Neurocognitive Status after Aortic Valve Replacement: Differences between TAVI and Surgery
Abstract
1. Background
2. Neurocognition: Cardiac Surgery
3. Neurocognition: TAVI vs. SAVR
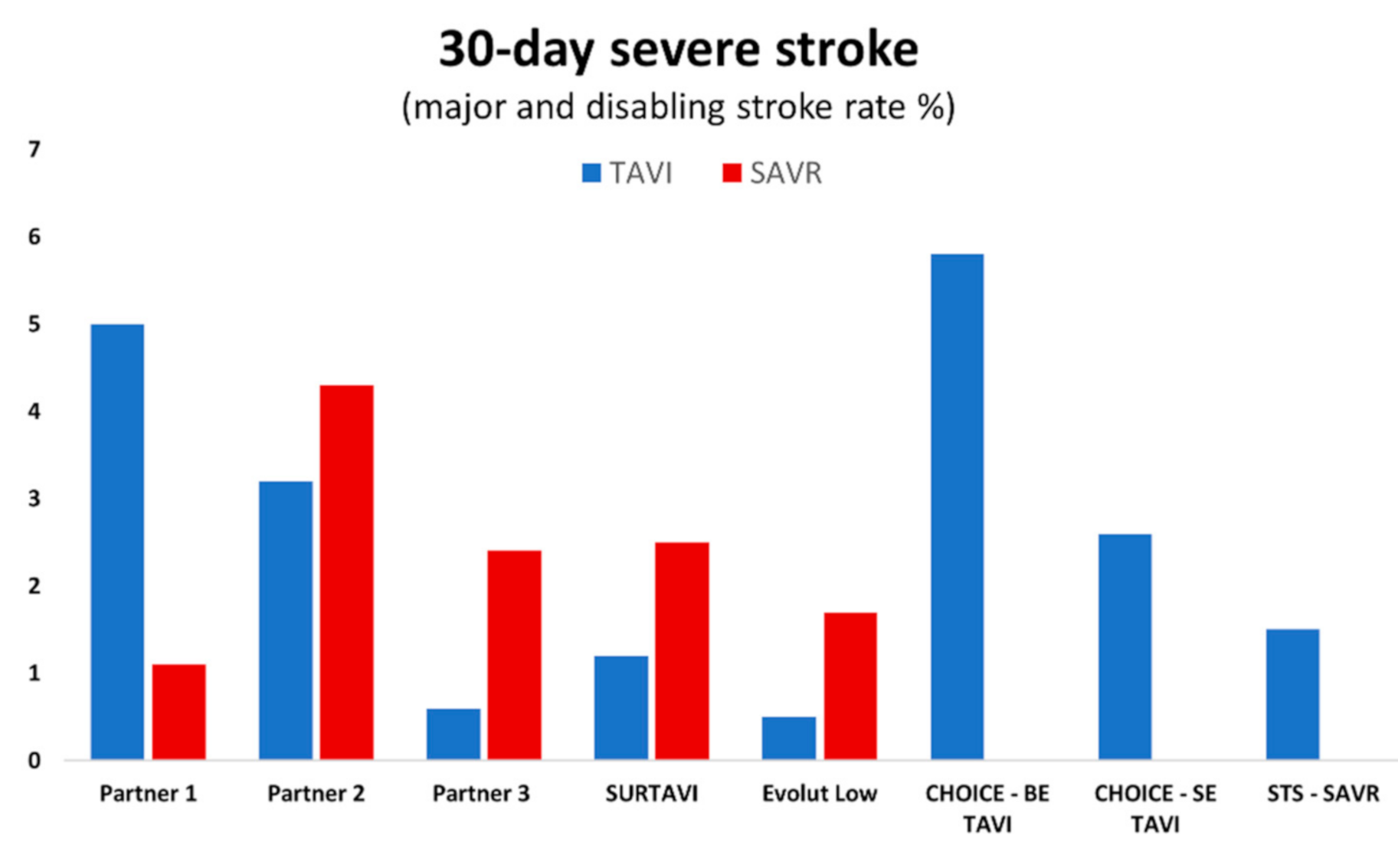
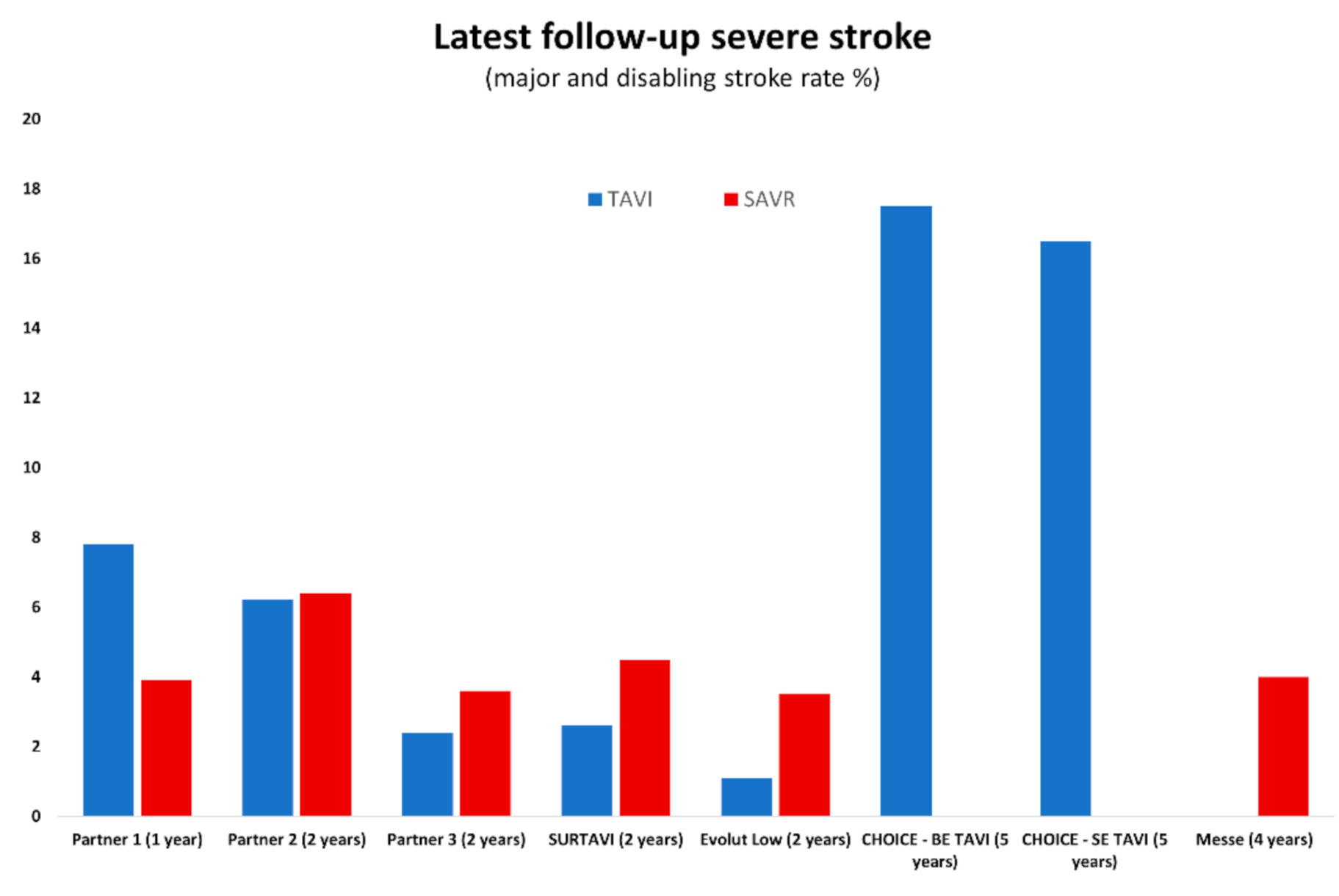
4. Stroke: TAVI vs. SAVR in Intermediate-Risk Populations
5. Stroke: TAVI vs. SAVR in Low-Risk Populations
6. Stroke: Balloon-Expandable vs. Self-Expanding Transcatheter Valves
7. Neurocognition and Stroke: Alternative Access TAVI and SAVR
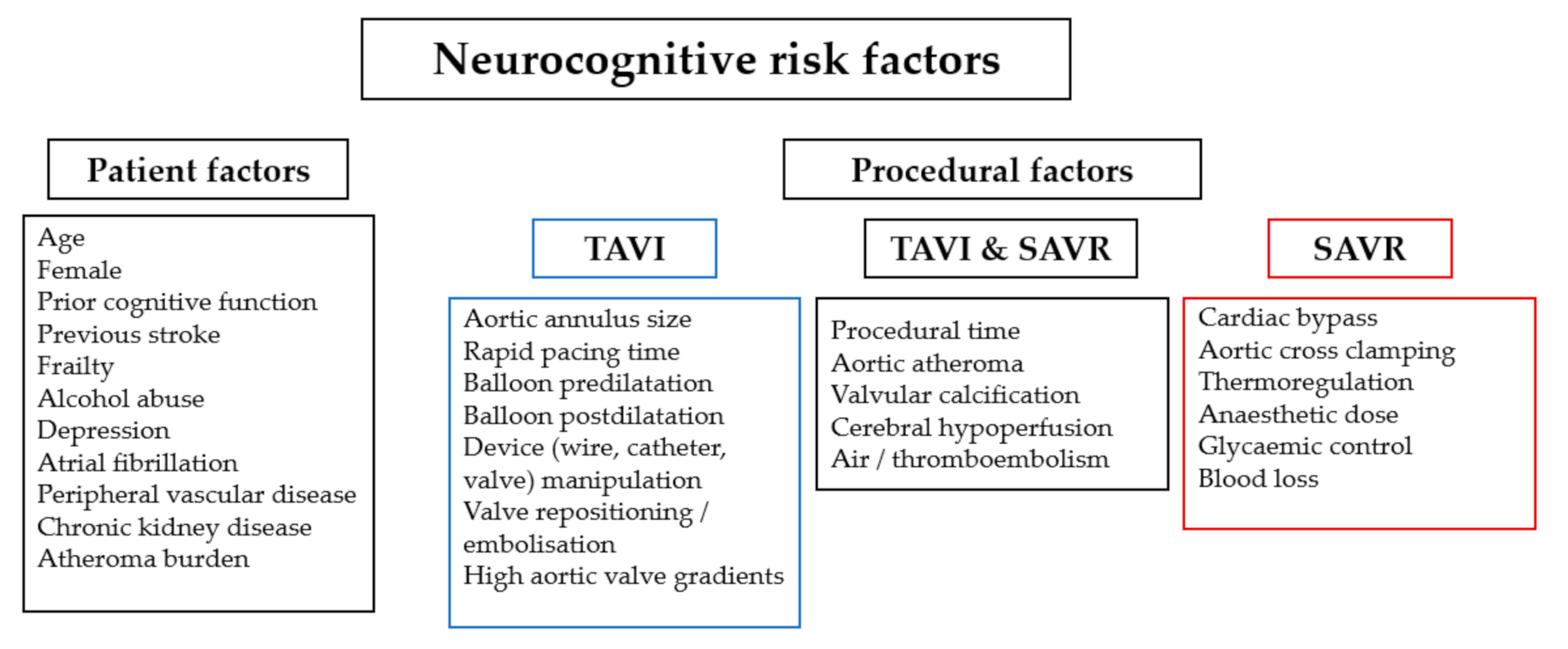
8. The Impact of Patient and Procedural Risk Factors
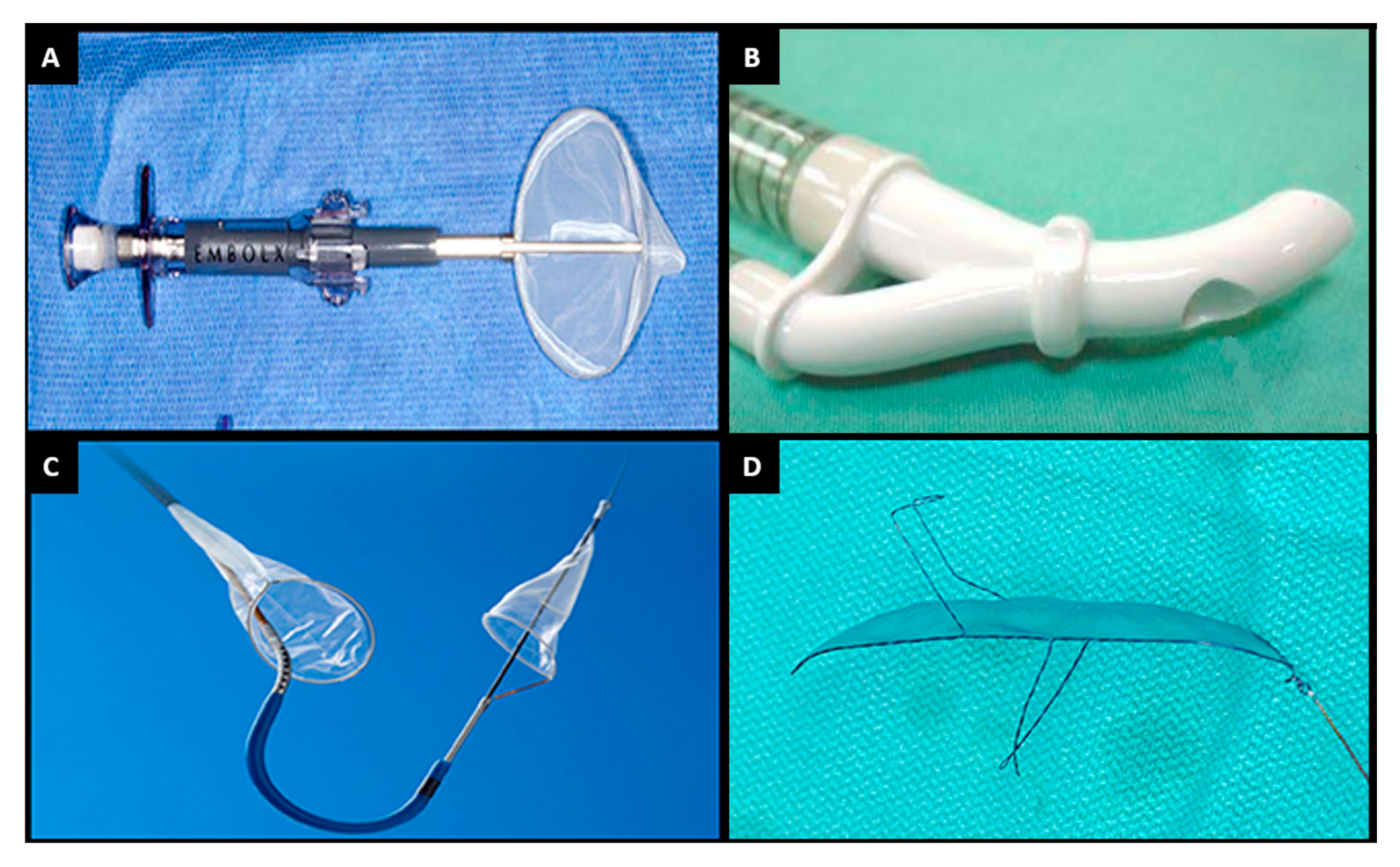
9. Cerebral Embolic Protection
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khan, M.M.; Herrmann, N.; Gallagher, D.; Gandell, D.; Fremes, S.E.; Wijeysundera, H.C.; Radhakrishnan, S.; Sun, Y.R.; Lanctôt, K.L. Cognitive Outcomes After Transcatheter Aortic Valve Implantation: A Metaanalysis. J. Am. Geriatr. Soc. 2017, 66, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Messé, S.R.; Acker, M.A.; Kasner, S.E.; Fanning, M.; Giovannetti, T.; Ratcliffe, S.J.; Bilello, M.; Szeto, W.Y.; Bavaria, J.E.; Hargrove, W.C., III; et al. Stroke after aortic valve surgery: Results from a prospective cohort. Circulation 2014, 129, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.T.; Delong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 2—Isolated Valve Surgery. Ann. Thorac. Surg. 2009, 88, S23–S42. [Google Scholar] [CrossRef]
- Shahian, D.M.; O’Brien, S.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.T.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 3—Valve Plus Coronary Artery Bypass Grafting Surgery. Ann. Thorac. Surg. 2009, 88, S43–S62. [Google Scholar] [CrossRef]
- Meredith, I.T.; Walters, D.L.; Dumonteil, N.; Worthley, S.G.; Tchétché, D.; Manoharan, G.; Blackman, D.J.; Rioufol, G.; Hildick-Smith, D.; Whitbourn, R.J.; et al. 1-Year Outcomes With the Fully Repositionable and Retrievable Lotus Transcatheter Aortic Replacement Valve in 120 High-Risk Surgical Patients with Severe Aortic Stenosis: Results of the REPRISE II Study. JACC Cardiovasc. Interv. 2016, 9, 376–384. [Google Scholar] [CrossRef]
- Kodali, S.; Thourani, V.H.; White, J.; Malaisrie, S.C.; Lim, S.; Greason, K.L.; Williams, M.; Guerrero, M.; Eisenhauer, A.C.; Kapadia, S.; et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur. Heart J. 2016, 37, 2252–2262. [Google Scholar] [CrossRef]
