Aortic Valve Disease and Associated Complex CAD: The Interventional Approach
Abstract
1. Introduction
2. Anatomical CAD Assessment in AS
2.1. Coronary Angiogram
2.2. Coronary CT Angiography
3. Functional CAD Assessment in Aortic Stenosis
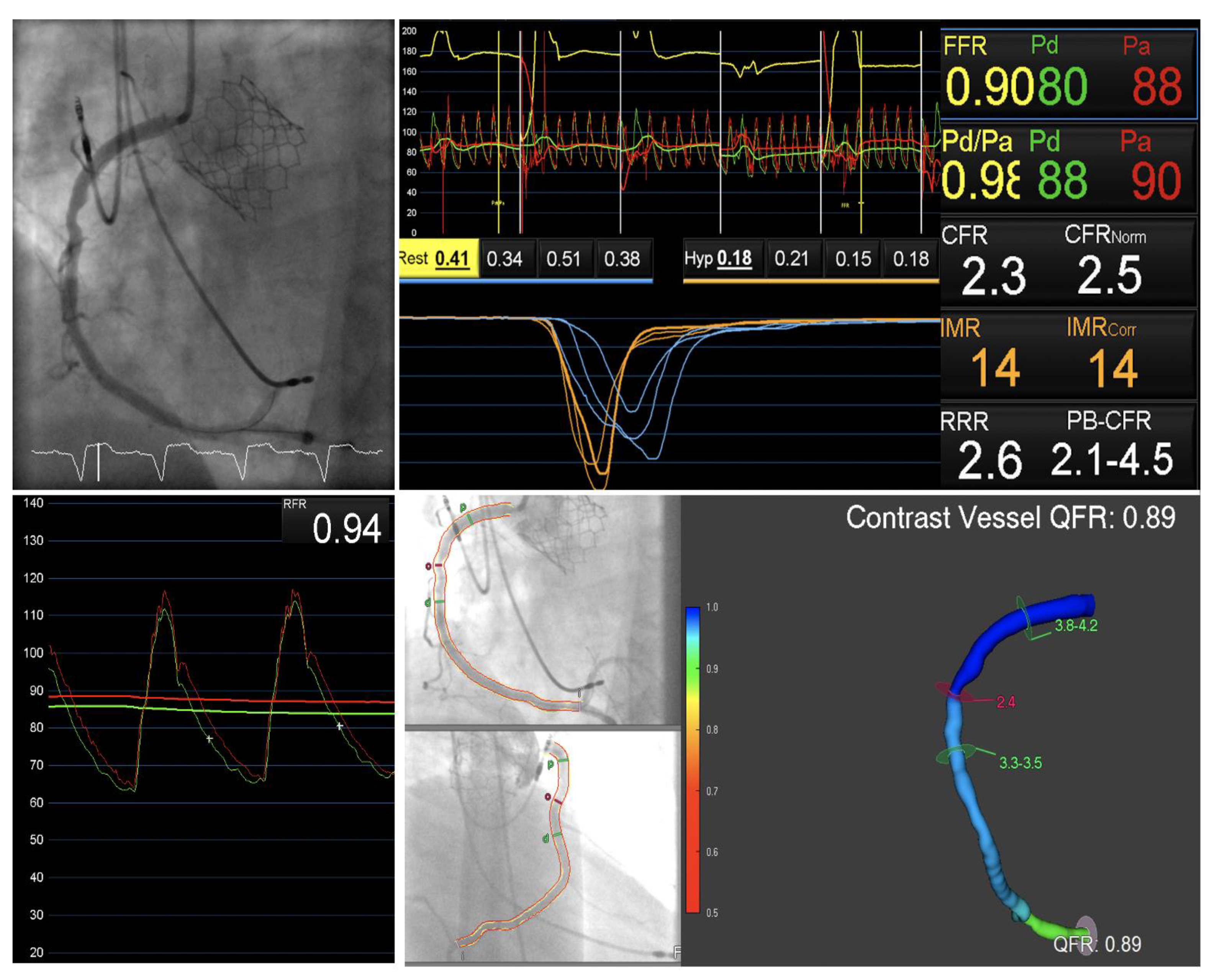
3.1. Invasive Physiological Assessment
3.2. Does Coronary Physiology Assessment Vary after TAVI?
3.3. Quantitative Flow Ratio in Presence of Aortic Stenosis
3.4. FFRCT in Presence of Aortic Stenosis
4. Impact of Myocardial Revascularization in Patients Undergoing TAVI
4.1. Prognostic Impact of CAD in Patients Undergoing TAVI
4.2. Benefits of PCI in Patients Undergoing TAVI
4.3. FFR Guided Revascularization
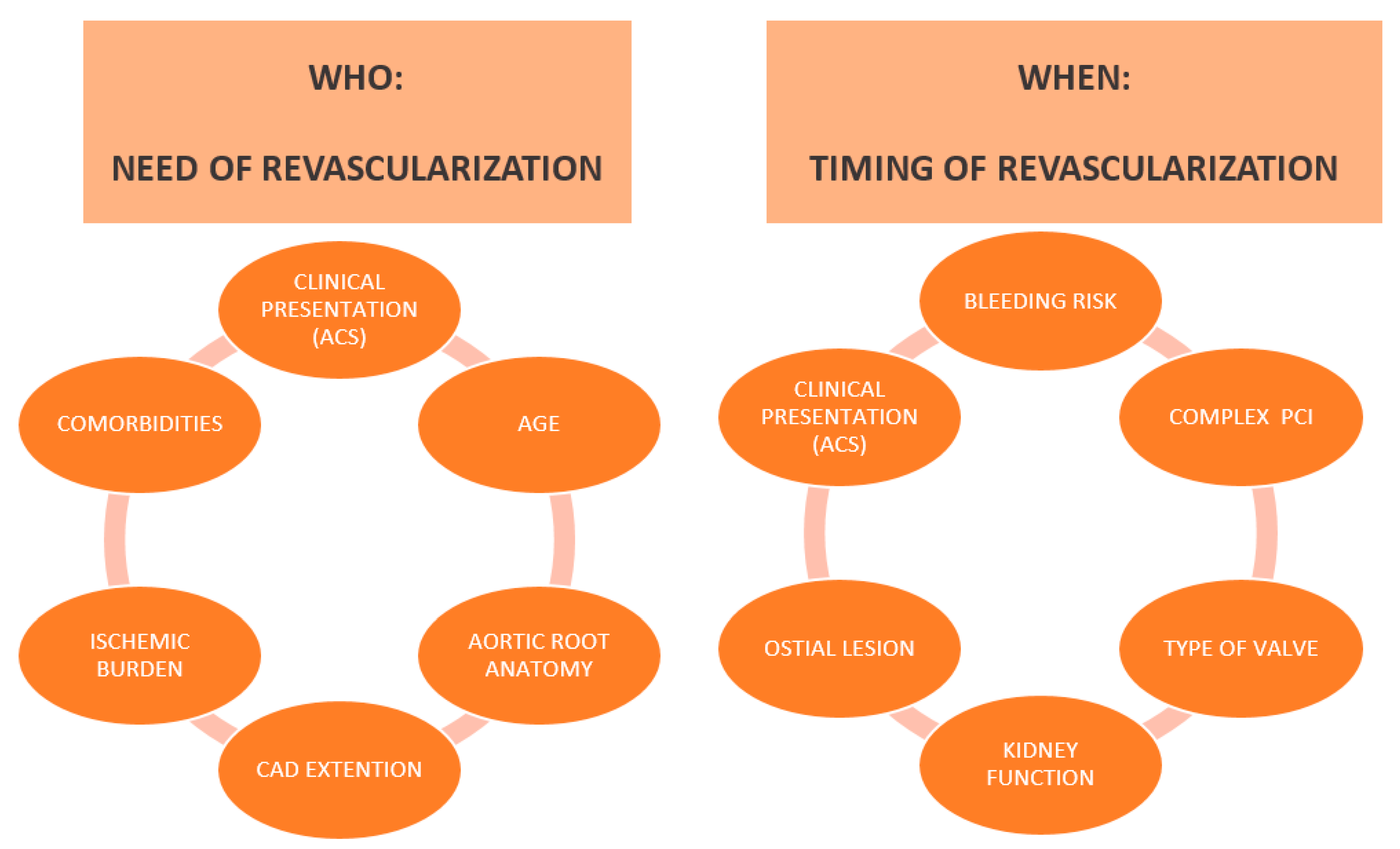
5. Timing of Percutaneous Coronary Intervention
5.1. PCI Upstream to TAVI
5.2. TAVI Upstream to PCI
5.3. PCI and Concomitant TAVI
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Dufresne, L.; Burr, H.; Ambikkumar, A.; Yasui, N.; Luk, K.; Ranatunga, D.K.; Whitmer, R.A.; Lathrop, M.; Engert, J.C.; et al. Association of LPA Variants With Aortic Stenosis: A Large-Scale Study Using Diagnostic and Procedural Codes From Electronic Health Records. JAMA Cardiol. 2018, 3, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Danson, E.; Hansen, P.; Sen, S.; Davies, J.; Meredith, I.; Bhindi, R. Assessment, treatment, and prognostic implications of CAD in patients undergoing TAVI. Nat. Rev. Cardiol. 2016, 13, 276–285. [Google Scholar] [CrossRef]
- Di Gioia, G.; Scarsini, R.; Strisciuglio, T.; De Biase, C.; Zivelonghi, C.; Franco, D.; De Bruyne, B.; Ribichini, F.; Barbato, E. Correlation between Angiographic and Physiologic Evaluation of Coronary Artery Narrowings in Patients With Aortic Valve Stenosis. Am. J. Cardiol. 2017, 120, 106–110. [Google Scholar] [CrossRef]
- van den Boogert, T.P.W.; Vendrik, J.; Claessen, B.E.P.M.; Baan, J.; Beijk, M.A.; Limpens, J.; Boekholdt, S.A.M.; Hoek, R.; Planken, R.N.; Henriques, J.P. CTCA for detection of significant coronary artery disease in routine TAVI work-up: A systematic review and meta-analysis. Neth. Heart J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Pontone, G.; Mushtaq, S.; Bartorelli, A.L.; Ballerini, G.; Bertella, E.; Segurini, C.; Conte, E.; Annoni, A.; Baggiano, A.; et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am. Heart J. 2014, 168, 332–339. [Google Scholar] [CrossRef]
- Hamdan, A.; Wellnhofer, E.; Konen, E.; Kelle, S.; Goitein, O.; Andrada, B.; Raanani, E.; Segev, A.; Barbash, I.; Klempfner, R.; et al. Coronary CT angiography for the detection of coronary artery stenosis in patients referred for transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2015, 9, 31–41. [Google Scholar] [CrossRef]
- Opolski, M.P.; Kim, W.K.; Liebetrau, C.; Walther, C.; Blumenstein, J.; Gaede, L.; Kempfert, J.; Van Linden, A.; Walther, T.; Hamm, C.W.; et al. Diagnostic accuracy of computed tomography angiography for the detection of coronary artery disease in patients referred for transcatheter aortic valve implantation. Clin. Res. Cardiol. 2015, 104, 471–480. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yamada, Y.; Hashimoto, M.; Okamura, T.; Yamada, M.; Yashima, F.; Hayashida, K.; Fukuda, K.; Jinzaki, M. CT imaging before transcatheter aortic valve implantation (TAVI) using variable helical pitch scanning and its diagnostic performance for coronary artery disease. Eur. Radiol. 2017, 27, 1963–1970. [Google Scholar] [CrossRef]
- Rossi, A.; De Cecco, C.N.; Kennon, S.R.O.; Zou, L.; Meinel, F.G.; Toscano, W.; Segreto, S.; Achenbach, S.; Hausleiter, J.; Schoepf, U.J.; et al. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2017, 11, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Strong, C.; Ferreira, A.; Teles, R.C.; Mendes, G.; Abecasis, J.; Cardoso, G.; Guerreiro, S.; Freitas, P.; Santos, A.C.; Saraiva, C.; et al. Diagnostic accuracy of computed tomography angiography for the exclusion of coronary artery disease in candidates for transcatheter aortic valve implantation. Sci. Rep. 2019, 9, 19942. [Google Scholar] [CrossRef] [PubMed]
- Chaikriangkrai, K.; Jhun, H.Y.; Shantha, G.P.S.; Abdulhak, A.B.; Tandon, R.; Alqasrawi, M.; Klappa, A.; Pancholy, S.; Deshmukh, A.; Bhama, J.; et al. Diagnostic Accuracy of Coronary Computed Tomography Before Aortic Valve Replacement: Systematic Review and Meta-Analysis. J. Thorac. Imaging 2018, 33, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Annoni, A.D.; Andreini, D.; Pontone, G.; Mancini, M.E.; Formenti, A.; Mushtaq, S.; Baggiano, A.; Conte, E.; Guglielmo, M.; Muscogiuri, G.; et al. CT angiography prior to TAVI procedure using third-generation scanner with wide volume coverage: Feasibility, renal safety and diagnostic accuracy for coronary tree. Br. J. Radiol. 2018, 91, 20180196. [Google Scholar] [CrossRef]
- Harris, B.S.; De Cecco, C.N.; Schoepf, U.J.; Steinberg, D.H.; Bayer, R.R.; Krazinski, A.W.; Dyer, K.T.; Sandhu, M.K.; Zile, M.R.; Meinel, F.G. Dual-source CT imaging to plan transcatheter aortic valve replacement: Accuracy for diagnosis of obstructive coronary artery disease. Radiology 2015, 275, 80–88. [Google Scholar] [CrossRef]
