Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia
Abstract
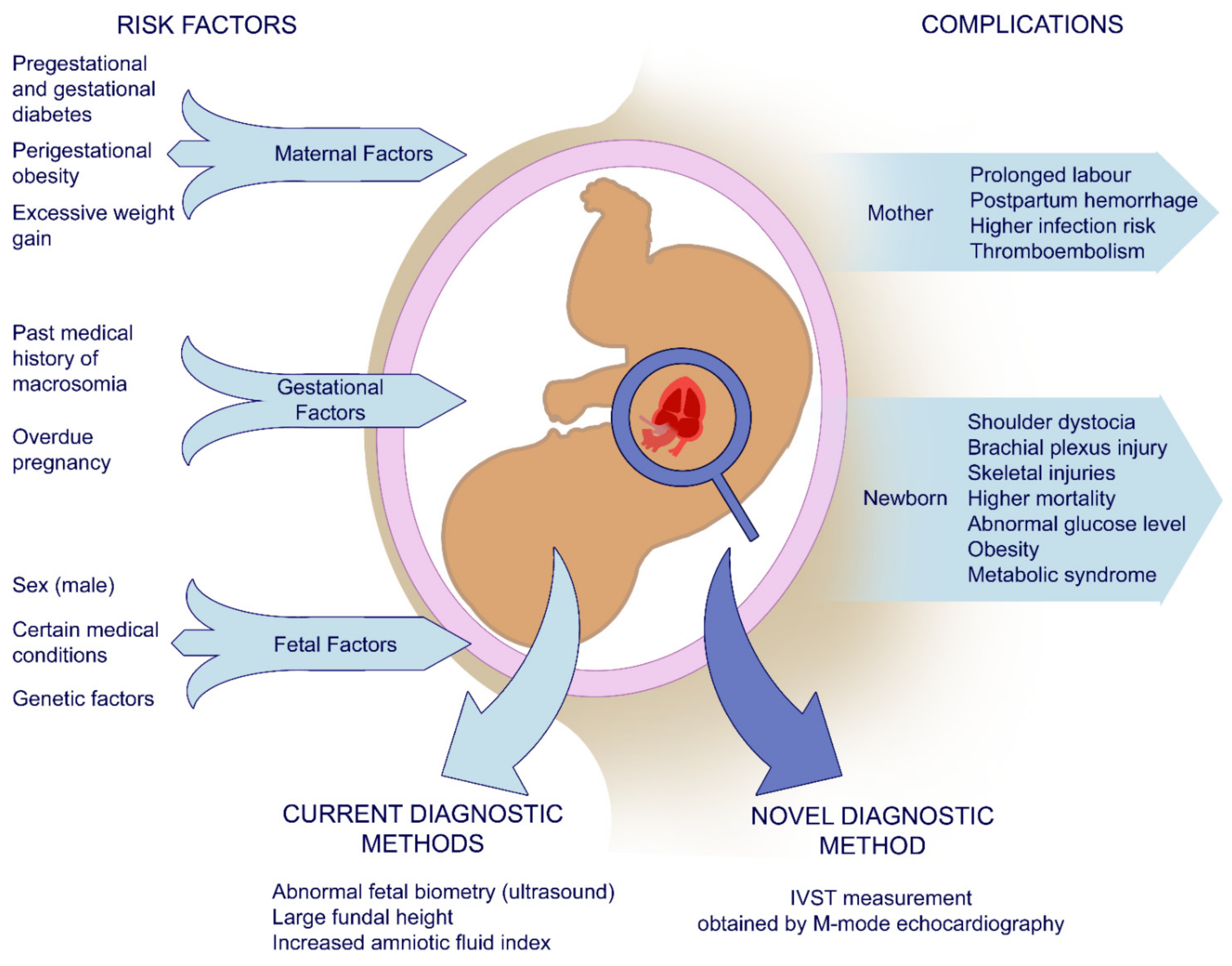
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurement Tools
2.3. Data Collection
2.4. Statistical Analysis
3. Results
Macrosomia Predictors
4. Discussion
5. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACOG Macrosomia. Obstet. Gynecol. 2020, 135, e18–e35. [CrossRef]
- Capobianco, G.; Gulotta, A.; Tupponi, G.; Dessole, F.; Pola, M.; Virdis, G.; Petrillo, M.; Mais, V.; Olzai, G.; Antonucci, R.; et al. Materno-Fetal and Neonatal Complications of Diabetes in Pregnancy: A Retrospective Study. J. Clin. Med. 2020, 9, 2707. [Google Scholar] [CrossRef]
- Gujski, M.; Szukiewicz, D.; Chołuj, M.; Sawicki, W.; Bojar, I. Fetal and Placental Weight in Pre-Gestational Maternal Obesity (PGMO) vs. Excessive Gestational Weight Gain (EGWG)—A Preliminary Approach to the Perinatal Outcomes in Diet-Controlled Gestational Diabetes Mellitus. J. Clin. Med. 2020, 9, 3530. [Google Scholar] [CrossRef]
- Keet, C.A.; Savage, J.H.; Seopaul, S.; Peng, R.D.; Wood, R.A.; Matsui, E.C. Temporal trends and racial/ethnic disparity in self-reported pediatric food allergy in the United States. Ann. Allergy Asthma Immunol. 2014, 112, 222–229.e3. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Laughon, S.K.; Kiely, M.; Brite, J.; Chen, Z.; Zhang, C. Gestational diabetes, pre-pregnancy obesity and pregnancy weight gain in relation to excess fetal growth: Variations by race/ethnicity. Diabetologia 2013, 56, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Respondek-Liberska, M.; Węgrzynowski, J.; Oszukowski, P.; Gulczyńska, E.; Nykiel, E.; Jakubowski, L.; Grzesiak, M.; Czichos, E.; Romanowicz, H. Fetal macrosomia, polyhydramnios and cardiac anomalies may be helpful to predict poor outcome in neonate–Case report of a possible fetal RASopathy with sonographic and neonatal findings and genetic evaluation. Prenat. Cardiol. 2017, 7, 73–82. [Google Scholar] [CrossRef][Green Version]
- Nkwabong, E.; Nzalli Tangho, G.R. Risk Factors for Macrosomia. J. Obstet. Gynecol. India 2015, 65, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Pates, J.A.; McIntire, D.D.; Casey, B.M.; Leveno, K.J. Predicting Macrosomia. J. Ultrasound Med. 2008, 27, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kayem, G.; Grangé, G.; Bréart, G.; Goffinet, F. Comparison of fundal height measurement and sonographically measured fetal abdominal circumference in the prediction of high and low birth weight at term. Ultrasound Obstet. Gynecol. 2009, 34, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, J.; Krekora, M.; Podciechowski, L.; Respondek, M. Prognostic values of biophysical methods in the intrauetrine monitoring of fetal well-being in pregnant women with insulin-dependent diabetes. Arch. Perinat. Med. 1996, 1, 37–44. [Google Scholar]
- Kafle, P.; Ansari, M.; Khanal, U. Fetal Cardiac Interventricular Septal Thickness at 28–37 Weeks of Gestation in Nepalese Population. Nepal. J. Radiol. 2013, 2, 36–42. [Google Scholar] [CrossRef]