- Knipp, S.C.; Matatko, N.; Wilhelm, H.; Schlamann, M.; Thielmann, M.; Lösch, C.; Diener, H.C.; Jakob, H. Cognitive Outcomes Three Years After Coronary Artery Bypass Surgery: Relation to Diffusion-Weighted Magnetic Resonance Imaging. Ann. Thorac. Surg. 2008, 85, 872–879. [Google Scholar] [CrossRef]
- Haussig, S.; Mangner, N.; Dwyer, M.G.; Lehmkuhl, L.; Lücke, C.; Woitek, F.; Holzhey, D.M.; Mohr, F.W.; Gutberlet, M.; Zivadinov, R.; et al. Effect of a Cerebral Protection Device on Brain Lesions Following Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis: The CLEAN-TAVI Randomized Clinical Trial. JAMA 2016, 316, 592–601. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve im-plantation: The Valve Academic Research Consortium-2 consensus document (VARC-2). Eur. J. Cardiothorac. Surg. 2012, 42, S45–S60. [Google Scholar] [CrossRef]
- Lansky, A.J.; Messé, S.R.; Brickman, A.M.; Dwyer, M.; van der Worp, H.B.; Lazar, R.M.; Pietras, C.G.; Abrams, K.J.; McFadden, E.; Petersen, N.H.; et al. Proposed Standardized Neurological Endpoints for Cardiovascular Clinical Trials: An Academic Research Consortium Initiative. J. Am. Coll. Cardiol. 2017, 69, 679–691. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.B.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Silverstein, A.; Krieger, H.P. Neurologic complications of cardiac surgery. Trans. Am. Neurol. Assoc. 1960, 85, 151–154. [Google Scholar] [CrossRef]
- Egerton, N.; Kay, J.H. Psychological Disturbances Associated with Open Heart Surgery. Br. J. Psychiatry 1964, 110, 433–439. [Google Scholar] [CrossRef]
- Sotaniemi, K.A. Long-term neurologic outcome after cardiac operation. Ann. Thorac. Surg. 1995, 59, 1336–1339. [Google Scholar] [CrossRef]
- Newman, M.F.; Kirchner, J.L.; Phillips-Bute, B.; Gaver, V.; Grocott, H.; Jones, R.H.; Mark, D.B.; Reves, J.G.; Blumenthal, J.A. Longitudinal Assessment of Neurocognitive Function after Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2001, 344, 395–402. [Google Scholar] [CrossRef]
- Berger, M.; Terrando, N.; Smith, S.K.; Browndyke, J.N.; Newman, M.F.; Mathew, J.P. Neurocognitive Function after Cardiac SurgeryFrom Phenotypes to Mechanisms. Anesthesiology 2018, 129, 829–851. [Google Scholar] [CrossRef]
- Zimpfer, D.; Czerny, M.; Kilo, J.; Kasimir, M.-T.; Madl, C.; Kramer, L.; Wieselthaler, G.M.; Wolner, E.; Grimm, M. Cognitive deficit after aortic valve replacement. Ann. Thorac. Surg. 2002, 74, 407–412. [Google Scholar] [CrossRef]