- Chieffo, A.; Giustino, G.; Spagnolo, P.; Panoulas, V.F.; Montorfano, M.; Latib, A.; Figini, F.; Agricola, E.; Gerli, C.; Franco, A.; et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2015, 8, e002025. [Google Scholar] [CrossRef]
- Joseph, J.; Kotronias, R.A.; Estrin-Serlui, T.; Cahill, T.J.; Kharbanda, R.K.; Newton, J.D.; Grebenik, C.; Dawkins, S.; Banning, A.P. Safety and operational efficiency of restructuring and redeploying a transcatheter aortic valve replacement service during the COVID-19 pandemic: The Oxford experience. Cardiovasc. Revasc. Med. 2020. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef]
- Vandeplas, A.; Willems, J.L.; Piessens, J.; De Geest, H. Frequency of angina pectoris and coronary artery disease in severe isolated valvular aortic stenosis. Am. J. Cardiol. 1988, 62, 117–120. [Google Scholar] [CrossRef]
- Julius, B.K.; Spillmann, M.; Vassalli, G.; Villari, B.; Eberli, F.R.; Hess, O.M. Angina pectoris in patients with aortic stenosis and normal coronary arteries. Mechanisms and pathophysiological concepts. Circulation 1997, 95, 892–898. [Google Scholar] [CrossRef]
- Marcus, M.L.; Doty, D.B.; Hiratzka, L.F.; Wright, C.B.; Eastham, C.L. Decreased coronary reserve: A mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N. Engl. J. Med. 1982, 307, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, S.M.; Park, S.J.; Jeong, D.S.; Woo, M.A.; Jung, S.H.; Lee, S.C.; Park, S.W.; Choe, Y.H.; Park, P.W.; et al. Coronary Microvascular Dysfunction as a Mechanism of Angina in Severe AS: Prospective Adenosine-Stress CMR Study. J. Am. Coll. Cardiol. 2016, 67, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Carabello, B.A. Why angina in aortic stenosis with normal coronary arteriograms? Circulation 2003, 107, 3121–3123. [Google Scholar] [CrossRef]
- Rajappan, K.; Rimoldi, O.E.; Dutka, D.P.; Ariff, B.; Pennell, D.J.; Sheridan, D.J.; Camici, P.G. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation 2002, 105, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Rajappan, K.; Rimoldi, O.E.; Camici, P.G.; Bellenger, N.G.; Pennell, D.J.; Sheridan, D.J. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation 2003, 107, 3170–3175. [Google Scholar] [CrossRef]
- Broyd, C.J.; Sen, S.; Mikhail, G.W.; Francis, D.P.; Mayet, J.; Davies, J.E. Myocardial ischemia in aortic stenosis: Insights from arterial pulse-wave dynamics after percutaneous aortic valve replacement. Trends Cardiovasc. Med. 2013, 23, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Sen, S.; Broyd, C.; Hadjiloizou, N.; Baksi, J.; Francis, D.P.; Foale, R.A.; Parker, K.H.; Hughes, A.D.; Chukwuemeka, A.; et al. Arterial pulse wave dynamics after percutaneous aortic valve replacement: Fall in coronary diastolic suction with increasing heart rate as a basis for angina symptoms in aortic stenosis. Circulation 2011, 124, 1565–1572. [Google Scholar] [CrossRef]
- Benenati, S.; De Maria, G.L.; Scarsini, R.; Porto, I.; Banning, A.P. Invasive “in the cath-lab” assessment of myocardial ischemia in patients with coronary artery disease: When does the gold standard not apply? Cardiovasc. Revasc. Med. 2018, 19, 362–372. [Google Scholar] [CrossRef]
- Eberli, F.R.; Ritter, M.; Schwitter, J.; Bortone, A.; Schneider, J.; Hess, O.M.; Krayenbuehl, H.P. Coronary reserve in patients with aortic valve disease before and after successful aortic valve replacement. Eur. Heart J. 1991, 12, 127–138. [Google Scholar] [CrossRef]
- Stoller, M.; Gloekler, S.; Zbinden, R.; Tueller, D.; Eberli, F.; Windecker, S.; Wenaweser, P.; Seiler, C. Left ventricular afterload reduction by transcatheter aortic valve implantation in severe aortic stenosis and its prompt effects on comprehensive coronary haemodynamics. EuroIntervention 2018, 14, 166–173. [Google Scholar] [CrossRef]
- Scarsini, R.; De Maria, G.L.; Di Gioia, G.; Kotronias, R.A.; Aurigemma, C.; Zimbardo, G.; Burzotta, F.; Leone, A.M.; Pesarini, G.; Trani, C.; et al. The Influence of Aortic Valve Obstruction on the Hyperemic Intracoronary Physiology: Difference Between Resting Pd/Pa and FFR in Aortic Stenosis. J. Cardiovasc. Transl. Res. 2019, 12, 539–550. [Google Scholar] [CrossRef]
- Ahmad, Y.; Götberg, M.; Cook, C.; Howard, J.P.; Malik, I.; Mikhail, G.; Frame, A.; Petraco, R.; Rajkumar, C.; Demir, O.; et al. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc. Interv. 2018, 11, 2019–2031. [Google Scholar] [CrossRef]
- Yamanaka, F.; Shishido, K.; Ochiai, T.; Moriyama, N.; Yamazaki, K.; Sugitani, A.; Tani, T.; Tobita, K.; Mizuno, S.; Tanaka, Y.; et al. Instantaneous Wave-Free Ratio for the Assessment of Intermediate Coronary Artery Stenosis in Patients With Severe Aortic Valve Stenosis: Comparison With Myocardial Perfusion Scintigraphy. JACC Cardiovasc. Interv. 2018, 11, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Pesarini, G.; Zivelonghi, C.; Piccoli, A.; Ferrero, V.; Lunardi, M.; Barbierato, M.; Caprioglio, F.; Vassanelli, C.; Ribichini, F. Coronary physiology in patients with severe aortic stenosis: Comparison between fractional flow reserve and instantaneous wave-free ratio. Int. J. Cardiol. 2017, 243, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Cantone, R.; Venturi, G.; De Maria, G.L.; Variola, A.; Braggio, P.; Lunardi, M.; Pesarini, G.; Ferdeghini, M.; Piccoli, A.; et al. Correlation between intracoronary physiology and myocardial perfusion imaging in patients with severe aortic stenosis. Int. J. Cardiol. 2019, 292, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Vendrik, J.; Eftekhari, A.; Howard, J.P.; Cook, C.; Rajkumar, C.; Malik, I.; Mikhail, G.; Ruparelia, N.; Hadjiloizou, N.; et al. Determining the Predominant Lesion in Patients With Severe Aortic Stenosis and Coronary Stenoses: A Multicenter Study Using Intracoronary Pressure and Flow. Circ. Cardiovasc. Interv. 2019, 12, e008263. [Google Scholar] [CrossRef]
