Eldridge, M.W. Heart rate recovery after maximal exercise is impaired in healthy young adults born preterm. Eur. J. Appl. Physiol. 2019, 119, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Vrijlandt, E.J.; Gerritsen, J.; Boezen, H.M.; Grevink, R.G.; Duiverman, E.J. Lung function and exercise capacity in young adults born prematurely. Am. J. Respir. Crit. Care Med. 2006, 173, 890–896. [Google Scholar] [CrossRef]
- Jacob, S.V.; Lands, L.C.; Coates, A.L.; Davis, G.M.; MacNeish, C.F.; Hornby, L.; Riley, S.P.; Outerbridge, E.W. Exercise ability in survivors of severe bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 1997, 155, 1925–1929. [Google Scholar] [CrossRef]
- Lovering, A.T.; Laurie, S.S.; Elliott, J.E.; Beasley, K.M.; Yang, X.; Gust, C.E.; Mangum, T.S.; Goodman, R.D.; Hawn, J.A.; Gladstone, I.M. Normal pulmonary gas exchange efficiency and absence of exercise-induced arterial hypoxemia in adults with bronchopulmonary dysplasia. J. Appl. Physiol. 2013, 115, 1050–1056. [Google Scholar] [CrossRef]
- Goss, K.N.; Cucci, A.R.; Fisher, A.J.; Albrecht, M.; Frump, A.; Tursunova, R.; Gao, Y.; Brown, M.B.; Petrache, I.; Tepper, R.S.; et al. Neonatal hyperoxic lung injury favorably alters adult right ventricular remodeling response to chronic hypoxia exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L797–L806. [Google Scholar] [CrossRef]