- Brækken, S.K.; Reinvang, I.; Russell, D.; Brucher, R.; Svennevig, J.L. Association between intraoperative cerebral microembolic signals and postoperative neuropsychological deficit: Comparison between patients with cardiac valve replacement and patients with coronary artery bypass grafting. J. Neurol. Neurosurg. Psychiatry 1998, 65, 573–576. [Google Scholar] [CrossRef]
- Knipp, S.C.; Matatko, N.; Schlamann, M.; Wilhelm, H.; Thielmann, M.; Forsting, M.; Diener, H.C.; Jakob, H. Small ischemic brain lesions after cardiac valve replacement detected by diffusion-weighted magnetic resonance imaging: Relation to neurocognitive function. Eur. J. Cardiothorac. Surg. 2005, 28, 88–96. [Google Scholar] [CrossRef]
- Gleason, T.G.; Schindler, J.T.; Adams, D.H.; Reardon, M.J.; Kleiman, N.S.; Caplan, L.R.; Conte, J.V.; Deeb, G.M.; Hughes, G.C.; Chenoweth, S.; et al. The risk and extent of neurologic events are equivalent for high-risk patients treated with transcatheter or surgical aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2016, 152, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.R.; Huded, C.P.; Kodali, S.K.; Svensson, L.G.; Tuzcu, E.M.; Baron, S.J.; Cohen, D.J.; Miller, D.C.; Thourani, V.H.; Herrmann, H.C.; et al. Stroke After Surgical Versus Transfemoral Transcatheter Aortic Valve Replacement in the PARTNER Trial. J. Am. Coll. Cardiol. 2018, 72, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Campelo-Parada, F.; Regueiro, A.; Del Trigo, M.; Chiche, O.; Chamandi, C.; Allende, R.; Cordoba-Soriano, J.G.; Paradis, J.-M.; De Larochellière, R.; et al. Serial Changes in Cognitive Function Following Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 68, 2129–2141. [Google Scholar] [CrossRef]
- Srinivasa, P.; Szerlip, M.; Al-Azizi, K.; Harrington, K.; Kodali, S.; Kapadia, S.; Lu, M.; Leon, M.B.; Mack, M.J. Neurocognitive Function Change in Low-Risk Patients Undergoing TAVR Versus SAVR. JACC Cardiovasc. Interv. 2020, 13, 2713–2714. [Google Scholar]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Makkar, R.; Svensson, L.G.; Kodali, S.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Landt, M.; Neumann, F.J.; Massberg, S.; Frerker, C.; Kurz, T.; Kaur, J.; Toelg, R.; Sachse, S.; Jochheim, D.; et al. 5-Year Outcomes After TAVR With Balloon-Expandable Versus Self-Expanding Valves: Results From the CHOICE Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 1071–1082. [Google Scholar] [CrossRef]