- Scarsini, R.; Pesarini, G.; Lunardi, M.; Piccoli, A.; Zanetti, C.; Cantone, R.; Bellamoli, M.; Ferrero, V.; Gottin, L.; Faggian, G.; et al. Observations from a real-time, iFR-FFR “hybrid approach” in patients with severe aortic stenosis and coronary artery disease undergoing TAVI. Cardiovasc. Revasc. Med. 2018, 19, 355–359. [Google Scholar] [CrossRef]
- De Maria, G.L.; Garcia-Garcia, H.M.; Scarsini, R.; Hideo-Kajita, A.; Gonzalo López, N.; Leone, A.M.; Sarno, G.; Daemen, J.; Shlofmitz, E.; Jeremias, A.; et al. Novel Indices of Coronary Physiology: Do We Need Alternatives to Fractional Flow Reserve? Circ. Cardiovasc. Interv. 2020, 13, e008487. [Google Scholar] [CrossRef] [PubMed]
- Pesarini, G.; Scarsini, R.; Zivelonghi, C.; Piccoli, A.; Gambaro, A.; Gottin, L.; Rossi, A.; Ferrero, V.; Vassanelli, C.; Ribichini, F. Functional Assessment of Coronary Artery Disease in Patients Undergoing Transcatheter Aortic Valve Implantation: Influence of Pressure Overload on the Evaluation of Lesions Severity. Circ. Cardiovasc. Interv. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Kotronias, R.A.; Scarsini, R.; Rajasundaram, S.; De Maria, G.L.; Ciofani, J.L.; Ribichini, F.; Kharbanda, R.K.; Banning, A.P. Transcatheter Aortic Valve Replacement Influence on Coronary Hemodynamics: A Quantitative Meta-Analysis and Proposed Decision-Making Algorithm. J. Invasive Cardiol. 2020, 32, 37–40. [Google Scholar]
- Scarsini, R.; Pesarini, G.; Zivelonghi, C.; Piccoli, A.; Ferrero, V.; Lunardi, M.; Gottin, L.; Zanetti, C.; Faggian, G.; Ribichini, F. Physiologic Evaluation of Coronary Lesions Using Instantaneous Wave-free Ratio (iFR) in Patients with Severe Aortic Stenosis Undergoing Trans-catheter Aortic Valve Implantation. EuroIntervention 2017. [Google Scholar] [CrossRef]
- Scarsini, R.; Lunardi, M.; Venturi, G.; Pighi, M.; Tavella, D.; Pesarini, G.; Ribichini, F. Long-term variations of FFR and iFR after transcatheter aortic valve implantation. Int. J. Cardiol. 2020, 317, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Vendrik, J.; Ahmad, Y.; Eftekhari, A.; Howard, J.P.; Wijntjens, G.W.M.; Stegehuis, V.E.; Cook, C.; Terkelsen, C.J.; Christiansen, E.H.; Koch, K.T.; et al. Long-Term Effects of Transcatheter Aortic Valve Implantation on Coronary Hemodynamics in Patients With Concomitant Coronary Artery Disease and Severe Aortic Stenosis. J. Am. Heart Assoc. 2020, 9, e015133. [Google Scholar] [CrossRef] [PubMed]
- Camuglia, A.C.; Syed, J.; Garg, P.; Kiaii, B.; Chu, M.W.; Jones, P.M.; Bainbridge, D.; Teefy, P.J. Invasively assessed coronary flow dynamics improve following relief of aortic stenosis with transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2014, 63, 1808–1809. [Google Scholar] [CrossRef][Green Version]
- Tu, S.; Westra, J.; Yang, J.; von Birgelen, C.; Ferrara, A.; Pellicano, M.; Nef, H.; Tebaldi, M.; Murasato, Y.; Lansky, A.; et al. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve from Diagnostic Coronary Angiography: The International Multicenter FAVOR Pilot Study. JACC Cardiovasc. Interv. 2016, 9, 2024–2035. [Google Scholar] [CrossRef]
- Westra, J.; Andersen, B.K.; Campo, G.; Matsuo, H.; Koltowski, L.; Eftekhari, A.; Liu, T.; Di Serafino, L.; Di Girolamo, D.; Escaned, J.; et al. Diagnostic Performance of In-Procedure Angiography-Derived Quantitative Flow Reserve Compared to Pressure-Derived Fractional Flow Reserve: The FAVOR II Europe-Japan Study. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Xu, B.; Tu, S.; Qiao, S.; Qu, X.; Chen, Y.; Yang, J.; Guo, L.; Sun, Z.; Li, Z.; Tian, F.; et al. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J. Am. Coll. Cardiol. 2017, 70, 3077–3087. [Google Scholar] [CrossRef]
- Westra, J.; Tu, S.; Campo, G.; Qiao, S.; Matsuo, H.; Qu, X.; Koltowski, L.; Chang, Y.; Liu, T.; Yang, J.; et al. Diagnostic performance of quantitative flow ratio in prospectively enrolled patients: An individual patient-data meta-analysis. Catheter. Cardiovasc. Interv. 2019, 94, 693–701. [Google Scholar] [CrossRef]
- Mejía-Rentería, H.; Nombela-Franco, L.; Paradis, J.M.; Lunardi, M.; Lee, J.M.; Amat-Santos, I.J.; Veiga Fernandez, G.; Kalra, A.; Bansal, E.J.; de la Torre Hernandez, J.M.; et al. Angiography-based quantitative flow ratio versus fractional flow reserve in patients with coronary artery disease and severe aortic stenosis. EuroIntervention 2020, 16, e285–e292. [Google Scholar] [CrossRef]