- Goss, K.N.; Kumari, S.; Tetri, L.H.; Barton, G.; Braun, R.K.; Hacker, T.A.; Eldridge, M.W. Postnatal Hyperoxia Exposure Durably Impairs Right Ventricular Function and Mitochondrial Biogenesis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 609–619. [Google Scholar] [CrossRef]
- Kumari, S.; Braun, R.K.; Tetri, L.H.; Barton, G.P.; Hacker, T.A.; Goss, K.N. Bimodal right ventricular dysfunction after postnatal hyperoxia exposure: Implications for the preterm heart. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1272–H1281. [Google Scholar] [CrossRef]
- Bates, M.L.; Farrell, E.T.; Eldridge, M.W. Abnormal ventilatory responses in adults born prematurely. N. Engl. J. Med. 2014, 370, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Bisgard, G.E.; Olson, E.B.; Wang, Z.Y.; Bavis, R.W.; Fuller, D.D.; Mitchell, G.S. Adult carotid chemoafferent responses to hypoxia after 1, 2, and 4 wk of postnatal hyperoxia. J. Appl. Physiol. 2003, 95, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G. Regulation of breathing and autonomic outflows by chemoreceptors. Compr. Physiol. 2014, 4, 1511–1562. [Google Scholar] [CrossRef] [PubMed]
- Haraldsdottir, K.; Watson, A.M.; Goss, K.N.; Beshish, A.G.; Pegelow, D.F.; Palta, M.; Tetri, L.H.; Barton, G.P.; Brix, M.D.; Centanni, R.M.; et al. Impaired autonomic function in adolescents born preterm. Physiol. Rep. 2018, 6, e13620. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Hoffman, J.I.E. Right ventricular architecture responsible for mechanical performance: Unifying role of ventricular septum. J. Thorac. Cardiovasc. Surg. 2014, 148, 3166–3171.e4. [Google Scholar] [CrossRef]