- Agarwal, S.; Parashar, A.; Kumbhani, D.J.; Svensson, L.G.; Krishnaswamy, A.; Tuzcu, E.M.; Kapadia, S.R. Comparative meta-analysis of balloon-expandable and self-expandable valves for transcatheter aortic valve replacement. Int. J. Cardiol. 2015, 197, 87–97. [Google Scholar] [CrossRef]
- Durko Andras, P.; Reardon Michael, J.; Kleiman Neal, S.; Popma, J.J.; Van Mieghem, N.M.; Gleason, T.G.; Bajwa, T.; O’Hair, D.; Brown, D.L.; Ryan, W.H.; et al. Neurological Complications After Transcatheter Versus Surgical Aortic Valve Replacement in Intermediate-Risk Patients. J. Am. Coll. Cardiol. 2018, 72, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Leon Martin, B.; Mack Michael, J.; Hahn Rebecca, T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Xie, A.; Tsai, Y.C.; Black, D.; Di Eusanio, M.; Yan, T.D. Ministernotomy or minithoracotomy for minimally invasive aortic valve replacement: A Bayesian network meta-analysis. Ann. Cardiothorac. Surg. 2015, 4, 3–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blackman, D.J.; Baxter, P.D.; Gale, C.P.; Moat, N.E.; MacCarthy, P.A.; Hildick-Smith, D.; Trivedi, U.; Cunningham, D.; De Belder, M.A.; Ludman, P.F.; et al. Do Outcomes from Transcatheter Aortic Valve Implantation Vary According to Access Route and Valve Type? The UK TAVI Registry. J. Interv. Cardiol. 2013, 27, 86–95. [Google Scholar] [CrossRef]
- Zhan, Y.; Saadat, S.; Soin, A.; Kawabori, M.; Chen, F.Y. A meta-analysis comparing transaxillary and transfemoral transcatheter aortic valve replacement. J. Thorac. Dis. 2019, 11, 5140–5151. [Google Scholar] [CrossRef]
- Dahle, T.G.; Kaneko, T.; McCabe, J.M. Outcomes Following Subclavian and Axillary Artery Access for Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 662–669. [Google Scholar] [CrossRef]
- Armijo, G.; Nombela-Franco, L.; Tirado-Conte, G. Cerebrovascular Events after Transcatheter Aortic Valve Implantation. Front. Cardiovasc. Med. 2018, 5, 104. [Google Scholar] [CrossRef]
- Davlouros, P.A.; Mplani, V.C.; Koniari, I.; Tsigkas, G.; Hahalis, G. Transcatheter aortic valve replacement and stroke: A comprehensive review. J. Geriatr. Cardiol. 2018, 15, 95–104. [Google Scholar]
- Antunes, P.E.; De Oliveira, J.F.; Antunes, M.J. Predictors of cerebrovascular events in patients subjected to isolated coronary surgery. The importance of aortic cross-clamping. Eur. J. Cardiothorac. Surg. 2003, 23, 328–333. [Google Scholar] [CrossRef][Green Version]