- Michail, M.; Ihdayhid, A.R.; Comella, A.; Thakur, U.; Cameron, J.D.; McCormick, L.M.; Gooley, R.P.; Nicholls, S.J.; Mathur, A.; Hughes, A.D.; et al. Feasibility and Validity of Computed Tomography-Derived Fractional Flow Reserve in Patients With Severe Aortic Stenosis: The CAST-FFR Study. Circ. Cardiovasc. Interv. 2020. [Google Scholar] [CrossRef]
- Czer, L.S.; Gray, R.J.; Stewart, M.E.; De Robertis, M.; Chaux, A.; Matloff, J.M. Reduction in sudden late death by concomitant revascularization with aortic valve replacement. J. Thorac. Cardiovasc. Surg. 1988, 95, 390–401. [Google Scholar] [CrossRef]
- Iung, B.; Drissi, M.F.; Michel, P.L.; de Pamphilis, O.; Tsezana, R.; Cormier, B.; Vahanian, A.; Acar, J. Prognosis of valve replacement for aortic stenosis with or without coexisting coronary heart disease: A comparative study. J. Heart Valve Dis. 1993, 2, 430–439. [Google Scholar] [PubMed]
- Kotronias, R.A.; Bray, J.H.; Scarsini, R.; Rajasundaram, S.; Terentes-Printzios, D.; De Maria, G.L.; Kharbanda, R.K.; Mamas, M.A.; Bagur, R.; Banning, A.P. Transcatheter aortic valve replacement and percutaneous coronary intervention versus surgical aortic valve replacement and coronary artery bypass grafting in patients with severe aortic stenosis and concomitant coronary artery disease: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2020, 96, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Dewey, T.M.; Brown, D.L.; Herbert, M.A.; Culica, D.; Smith, C.R.; Leon, M.B.; Svensson, L.G.; Tuzcu, M.; Webb, J.G.; Cribier, A.; et al. Effect of concomitant coronary artery disease on procedural and late outcomes of transcatheter aortic valve implantation. Ann. Thorac. Surg. 2010, 89, 758–767; discussion 767. [Google Scholar] [CrossRef]
- Franzone, A.; Stortecky, S.; Räber, L.; Heg, D.; Yamaji, K.; Piccolo, R.; Asami, M.; Lanz, J.; Praz, F.; Koskinas, K.; et al. Effects of coronary artery disease in patients undergoing transcatheter aortic valve implantation: A study of age- and gender-matched cohorts. Int. J. Cardiol. 2017, 243, 150–155. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Stortecky, S.; Cao, D.; Rat-Wirtzler, J.; O’Sullivan, C.J.; Gloekler, S.; Buellesfeld, L.; Khattab, A.A.; Nietlispach, F.; Pilgrim, T.; et al. Coronary artery disease severity and aortic stenosis: Clinical outcomes according to SYNTAX score in patients undergoing transcatheter aortic valve implantation. Eur. Heart J. 2014, 35, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Regev, E.; Chen, S.; Assali, A.; Barbash, I.M.; Planer, D.; Vaknin-Assa, H.; Guetta, V.; Vukasinovic, V.; Orvin, K.; et al. The Prognostic Effects of Coronary Disease Severity and Completeness of Revascularization on Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 1428–1435. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Zahn, R.; Horack, M.; Gerckens, U.; Schuler, G.; Sievert, H.; Naber, C.; Voehringer, M.; Schäfer, U.; Senges, J.; et al. Transcatheter aortic valve implantation in patients with and without concomitant coronary artery disease: Comparison of characteristics and early outcome in the German multicenter TAVI registry. Clin. Res. Cardiol. 2012, 101, 973–981. [Google Scholar] [CrossRef]
- Snow, T.M.; Ludman, P.; Banya, W.; DeBelder, M.; MacCarthy, P.M.; Davies, S.W.; Di Mario, C.; Moat, N.E. Management of concomitant coronary artery disease in patients undergoing transcatheter aortic valve implantation: The United Kingdom TAVI Registry. Int. J. Cardiol. 2015, 199, 253–260. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Conrotto, F.; Giordana, F.; Moretti, C.; D’Amico, M.; Salizzoni, S.; Omedè, P.; La Torre, M.; Thomas, M.; Khawaja, Z.; et al. Mid-term prognostic value of coronary artery disease in patients undergoing transcatheter aortic valve implantation: A meta-analysis of adjusted observational results. Int. J. Cardiol. 2013, 168, 2528–2532. [Google Scholar] [CrossRef]
- Kotronias, R.A.; Kwok, C.S.; George, S.; Capodanno, D.; Ludman, P.F.; Townend, J.N.; Doshi, S.N.; Khogali, S.S.; Généreux, P.; Herrmann, H.C.; et al. Transcatheter Aortic Valve Implantation With or Without Percutaneous Coronary Artery Revascularization Strategy: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Redwood, S. The percutaneous Coronary intervention prior to transcatheter aortic valve Implantation: The activation trial. In Abstract, PCR Valves e-Course 2020. 2020. Available online: https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2020/PCR-Valves-e-Course-Hotline-I (accessed on 31 January 2021).
- Khawaja, M.Z.; Wang, D.; Pocock, S.; Redwood, S.R.; Thomas, M.R. The percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) trial: Study protocol for a randomized controlled trial. Trials 2014, 15, 300. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Al-Lamee, R.; Thompson, D.; Dehbi, H.M.; Sen, S.; Tang, K.; Davies, J.; Keeble, T.; Mielewczik, M.; Kaprielian, R.; Malik, I.S.; et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet 2018, 391, 31–40. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Baudhuin, T.; Melin, J.A.; Pijls, N.H.; Sys, S.U.; Bol, A.; Paulus, W.J.; Heyndrickx, G.R.; Wijns, W. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation 1994, 89, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Möbius-Winkler, S.; Mobius-Winckler, S.; Rioufol, G.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef]