| Term | Preterm | p-Value | |
|---|---|---|---|
| Baseline Adult Characteristics | |||
| Number, Participants (N) | 18 | 32 | |
| Current age, years | 25.4 (4.0) | 26.1 (4.2) | 0.58 |
| Female sex | 10 (56%) | 23 (72%) | 0.25 |
| Race, white | 17 (94%) | 30 (94%) | 0.92 |
| Height (inches) | 68.1 (3.4) | 65.6 (2.8) | <0.01 |
| Weight (kg) | 69.9 (9.6) | 74.5 (24.1) | 0.44 |
| Body mass index (kg/m2) | 23.3 (1.9) | 26.7 (8.5) | 0.10 |
| Body surface area (m2) | 1.83 (0.17) | 1.84 (0.29) | 0.91 |
| Neonatal Characteristics | |||
| Gestational age (weeks) | 39.8 (1.0) | 29.2 (2.5) | <0.01 |
| Birth weight (g) | 3397 (572) | 1225 (397) | <0.01 |
| Bronchopulmonary dysplasia | n/a | 10 (32%) | |
| Days on assisted ventilation | n/a | 12.4 (14.0) | |
| Patent ductus arteriosus | n/a | 12 (38%) |
| Normoxia | Hypoxia | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Term (N = 18) | Preterm (N = 32) | (Normoxia) p | Term (N = 17) | Preterm (N = 28) | (Hypoxia) p | (Interaction) p |
| SBP (mmHg) | 123 (11) | 126 (18) | 0.50 | 126 (18) | 125 (17) | 0.78 | 0.24 |
| DBP (mmHg) | 72 (7) | 75 (10) | 0.23 | 74 (7) | 74 (11) | 0.94 | 0.15 |
| SpO2 (%) | 98.1 (1.1) | 98.0 (1.1) | 0.87 | 83 (5) | 82 (8) | 0.51 | 0.35 |
| HR (b/min) | 67 (13) | 70 (11) | 0.47 | 72 (13) | 81 (15) | 0.05 | 0.12 |
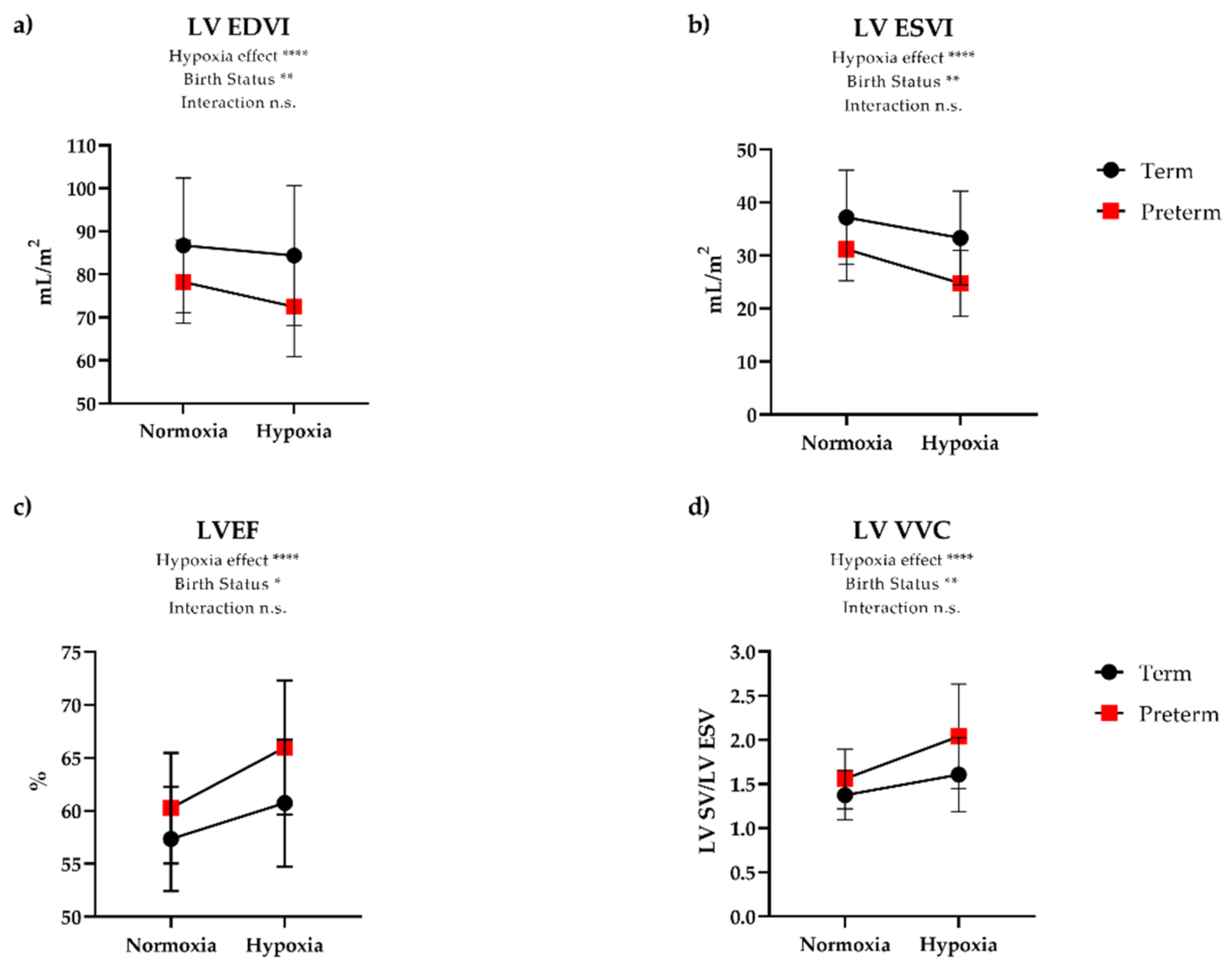