- Nombela-Franco, L.; Webb, J.G.; De Jaegere, P.P.; Toggweiler, S.; Nuis, R.-J.; Dager, A.E.; Amat-Santos, I.J.; Cheung, A.; Ye, J.; Binder, R.K.; et al. Timing, Predictive Factors, and Prognostic Value of Cerebrovascular Events in a Large Cohort of Patients Undergoing Transcatheter Aortic Valve Implantation. Circulation 2012, 126, 3041–3053. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; El Faquir, N.; Rahhab, Z.; Rodríguez-Olivares, R.; Wilschut, J.; Ouhlous, M.; Galema, T.W.; Geleijnse, M.L.; Kappetein, A.-P.; Schipper, M.E.; et al. Incidence and Predictors of Debris Embolizing to the Brain During Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2015, 8, 718–724. [Google Scholar] [CrossRef]
- Kroon, H.; von der Thusen, J.H.; Ziviello, F.; van Wiechen, M.; Ooms, J.F.; Kardys, I.; Schipper, M.; van Gils, L.; Daemen, J.; de Jaegere, P.; et al. Heterogeneity of debris captured by cerebral embolic protection filters during TAVI. Eurointervention 2021, 16, 1141–1147. [Google Scholar] [CrossRef]
- Grodecki, K.; Tamarappoo, B.K.; Huczek, Z.; Jedrzejczyk, S.; Cadet, S.; Kwiecinski, J.; Rymuza, B.; Parma, R.; Olasinska-Wisniewska, A.; Fijalkowska, J.; et al. Non-calcific aortic tissue quantified from computed tomography angiography improves diagnosis and prognostication of patients referred for transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2020. [Google Scholar] [CrossRef]
- Mack, M.J.; Acker, M.A.; Gelijns, A.C.; Overbey, J.R.; Parides, M.K.; Browndyke, J.N.; Groh, M.A.; Moskowitz, A.J.; Jeffries, N.O.; Ailawadi, G.; et al. Effect of Cerebral Embolic Protection Devices on CNS Infarction in Surgical Aortic Valve Replacement: A Randomized Clinical Trial. JAMA 2017, 318, 536–547. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Kodali, S.; Makkar, R.; Mehran, R.; Lazar, R.M.; Zivadinov, R.; Dwyer, M.G.; Jilaihawi, H.; Virmani, R.; Anwaruddin, S.; et al. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2017, 69, 367–377. [Google Scholar] [CrossRef]