- Davies, J.E.; Sen, S.; Dehbi, H.M.; Al-Lamee, R.; Petraco, R.; Nijjer, S.S.; Bhindi, R.; Lehman, S.J.; Walters, D.; Sapontis, J.; et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N. Engl. J. Med. 2017, 376, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Götberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.E.; Öhagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N. Engl. J. Med. 2017, 376, 1813–1823. [Google Scholar] [CrossRef]
- Di Gioia, G.; Pellicano, M.; Toth, G.G.; Casselman, F.; Adjedj, J.; Van Praet, F.; Ferrara, A.; Stockman, B.; Degrieck, I.; Bartunek, J.; et al. Fractional Flow Reserve-Guided Revascularization in Patients with Aortic Stenosis. Am. J. Cardiol. 2016, 117, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, M.; Scarsini, R.; Venturi, G.; Pesarini, G.; Pighi, M.; Gratta, A.; Gottin, L.; Barbierato, M.; Caprioglio, F.; Piccoli, A.; et al. Physiological Versus Angiographic Guidance for Myocardial Revascularization in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2019, 8, e012618. [Google Scholar] [CrossRef] [PubMed]
- Lopez Otero, D.; Avila-Carrillo, A.; Gonzalez Ferreiro, R.; Cid Menendez, A.; Iglesias Alvarez, D.; Alvarez Rodriguez, L.; Antunez Muinos, P.; Alvarez, B.C.; Sanmartin Pena, X.C.; Gomez Perez, F.; et al. Impact of Coronary Revascularization in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2019, 123, 948–955. [Google Scholar] [CrossRef]
- Huczek, Z.; Zbronski, K.; Grodecki, K.; Scislo, P.; Rymuza, B.; Kochman, J.; Dabrowski, M.; Witkowski, A.; Wojakowski, W.; Parma, R.; et al. Concomitant coronary artery disease and its management in patients referred to transcatheter aortic valve implantation: Insights from the POL-TAVI Registry. Catheter. Cardiovasc. Interv. 2018, 91, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.; Nombela-Franco, L.; Jimenez-Quevedo, P.; Biagioni, C.; Salinas, P.; Aldazabal, A.; Cerrato, E.; Gonzalo, N.; Del Trigo, M.; Nunez-Gil, I.; et al. The Value of the SYNTAX Score II in Predicting Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. Rev. Esp. Cardiol. (Engl. Ed.) 2018, 71, 628–637. [Google Scholar] [CrossRef]
- Shamekhi, J.; Stundl, A.; Weber, M.; Mellert, F.; Welz, A.; Grube, E.; Nickenig, G.; Werner, N.; Sinning, J.M. Impact of coronary artery disease in patients undergoing transfemoral transcatheter aortic valve implantation. Int. J. Cardiol. 2017, 245, 215–221. [Google Scholar] [CrossRef]
- Ahad, S.; Wachter, K.; Rustenbach, C.; Stan, A.; Hill, S.; Schaufele, T.; Ursulescu, A.; Franke, U.F.W.; Baumbach, H. Concomitant therapy: Off-pump coronary revascularization and transcatheter aortic valve implantation. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 12–17. [Google Scholar] [CrossRef]
- Paradis, J.M.; White, J.M.; Genereux, P.; Urena, M.; Doshi, D.; Nazif, T.; Hahn, R.; George, I.; Khalique, O.; Harjai, K.; et al. Impact of Coronary Artery Disease Severity Assessed With the SYNTAX Score on Outcomes Following Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Manoly, I.; Hasan, R.; Brazier, A.; Farooq, V.; Thompson, T.; Karunaratne, D.; Naylor, H.; Fraser, D. Feasibility of hybrid off pump artery bypass grafting and transaortic transcatheter aortic valve implantation: A case series. Catheter. Cardiovasc. Interv. 2017, 89, 1273–1279. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Verardi, R.; Visconti, M.; Conrotto, F.; Scacciatella, P.; Dziewierz, A.; Stefanini, G.G.; Paradis, J.M.; Omede, P.; Kodali, S.; et al. Independent impact of extent of coronary artery disease and percutaneous revascularisation on 30-day and one-year mortality after TAVI: A meta-analysis of adjusted observational results. EuroIntervention 2018, 14, e1169–e1177. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Zusman, O.; Codner, P.; Assali, A.; Kornowski, R. Impact of Coronary Artery Revascularization Completeness on Outcomes of Patients With Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis of Studies Using the Residual SYNTAX Score (Synergy Between PCI With Taxus and Cardiac Surgery). Circ. Cardiovasc. Interv. 2018, 11, e006000. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Thawabi, M.; Haik, N.; Haik, B.J.; Chen, C.; Cohen, M.; Russo, M. Impact of Coronary Artery Disease on Postoperative Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement (TAVR): Is Preoperative Coronary Revascularization Necessary? J. Invasive Cardiol. 2016, 28, E179–E184. [Google Scholar] [PubMed]
- Penkalla, A.; Kempfert, J.; Unbehaun, A.; Buz, S.; Drews, T.; Pasic, M.; Falk, V. Transcatheter Aortic Valve Implantation in Nonagenarians. Innovations 2016, 11, 390–395. [Google Scholar] [CrossRef]