- Gabryelska, A.; Szmyd, B.; Panek, M.; Szemraj, J.; Kuna, P.; Białasiewicz, P. Serum hypoxia–inducible factor–1α protein level as a diagnostic marker of obstructive sleep apnea. Polish Arch. Intern. Med. 2020, 130. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K. Births: Final Data for 2018. Natl. Vital. Stat. Rep. 2019, 68, 1–47. [Google Scholar] [PubMed]
- Jiang, H.; Wen, Y.; Hu, L.; Miao, T.; Zhang, M.; Dong, J. Serum MicroRNAs as Diagnostic Biomarkers for Macrosomia. Reprod. Sci. 2015, 22, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Nahavandi, S.; Seah, J.; Shub, A.; Houlihan, C.; Ekinci, E.I. Biomarkers for macrosomia prediction in pregnancies affected by diabetes. Front. Endocrinol. 2018, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Sibiak, R.; Jankowski, M.; Gutaj, P.; Mozdziak, P.; Kempisty, B.; Wender-Ożegowska, E. Placental Lactogen as a Marker of Maternal Obesity, Diabetes, and Fetal Growth Abnormalities: Current Knowledge and Clinical Perspectives. J. Clin. Med. 2020, 9, 1142. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhang, H.; Shi, F.; Guo, J.; Wang, S.; Zhang, B. Ensemble Learning to Improve the Prediction of Fetal Macrosomia and Large-for-Gestational Age. J. Clin. Med. 2020, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Malin, G.; Bugg, G.; Takwoingi, Y.; Thornton, J.; Jones, N. Antenatal magnetic resonance imaging versus ultrasound for predicting neonatal macrosomia: A systematic review and meta-analysis. BJOG An Int. J. Obstet. Gynaecol. 2016, 123, 77–88. [Google Scholar] [CrossRef]
- Karuga, F.F.; Szmyd, B.; Respondek-Liberska, M. Fetal congenital heart disease and fetal position-are they related? Prenat. Cardiol. 2020. [Google Scholar] [CrossRef]
- Szmyd, B.; Karuga, F.; Gach, A.; Moszura, T.; Kopala, M.; Respondek-Liberska, M. Complex cardiovascular defects in a male infant with Williams syndrome juxtaposed with results of a preliminary survey illustrating other patients’ ouctomes. Kardiol. Pol. 2021. [Google Scholar] [CrossRef] [PubMed]


| Proposed Tests | IVST ≥ 4.7 mm | LGA in US Exam | IVST ≥ 4.7 mm and/or LGA in US Exam | |
|---|---|---|---|---|
| Predictive Values | ||||
| Sensitivity | 71.43% | 46.43% | 78.57% | |
| Specificity | 61.25% | 77.12% | 57.20% | |
| Negative predictive value | 95.40% | 93.30% | 96.27% | |
| Positive predictive value | 16.00% | 17.33% | 15.94% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szmyd, B.; Biedrzycka, M.; Karuga, F.F.; Rogut, M.; Strzelecka, I.; Respondek-Liberska, M. Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia. J. Clin. Med. 2021, 10, 949. https://doi.org/10.3390/jcm10050949
Szmyd B, Biedrzycka M, Karuga FF, Rogut M, Strzelecka I, Respondek-Liberska M. Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia. Journal of Clinical Medicine. 2021; 10(5):949. https://doi.org/10.3390/jcm10050949
Chicago/Turabian StyleSzmyd, Bartosz, Małgorzata Biedrzycka, Filip Franciszek Karuga, Magdalena Rogut, Iwona Strzelecka, and Maria Respondek-Liberska. 2021. "Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia" Journal of Clinical Medicine 10, no. 5: 949. https://doi.org/10.3390/jcm10050949
APA StyleSzmyd, B., Biedrzycka, M., Karuga, F. F., Rogut, M., Strzelecka, I., & Respondek-Liberska, M. (2021). Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia. Journal of Clinical Medicine, 10(5), 949. https://doi.org/10.3390/jcm10050949