- Seeger, J.; Gonska, B.; Otto, M.; Rottbauer, W.; Wöhrle, J. Cerebral Embolic Protection During Transcatheter Aortic Valve Replacement Significantly Reduces Death and Stroke Compared with Unprotected Procedures. JACC Cardiovasc. Interv. 2017, 10, 2297–2303. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; Van Gils, L.; Ahmad, H.; Van Kesteren, F.; Van Der Werf, H.W.; Brueren, G.; Storm, M.; Lenzen, M.; Daemen, J.; van den Heuvel, A.F.M.; et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: The randomised MISTRAL-C trial. Eurointervention 2016, 12, 499–507. [Google Scholar] [CrossRef]
- Boston Scientific Corporation. PROTECTED TAVR: Stroke PROTECTion With SEntinel During Transcatheter. Clinical Trial Registration NCT04149535, clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04149535 (accessed on 11 February 2021).
- Kharbanda, R. The Role of Cerebral Embolic Protection in Preventing Strokes and Improving other Health Outcomes in Patients Receiving a Replacement Heart Valve. Available online: https://www.isrctn.com/ISRCTN16665769?q=&filters=conditionCategory:Circulatory%20System,trialStatus:Ongoing&sort=&offset=1&totalResults=150&page=1&pageSize=10&searchType=basic-search (accessed on 11 February 2021). [CrossRef]
- Lansky, A.J.; Schofer, J.; Tchetche, D.; Stella, P.; Pietras, C.G.; Parise, H.; Abrams, K.; Forrest, J.K.; Cleman, M.; Reinöhl, J.; et al. A prospective randomized evaluation of the TriGuardTM HDH embolic DEFLECTion device during transcatheter aortic valve implantation: Results from the DEFLECT III trial. Eur. Heart J. 2015, 36, 2070–2078. [Google Scholar] [CrossRef]
- Nazif, T.M.; Moses, J.; Sharma, R.; Dhoble, A.; Rovin, J.; Brown, D.; Horwitz, P.; Makkar, R.; Stoler, R.; Forrest, J.; et al. Randomized Evaluation of TriGuard 3 Cerebral Embolic Protection After Transcatheter Aortic Valve Replacement: REFLECT II. JACC Cardiovasc. Interv. 2021, 14, 515–527. [Google Scholar] [CrossRef]
- Panchal, H.B.; Paul, T.K. Editorial commentary: Use of cerebral embolic protection devices during transcatheter aortic valve replacement. Trends Cardiovasc. Med. 2018, 28, 419–420. [Google Scholar] [CrossRef]
| American Heart Association/American Stroke Association [14] | Valve Academic Research Consortium-2 [12] | NeuroARC [13] |
|---|---|---|
| Definition of CNS infarction: CNS infarction is brain, spinal cord, or retinal cell death attributable to ischaemia, based on: 1. Pathological, imaging, or other objective evidence of cerebral, spinal cord, or retinal focal ischaemic injury in a defined vascular distribution; or 2. Clinical evidence of cerebral, spinal cord, or retinal focal ischaemic injury based on symptoms persisting ≥24 h or until death, and