- Kobayashi, J.; Shimahara, Y.; Fujita, T.; Kanzaki, H.; Amaki, M.; Hata, H.; Kume, Y.; Yamashita, K.; Okada, A. Early Results of Simultaneous Transaortic Transcatheter Aortic Valve Implantation and Total Arterial Off-Pump Coronary Artery Revascularization in High-Risk Patients. Circ. J. 2016, 80, 1946–1950. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Stortecky, S.; Franzone, A.; O’Sullivan, C.J.; Praz, F.; Zuk, K.; Raber, L.; Pilgrim, T.; Moschovitis, A.; Fiedler, G.M.; et al. Post-Procedural Troponin Elevation and Clinical Outcomes Following Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Witberg, G.; Lavi, I.; Harari, E.; Shohat, T.; Orvin, K.; Codner, P.; Vaknin-Assa, H.; Assali, A.; Kornowski, R. Effect of coronary artery disease severity and revascularization completeness on 2-year clinical outcomes in patients undergoing transcatether aortic valve replacement. Coron. Artery Dis. 2015, 26, 573–582. [Google Scholar] [CrossRef]
- O’Sullivan, C.J.; Englberger, L.; Hosek, N.; Heg, D.; Cao, D.; Stefanini, G.G.; Stortecky, S.; Gloekler, S.; Spitzer, E.; Tuller, D.; et al. Clinical outcomes and revascularization strategies in patients with low-flow, low-gradient severe aortic valve stenosis according to the assigned treatment modality. JACC Cardiovasc. Interv. 2015, 8, 704–717. [Google Scholar] [CrossRef]
- Penkalla, A.; Pasic, M.; Drews, T.; Buz, S.; Dreysse, S.; Kukucka, M.; Mladenow, A.; Hetzer, R.; Unbehaun, A. Transcatheter aortic valve implantation combined with elective coronary artery stenting: A simultaneous approachdagger. Eur. J. Cardiothorac. Surg. 2015, 47, 1083–1089. [Google Scholar] [CrossRef]
- Khawaja, M.Z.; Asrress, K.N.; Haran, H.; Arri, S.; Nadra, I.; Bolter, K.; Wilson, K.; Clack, L.; Hancock, J.; Young, C.P.; et al. The effect of coronary artery disease defined by quantitative coronary angiography and SYNTAX score upon outcome after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention 2015, 11, 450–455. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; van der Boon, R.M.; Faqiri, E.; Diletti, R.; Schultz, C.; van Geuns, R.J.; Serruys, P.W.; Kappetein, A.P.; van Domburg, R.T.; de Jaegere, P.P. Complete revascularization is not a prerequisite for success in current transcatheter aortic valve implantation practice. JACC Cardiovasc. Interv. 2013, 6, 867–875. [Google Scholar] [CrossRef]
- Saia, F.; Palmerini, T.; Compagnone, M.; Battistini, P.; Moretti, C.; Taglieri, N.; Marcelli, C.; Bruno, A.G.; Ghetti, G.; Corsini, A.; et al. Coronary artery disease and reasonably incomplete coronary revascularization in high-risk patients undergoing transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2020, 95, 19–27. [Google Scholar] [CrossRef]
- Kleczynski, P.; Dziewierz, A.; Bagienski, M.; Rzeszutko, L.; Sorysz, D.; Trebacz, J.; Sobczynski, R.; Tomala, M.; Gackowski, A.; Dudek, D. Impact of Coronary Artery Disease Burden on 12-Month Mortality of Patients After Transcatheter Aortic Valve Implantation. J. Interv. Cardiol. 2016, 29, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.S.; Ige, M.; Tuzcu, E.M.; Ellis, S.G.; Stewart, W.J.; Svensson, L.G.; Lytle, B.W.; Kapadia, S.R. Severe aortic stenosis and coronary artery disease—Implications for management in the transcatheter aortic valve replacement era: A comprehensive review. J. Am. Coll. Cardiol. 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Singh, V.; Mendirichaga, R.; Inglessis-Azuaje, I.; Palacios, I.F.; O’Neill, W.W. The Role of Impella for Hemodynamic Support in Patients with Aortic Stenosis. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Kotronias, R.A.; Scarsini, R.; Gibbs, T.; De Maria, G.L.; Rajasundaram, S.; Langrish, J.P.; Lucking, A.J.; Channon, K.M.; Kharbanda, R.K.; Banning, A.P. Safety of Rotational Atherectomy Using the Radial Access in Patients With Severe Aortic Stenosis. Am. J. Cardiol. 2019, 124, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Benenati, S.; Scarsini, R.; De Maria, G.L.; Banning, A.P. Rescue aortic balloon valvuloplasty during procedural cardiac arrest while treating critical left main stem stenosis: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- van Rosendael, P.J.; van der Kley, F.; Kamperidis, V.; Katsanos, S.; Al Amri, I.; Regeer, M.; Schalij, M.J.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Timing of staged percutaneous coronary intervention before transcatheter aortic valve implantation. Am. J. Cardiol. 2015, 115, 1726–1732. [Google Scholar] [CrossRef]
- Schneeberger, Y.; Schaefer, A.; Schofer, N.; Silaschi, M.; Deuschl, F.; Blankenberg, S.; Reichenspurner, H.; Treede, H.; Schäfer, U.; Charitos, E.I.; et al. Transcatheter aortic valve implantation utilizing a non-occlusive balloon for predilatation. Int. J. Cardiol. 2019, 275, 65–69. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Matsumoto, Y.; Suzuki, H.; Takanami, K.; Kikuchi, Y.; Takahashi, J.; Miyata, S.; Tomita, N.; Kumagai, K.; Taki, Y.; et al. Transcatheter aortic valve implantation and cognitive function in elderly patients with severe aortic stenosis. EuroIntervention 2020, 15, e1580–e1587. [Google Scholar] [CrossRef]
- Muratori, M.; Fusini, L.; Tamborini, G.; Gripari, P.; Delgado, V.; Marsan, N.A.; Ghulam Ali, S.; Barbier, P.; Bartorelli, A.L.; Alamanni, F.; et al. Sustained favourable haemodynamics 1 year after TAVI: Improvement in NYHA functional class related to improvement of left ventricular diastolic function. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1269–1278. [Google Scholar] [CrossRef]
- De Maria, G.L.; Scarsini, R.; Banning, A.P. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc. Interv. 2019, 12, 1465–1478. [Google Scholar] [CrossRef]