| LV EF (%) | 57 (5) | 60 (5) | 0.06 | 61 (6) | 66 (6) | <0.01 | 0.25 |
| LV CI (L/min/m2) | 3.29 (0.78) | 3.26 (0.59) | 0.89 | 3.60 (0.61) | 3.81 (0.76) | 0.34 | 0.30 |
| LV EDVI (mL/m2) | 86.7 (15.6) | 78.2 (9.6) | 0.02 | 84.4 (16.3) | 72.5 (11.6) | <0.01 | 0.06 |
| LV ESVI (mL/m2) | 37.2 (8.9) | 31.2 (6.0) | <0.01 | 33.3 (8.8) | 24.8 (6.2) | <0.01 | 0.11 |
| LV SVI (mL/m2) | 49.5 (8.9) | 47.0 (6.6) | 0.26 | 51.1 (10.5) | 47.7 (8.5) | 0.25 | 0.54 |
| LV VVC (SV/ESV) | 1.37 (0.28) | 1.56 (0.34) | 0.06 | 1.61 (0.42) | 2.04 (0.59) | 0.01 | 0.12 |
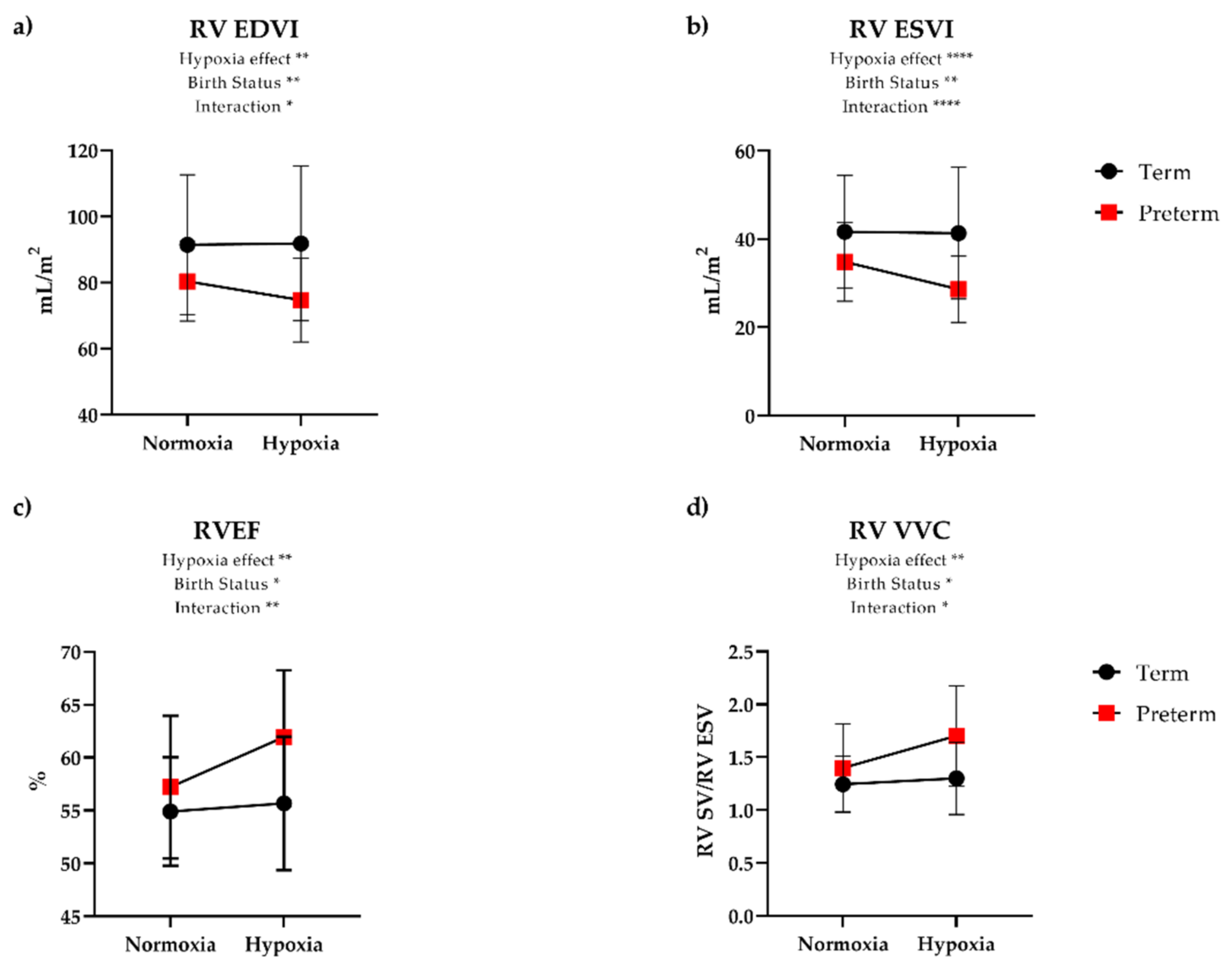
| RV EF (%) | 55 (5) | 57 (7) | 0.21 | 56 (6) | 62 (6) | <0.01 | 0.01 |
| RV CI (mL/min/m2) | 3.28 (0.70) | 3.15 (0.54) | 0.48 | 3.55 (0.60) | 3.67 (0.72) | 0.56 | 0.23 |
| RV EDVI (mL/m2) | 91.4 (21.2) | 80.4 (12.0) | 0.02 | 91.8 (23.4) | 74.7 (12.6) | <0.01 | 0.01 |
| RV ESVI (mL/m2) | 41.6 (12.7) | 34.8 (8.9) | 0.03 | 41.3 (14.9) | 28.6 (7.5) | <0.01 | <0.01 |
| RV SVI (mL/m2) | 49.8 (10.0) | 45.6 (6.5) | 0.08 | 50.5 (11.0) | 46.0 (8.1) | 0.12 | 0.84 |
| RV VVC (SV ESV) | 1.25 (0.27) | 1.40 (0.42) | 0.16 | 1.30 (0.34) | 1.70 (0.47) | <0.01 | 0.01 |
| Normoxia | Hypoxia | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Term (N = 18) | Preterm (N = 32) | (Normoxia) p | Term (N = 16) | Preterm (N = 27) | (Hypoxia) p | (Interaction) p |
| LV peak radial strain (%) | 23.4 (9.1) | 27.0 (9.3) | 0.19 | 25.4 (9.8) | 32.8 (10.6) | 0.03 | 0.26 |