other aetiologies excluded. | Disabling stroke: An mRS score of 2 or more at 90 days and an increase in at least one mRS category from an individual’s pre-stroke baseline. | Type 1.a Ischaemic stroke: Sudden onset of neurological signs or symptoms fitting a focal or multifocal vascular territory within the brain, spinal cord, or retina, that: a. CNS infarction in the corresponding vascular territory (with or without haemorrhage); or b. Absence of other apparent causes (including haemorrhage), even if no evidence of acute ischaemia in the corresponding vascular territory is detected; or c. Symptoms lasting <24 h, with pathology or neuroimaging confirmation of CNS infarction in the corresponding vascular territory. |
| Definition of ischaemic stroke: An episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction. | Non-disabling stroke: An mRS score of <2 at 90 days or one that does not result in an increase in at least one mRS category from an individual’s pre-stroke baseline. | Type 2.a Covert CNS infarction: Brain, spinal cord, or retinal cell death attributable to focal or multifocal ischaemia, on the basis of neuroimaging or pathological evidence of CNS infarction, without a history of acute neurological symptoms consistent with the lesion location. |
| Definition of silent CNS infarction: Imaging or neuropathological evidence of CNS infarction, without a history of acute neurological dysfunction attributable to the lesion. | Stroke: duration of a focal or global neurological deficit ≥24 h; or 24 h if available neuroimaging documents a new haemorrhage or infarct; or the neurological deficit results in death. | Type 3.a TIA: Transient focal neurological signs or symptoms (lasting <24 h) presumed to be due to focal brain, spinal cord, or retinal ischaemia, but without evidence of acute infarction by neuroimaging or pathology (or in the absence of imaging). |
| Intermediate-Risk Patients | Low-Risk Patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PARTNER-2 | SURTAVI | PARTNER-3 | Evolut Low-Risk | |||||||||
| TAVI | SAVR | TAVI | SAVR | TAVI | SAVR | TAVI | SAVR | |||||
| 30 days | 3.2% | 4.3% | p = NS | 1.2% | 2.5% | p = NS | 0.0% | 0.4% | p = NS | 0.5% | 1.7% | p < 0.05 |
| 2 years | 6.2% | 6.4% | p = NS | 2.6% | 4.5% | p = NS | 0.6% | 0.6% | p = NS | 1.1% | 3.5% | p < 0.05 |
| Embol-X® | CardioGard® | Sentinel® | TriGUARD® | |
|---|---|---|---|---|