- Lunardi, M.; Pighi, M.; Venturi, G.; Del Sole, P.A.; Pesarini, G.; Mainardi, A.; Scarsini, R.; Ferrero, V.; Gottin, L.; Ribichini, F. Short-and-Long-Term Outcomes after Coronary Rotational Atherectomy in Patients Treated with Trans-Catheter Aortic Valve Implantation. J. Clin. Med. 2020, 10, 112. [Google Scholar] [CrossRef]
- Venturi, G.; Pighi, M.; Pesarini, G.; Ferrero, V.; Lunardi, M.; Castaldi, G.; Setti, M.; Benini, A.; Scarsini, R.; Ribichini, F.L. Contrast-Induced Acute Kidney Injury in Patients Undergoing TAVI Compared With Coronary Interventions. J. Am. Heart Assoc. 2020, 9, e017194. [Google Scholar] [CrossRef]
- Barbanti, M.; Costa, G.; Picci, A.; Criscione, E.; Reddavid, C.; Valvo, R.; Todaro, D.; Deste, W.; Condorelli, A.; Scalia, M.; et al. Coronary Cannulation After Transcatheter Aortic Valve Replacement: The RE-ACCESS Study. JACC Cardiovasc. Interv. 2020, 13, 2542–2555. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.L.; Zaid, S.; Fuchs, A.; Yamabe, T.; Yazdchi, F.; Gupta, E.; Ahmad, H.; Kofoed, K.F.; Goldberg, J.B.; Undemir, C.; et al. Alignment of Transcatheter Aortic-Valve Neo-Commissures (ALIGN TAVR): Impact on Final Valve Orientation and Coronary Artery Overlap. JACC Cardiovasc. Interv. 2020, 13, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Yoon, S.H.; Flint, N.; Sharma, R.; Chakravarty, T.; Kaewkes, D.; Patel, V.; Nakamura, M.; Cheng, W.; Makkar, R. Timing and Outcomes of Percutaneous Coronary Intervention in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 125, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Wenaweser, P.; Pilgrim, T.; Guerios, E.; Stortecky, S.; Huber, C.; Khattab, A.A.; Kadner, A.; Buellesfeld, L.; Gloekler, S.; Meier, B.; et al. Impact of coronary artery disease and percutaneous coronary intervention on outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention 2011, 7, 541–548. [Google Scholar] [CrossRef]
- Barbanti, M.; Todaro, D.; Costa, G.; Pilato, G.; Picci, A.; Gulino, S.; Capranzano, P.; La Spina, K.; Di Simone, E.; D’Arrigo, P.; et al. Optimized Screening of Coronary Artery Disease With Invasive Coronary Angiography and Ad Hoc Percutaneous Coronary Intervention During Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, F.Y.; Huang, B.T.; Xiong, T.Y.; Pu, X.B.; Chen, S.J.; Chen, M.; Feng, Y. The safety of concomitant transcatheter aortic valve replacement and percutaneous coronary intervention: A systematic review and meta-analysis. Medicine 2017, 96, e8919. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Patients (n) | Definition Significant Stenosis | Vessels Evaluated | Sn (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Strong et al. [13] | Retrospective cohort | 200 | >50% stenosis | Native | 100 | 42 | 48 | 100 |
| Chaikriangkrai et al. [14] | Systematic review and meta-analysis | 1498 | >50% stenosis | All | 96 | 74 | – | – |
| Andreini et al. [8] | Prospective cohort | 325 | >50% stenosis | Native (no stents) | 91 | 99 | 8 | 100 |
| Native (stented) | 94 | 87 | 67 | 98 | ||||
| CABG | 90 | 91 | 81 | 95 | ||||
| Annoni et al. [15] | Prospective cohort | 115 | – | All | 97 | 85 | 62 | 99 |
| Rossi et al. [12] | Prospective Cohort | 140 | >50% stenosis | All | 91 | 55 | 59 | 90 |
| >70% stenosis | All | 78 | 74 | 37 | 95 | |||
| Matsumoto et al. [11] | Prospective cohort | 60 | >50% stenosis | All | 92 | 58 | 41 | 91 |
| Hamdan et al. [9] | Prospective cohort | 115 | >50% stenosis | Native | 93 | 73 | 62 | 96 |
| CABG | 100 | 75 | 95 | 100 | ||||
| Opolski et al. [10] | Prospective cohort | 475 | >50% stenosis | All | 98 | 37 | 67 | 94 |
| Harris et al. [16] | Retrospective cohort | 100 | >50% stenosis | All | 99 | 56 | 86 | 94 |
| Chieffo et al. [17] | Retrospective cohort | 491 | – | All | – | – | 48 | – |
| Study | Study Design | Patients (n) | Reference Standard | Optimal FFR by ROC | Optimal iFR by ROC | Cut-Offs Tested | Sn (%) | Sp (%) | NPV (%) | PPV (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Scarsini et al. [36] | Prospective cohort | 28 | Adenosine SPECT | 0.78 | – | FFR < 0.78 | 87 | 88 | 92 | 81 |
| iFR < 0.82 | 80 | 69 | 86 | 60 | ||||||
| Yamanaka et al. [34] | Prospective cohort | 95 | Adenosine SPECT | 0.82 | 0.82 | – | – | – | – | – |
| Scarsini et al. [35] | Prospective cohort | 252 (85 AS) | FFR ≤ 0.80 | – | 0.83 | iFR < 0.83 | 72 | 84 | 96 | 78 |
| Study | Design | Patients (n) | Follow-Up (Months) | Stratification | Outcome(s) | |
|---|---|---|---|---|---|---|
| Lopez Otero et al. [77] | Retrospective cohort | 349 (187 CAD) | 35.2 (mean) | No CAD | MACE (p = 0.91) | 39% |
| rSS = 0 | 45% | |||||
| 0 < rSS < 8 | 40% | |||||
| rSS ≥ 8 | 47% | |||||
| Huczek et al. [78] | Registry | 896 (462 CAD) | 1 | No CAD | Mortality (p = 0.14) | 5.1% |
| bSS < 22 | 8.9% | |||||
| bSS ≥ 22 | 6.9% | |||||
| Witberg et al. [58] | Retrospective cohort | 1270 (453 CAD) | 22.8 (median) | No CAD | Mortality (p < 0.001) | 21.9% |
| bSS < 22 | 26.1% | |||||
| bSS ≥ 22 | 51.9% | |||||
| No CAD | Mortality (p < 0.001) | 21.9% | ||||
| rSS < 8 | 23.2% | |||||
| rSS > 8 | 45.1% | |||||
| Ryan et al. [79] | Prospective cohort | 402 (193 CAD) | 12 | SS-II < 37.4 | MACE (p = 0.001) | 13.4% |
