The Angiogenic Potential of Mesenchymal Stem Cells from the Hair Follicle Outer Root Sheath
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Differentiation of MSCORS and Adipose-Derived MSCs (ADMSCs)
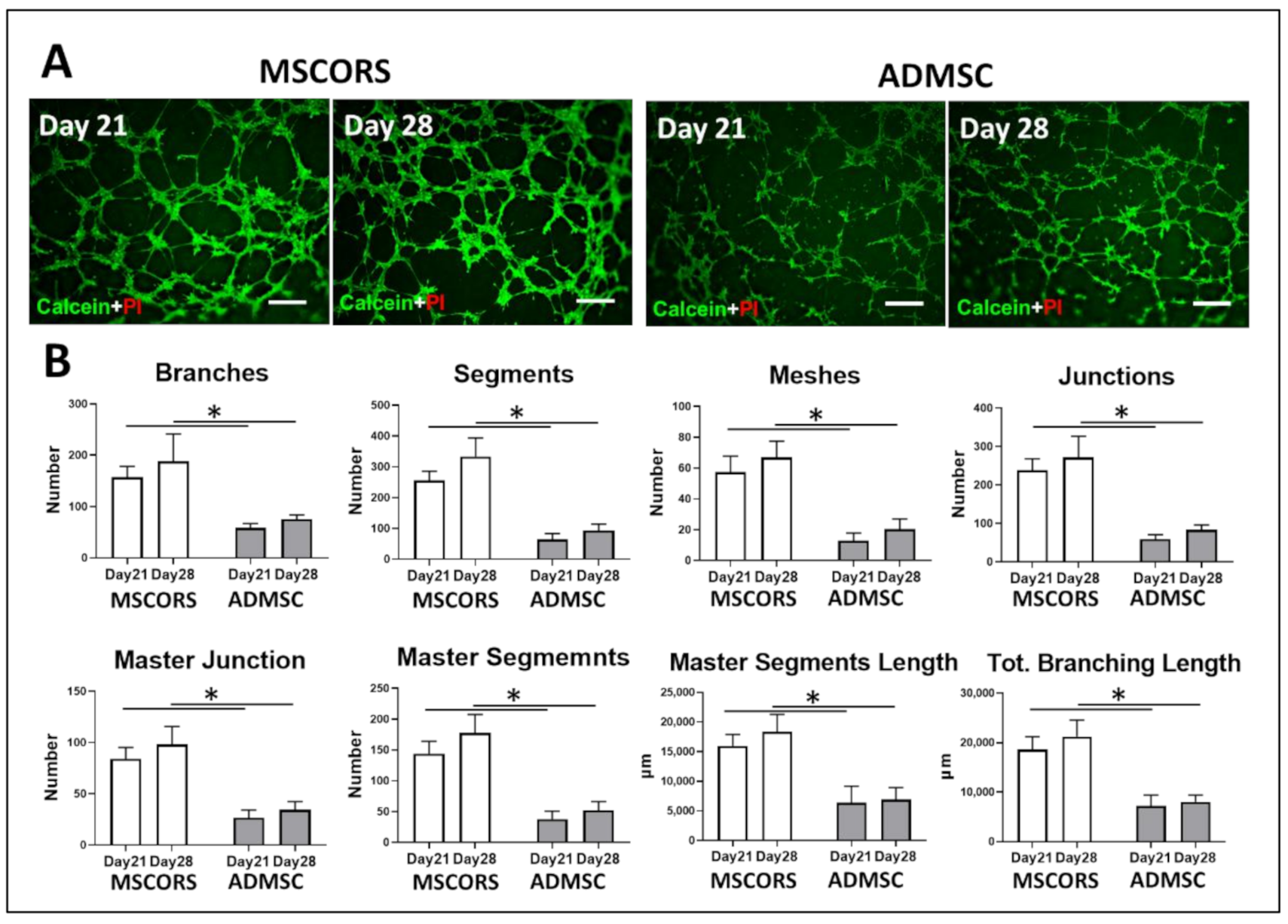
2.2. Tube-Forming Assay