| Manufacturer | Edwards Lifesciences, USA | CardioGard, Israel | Boston Scientific, MA, USA | Keystone Heart, Israel |
| Filter | Heparin-coated polyester mesh filter; pore size: 120 µm | Suction sideport adjacent to aortic perfusion cannula | Two oval coned mesh filters; pore size: 140 µm | Nitinol frame and mesh filter; pore size: 130 µm |
| Delivery | Direct aortic cannulation, above cross-clamp | 24 Fr direct aortic cannulation | 6 Fr radial | 9 Fr femoral |
| Primary mechanism | Filter and capture | Particulate and gaseous suction-based extraction | Filter and capture | Deflection |
| Coverage | Ascending aorta distal to cross-clamp | Ascending aorta distal to cross-clamp | Brachiocephalic and left common carotid arteries | Brachiocephalic, left common carotid, left subclavian arteries |
| Pertinent trial | Mack et al. No benefit vs. conventional therapy | Mack et al. No benefit vs. conventional therapy. Lower rates of in-hospital delirium (p < 0.05) | SENTINEL-Non-inferior MACCE (p = NS) Less stroke numerically but not-significant (p = NS) | DEFLECT III-fewer ischaemic brain lesions (p < 0.05), reduced neurological deficits on NIHSS (p < 0.05), improved neurocognition |
| Recent/ongoing trials | Nil recruiting | Nil recruiting | BHF-Protect and PROTECTED TAVR | REFLECT II trial was terminated early due to safety concerns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aroney, N.; Patterson, T.; Allen, C.; Redwood, S.; Prendergast, B. Neurocognitive Status after Aortic Valve Replacement: Differences between TAVI and Surgery. J. Clin. Med. 2021, 10, 1789. https://doi.org/10.3390/jcm10081789
Aroney N, Patterson T, Allen C, Redwood S, Prendergast B. Neurocognitive Status after Aortic Valve Replacement: Differences between TAVI and Surgery. Journal of Clinical Medicine. 2021; 10(8):1789. https://doi.org/10.3390/jcm10081789
Chicago/Turabian StyleAroney, Nicholas, Tiffany Patterson, Christopher Allen, Simon Redwood, and Bernard Prendergast. 2021. "Neurocognitive Status after Aortic Valve Replacement: Differences between TAVI and Surgery" Journal of Clinical Medicine 10, no. 8: 1789. https://doi.org/10.3390/jcm10081789
APA StyleAroney, N., Patterson, T., Allen, C., Redwood, S., & Prendergast, B. (2021). Neurocognitive Status after Aortic Valve Replacement: Differences between TAVI and Surgery. Journal of Clinical Medicine, 10(8), 1789. https://doi.org/10.3390/jcm10081789