| 37.4 ≤ SS-II ≤ 44.0 | 14.9% | |||||
| SS-II > 44.0 | 31.3% | |||||
| Shamekhi et al. [80] | Prospective cohort | 666 (437 CAD) | 24.7 (mean) | No CAD | Mortality (p = 0.001) | 26.2% |
| bSS < 24 | 34.8% | |||||
| bSS ≥ 24 | 46.8% | |||||
| No CAD | Mortality (p = 0.01) | 25.9% | ||||
| rSS ≤ 3 | 31.4% | |||||
| rSS >3 | 41.5% | |||||
| 0 < SS-II < 37 | Mortality (p < 0.001) | 27% | ||||
| 37 ≤ SS-II < 47 | 23.5% | |||||
| 47 ≤ SS-II < 55 | 33.5% | |||||
| SS-II ≥ 55 | 49.2% | |||||
| Ahad et al. [81] | Retrospective cohort | 70 (all CAD) | 24 | Mean bSS = 29.0 | Mortality | 31.6% |
| Paradis et al. [82] | Retrospective cohort | 377 (295 CAD) | 12 | No CAD | MACE (p = 0.61) | 26.8% |
| bSS ≤ 23 | 23.3% | |||||
| 23 ≤ bSS ≤ 32 | 16.7% | |||||
| bSS ≥ 33 | 22.0% | |||||
| No CAD | MACE (p = 0.01) | 26.8% | ||||
| rSS < 8 | 0% | |||||
| rSS ≥ 8 | 10.8% | |||||
| Manoly et al. [83] | Case series | 4 (all CAD) | 12 (median) | Mean bSS = 20.6 | Mortality | 25% |
| D’Ascenzo et al. [84] | Meta-analysis | 8334 (3994 CAD) | 12 | bSS >22 versus bSS <22 | Mortality (p = 0.001) | OR 1.71 (1.24–2.36) |
| PCI with rSS <8 versus PCI not performed | Mortality (p = 0.04) | OR 0.34 (0.12–0.93) | ||||
| Witberg et al. [85] | Meta-analysis | 3107 (1645 CAD) | 8.4–36 | High rSS versus no CAD | Mortality (p < 0.01) | OR 1.85 (1.42–2.40) |
| High rSS versus low rSS | Mortality (p < 0.001) | OR 1.69 (1.26–2.28) | ||||
| Low rSS versus no CAD | Mortality (p = 0.33) | OR 1.11 (0.89–1.39) | ||||
| Chauhan et al. [86] | Retrospective cohort | 238 (99 CAD) | 14.9 (mean) | bSS ≤ 2 | Mortality, MACE, revascularization(p = 0.27) | 21.3% |
| 3 ≤ bSS ≤ 10 | 16.3% | |||||
| bSS ≥ 11 | 21.2% | |||||
| Penkalla et al. [87] | Prospective cohort | 40 (28 CAD) | 12 and 60 | Mean bSS = 7.6 | Mortality | 41.4 and 69.6% |
| Kobayashi et al. [88] | Case series | 12 (all CAD) | Intra-hospital | Mean bSS = 22.4 | Mortality, MACE | 0% |
| Koskinas et al. [89] | Retrospective cohort | 577 (367 CAD) | 24 | No CAD and cTnT <15 × ULN | Mortality | 11.6% |
| bSS > 22 and cTnT >15 × ULN | 41.1% | |||||
| Witberg et al. [90] | Registry | 287 (49 CAD) | 24 | No CAD a | MACE (p = 0.19 a–b and p = 0.002 a–c) | 16.1% |
| bSS ≤ 22 b | 24.4% | |||||
| bSS > 22 c | 75% | |||||
| No CAD a | MACE (p = 0.606 a–b and p = 0.001 a–c) | 16.1% | ||||
| cSS ≤ 63 b | 18.7% | |||||
| cSS > 63 c | 41.2% | |||||
| No CAD a | MACE (p = 0.196 a–b and p < 0.001 a–c) | 16.1% | ||||
| 0 < rSS < 9 b | 8.6% | |||||
| rSS ≥ 9 c | 78.6% | |||||
| O’Sullivan et al. [91] | Registry | 108 (80 CAD) | 12 | Mean bSS = 16.1 | Mortality | 25.1% |
| Penkalla et al. [92] | Cohort | 593 (308 CAD) | 1 | No CAD | Mortality (p = 0.61) | 5.3% |
| CAD, no PCI, mean bSS = 5.7 | 3.9% | |||||
| CAD, PCI, mean bSS = 8.0 | 2.6% | |||||
| Khawaja et al. [93] | Retrospective cohort | 271 (93 CAD) | 1 and 12 | 0 < bSS ≤ 22 | Mortality (p = 0.007) | 5.2 and 23.3% |
| 22 < bSS ≤ 32 | 11.1 and 22.2% | |||||
| bSS > 33 | 14.3 and 57.1% | |||||
| Stefanini et al. [57] | Registry | 445 (287 CAD) | 12 | No CAD | MACE (p = 0.016) | 12.5% |
| 0 ≤ bSS ≤ 22 | 16.1% | |||||
| bSS >22 | 29.6% | |||||
| No CAD | MACE (p = 0.043) | 12.5% | ||||
| 0 ≤ rSS ≤ 14 | 16.5% | |||||
| rSS > 14 | 26.3% | |||||
| Van Mieghem et al. [94] | Prospective cohort | 263 (124 CAD) | 16 (median) | rSS = 0 | Mortality (p = 0.85) | 20.1% |
| rSS > 0 | 22.6 % | |||||
| Saia et al. [95] | Prospective cohort | 540 (291 CAD) | 58 (median) | CR IR | Mortality (p = 0.45) | 2.9% 4.6% |
| Kleczynski et al. [96] | Cohort | 101 | 12 | CR IR | Mortality (p = < 0.001) | 7.1% 75% |
| Advantages | Disadvantages | Preferred Clinical Scenario | |
|---|---|---|---|
| PCI before TAVI |
|
|
|
| Concomitant PCI and TAVI |
|
|
|
| PCI post TAVI |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, F.; Scarsini, R.; Kotronias, R.A.; Terentes-Printzios, D.; Burrage, M.K.; Bray, J.J.H.; Ciofani, J.L.; Venturi, G.; Pighi, M.; De Maria, G.L.; et al. Aortic Valve Disease and Associated Complex CAD: The Interventional Approach. J. Clin. Med. 2021, 10, 946. https://doi.org/10.3390/jcm10050946
Marin F, Scarsini R, Kotronias RA, Terentes-Printzios D, Burrage MK, Bray JJH, Ciofani JL, Venturi G, Pighi M, De Maria GL, et al. Aortic Valve Disease and Associated Complex CAD: The Interventional Approach. Journal of Clinical Medicine. 2021; 10(5):946. https://doi.org/10.3390/jcm10050946
Chicago/Turabian StyleMarin, Federico, Roberto Scarsini, Rafail A. Kotronias, Dimitrios Terentes-Printzios, Matthew K. Burrage, Jonathan J. H. Bray, Jonathan L. Ciofani, Gabriele Venturi, Michele Pighi, Giovanni L. De Maria, and et al. 2021. "Aortic Valve Disease and Associated Complex CAD: The Interventional Approach" Journal of Clinical Medicine 10, no. 5: 946. https://doi.org/10.3390/jcm10050946
APA StyleMarin, F., Scarsini, R., Kotronias, R. A., Terentes-Printzios, D., Burrage, M. K., Bray, J. J. H., Ciofani, J. L., Venturi, G., Pighi, M., De Maria, G. L., & Banning, A. P. (2021). Aortic Valve Disease and Associated Complex CAD: The Interventional Approach. Journal of Clinical Medicine, 10(5), 946. https://doi.org/10.3390/jcm10050946