| LV radial systolic SR (%/s) | 95.2 (41.3) | 107.8 (40.8) | 0.30 | 102.8 (43.4) | 142.4 (59.9) | 0.03 | 0.06 |
| LV radial diastolic SR (%/s) | −103.4 (52.5) | −118.2 (58.6) | 0.38 | −128.8 (71.1) | −157.4 (80.3) | 0.24 | 0.50 |
| LV peak circumferential strain (%) | −16.2 (2.7) | −17.9 (3.3) | 0.08 | −16.7 (3.4) | −19.6 (3.2) | <0.01 | 0.33 |
| LV circumferential systolic SR (%/s) | −77.2 (15.7) | −81.7 (16.9) | 0.36 | −83.5 (23.0) | −102.3 (24.1) | 0.02 | 0.04 |
| LV circumferential diastolic SR (%/s) | 68.0 (18.7) | 70.7 (19.2) | 0.63 | 72.2 (27.3) | 88.7 (31.3) | 0.09 | 0.06 |
| RV peak circumferential strain (%) | −8.1 (1.8) | −10.6 (2.6) | <0.01 | −8.1 (2.0) | −11.4 (2.2) | <0.01 | 0.42 |
| RV circumferential systolic SR (%/s) | −39.0 (13.2) | −47.9 (14.1) | 0.03 | −41.5 (12.2) | −57.3 (16.3) | <0.01 | 0.06 |
| RV circumferential diastolic SR (%/s) | 33.2 (12.8) | 40.6 (13.8) | 0.07 | 32.6 (11.6) | 40.0 (9.2) | 0.03 | 0.90 |
| Normoxia | Hypoxia | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Term (N = 18) | Preterm (N = 32) | (Normoxia) p | Term (N = 17) | Preterm (N = 28) | (Hypoxia) p | (Interaction) p |
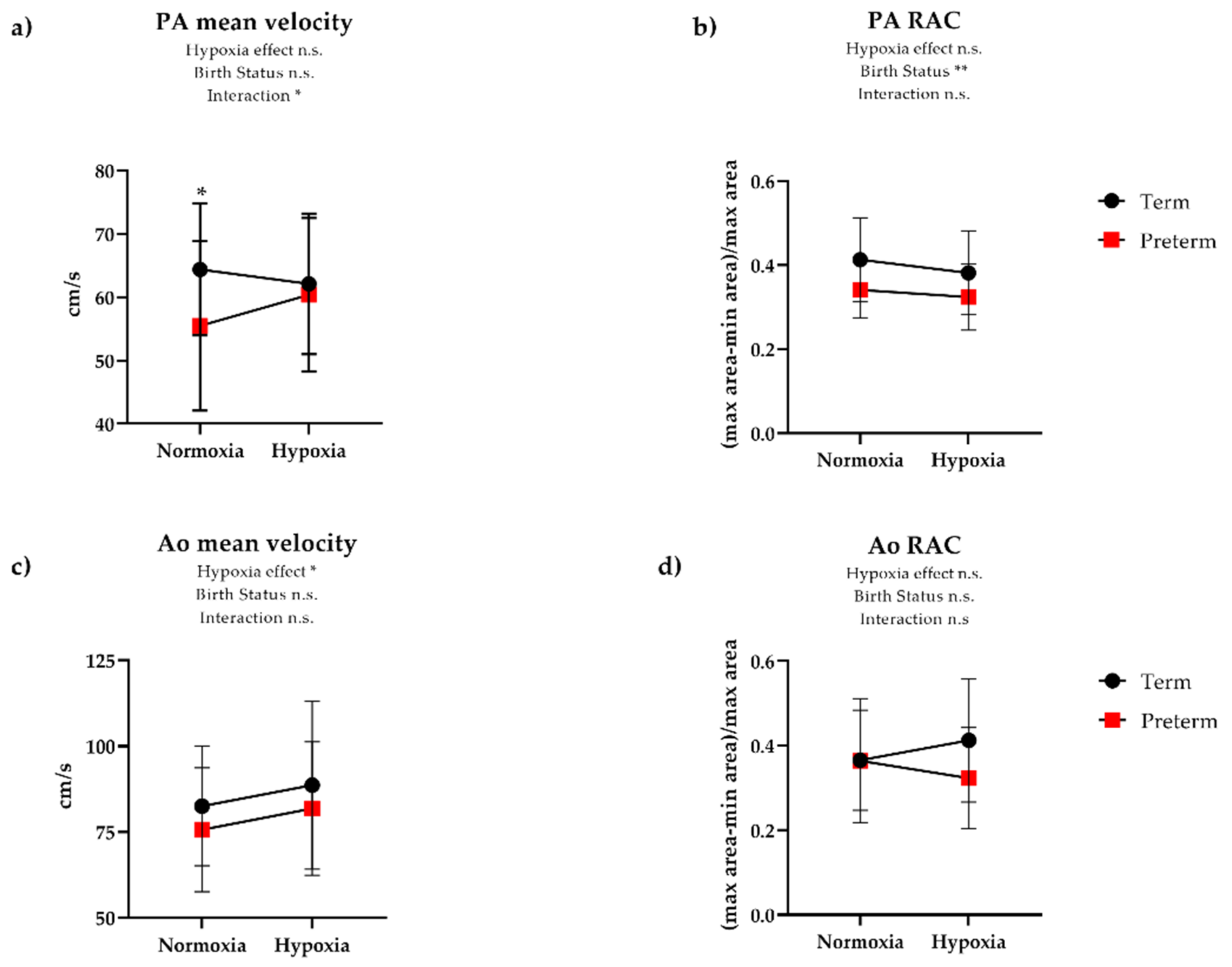
| Ao mean velocity (cm/s) | 83 (17) | 76 (18) | 0.21 | 89 (24) | 82 (19) | 0.32 | 0.72 |
| Ao diameter (mm) | 26.7 (2.6) | 26.6 (3.6) | 0.86 | 26.9 (3.1) | 26.1 (3.0) | 0.39 | 0.81 |
| Ao RAC | 0.37 (0.12) | 0.36 (0.15) | 0.98 | 0.41 (0.15) | 0.32 (0.12) | 0.03 | 0.10 |
| PA mean velocity (cm/s) | 64 (10) | 55 (13) | 0.02 | 62 (11) | 60 (12) | 0.64 | 0.03 |
| PA diameter (mm) | 27.0 (7.5) | 29.0 (3.0) | 0.19 | 29.2 (4.2) | 28.8 (3.1) | 0.69 | 0.14 |
| PA RAC | 0.41 (0.10) | 0.34 (0.07) | <0.01 | 0.38 (0.10) | 0.32 (0.08) | 0.04 | 0.74 |
| Ao:PA diameter | 0.94 (0.13) | 0.92 (0.10) | 0.45 | 0.93 (0.12) | 0.91 (0.12) | 0.63 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barton, G.P.; Corrado, P.A.; Francois, C.J.; Chesler, N.C.; Eldridge, M.W.; Wieben, O.; Goss, K.N. Exaggerated Cardiac Contractile Response to Hypoxia in Adults Born Preterm. J. Clin. Med. 2021, 10, 1166. https://doi.org/10.3390/jcm10061166
Barton GP, Corrado PA, Francois CJ, Chesler NC, Eldridge MW, Wieben O, Goss KN. Exaggerated Cardiac Contractile Response to Hypoxia in Adults Born Preterm. Journal of Clinical Medicine. 2021; 10(6):1166. https://doi.org/10.3390/jcm10061166
Chicago/Turabian StyleBarton, Gregory P., Philip A. Corrado, Christopher J. Francois, Naomi C. Chesler, Marlowe W. Eldridge, Oliver Wieben, and Kara N. Goss. 2021. "Exaggerated Cardiac Contractile Response to Hypoxia in Adults Born Preterm" Journal of Clinical Medicine 10, no. 6: 1166. https://doi.org/10.3390/jcm10061166
APA StyleBarton, G. P., Corrado, P. A., Francois, C. J., Chesler, N. C., Eldridge, M. W., Wieben, O., & Goss, K. N. (2021). Exaggerated Cardiac Contractile Response to Hypoxia in Adults Born Preterm. Journal of Clinical Medicine, 10(6), 1166. https://doi.org/10.3390/jcm10061166