2.3. Quantitative ImageJ Analysis for Angiogenic Assay
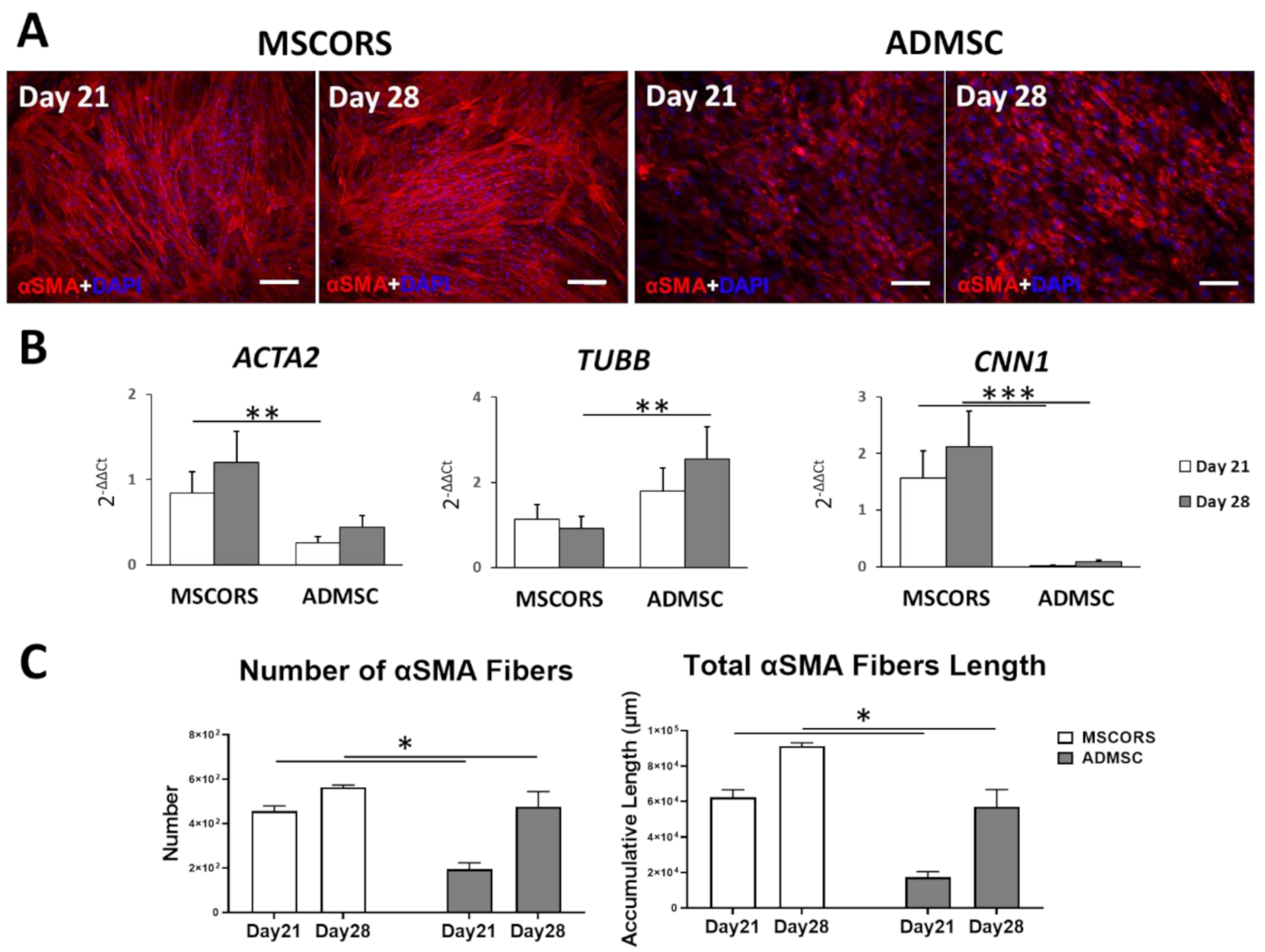
2.4. Immunostaining
2.5. Quantitative Analysisof the Length of Immunostained αSMA Fibers Using ImageJ software
2.6. qRT-PCR to Determine Gene Expression of Endothelial and Smooth Muscle Cell Markers
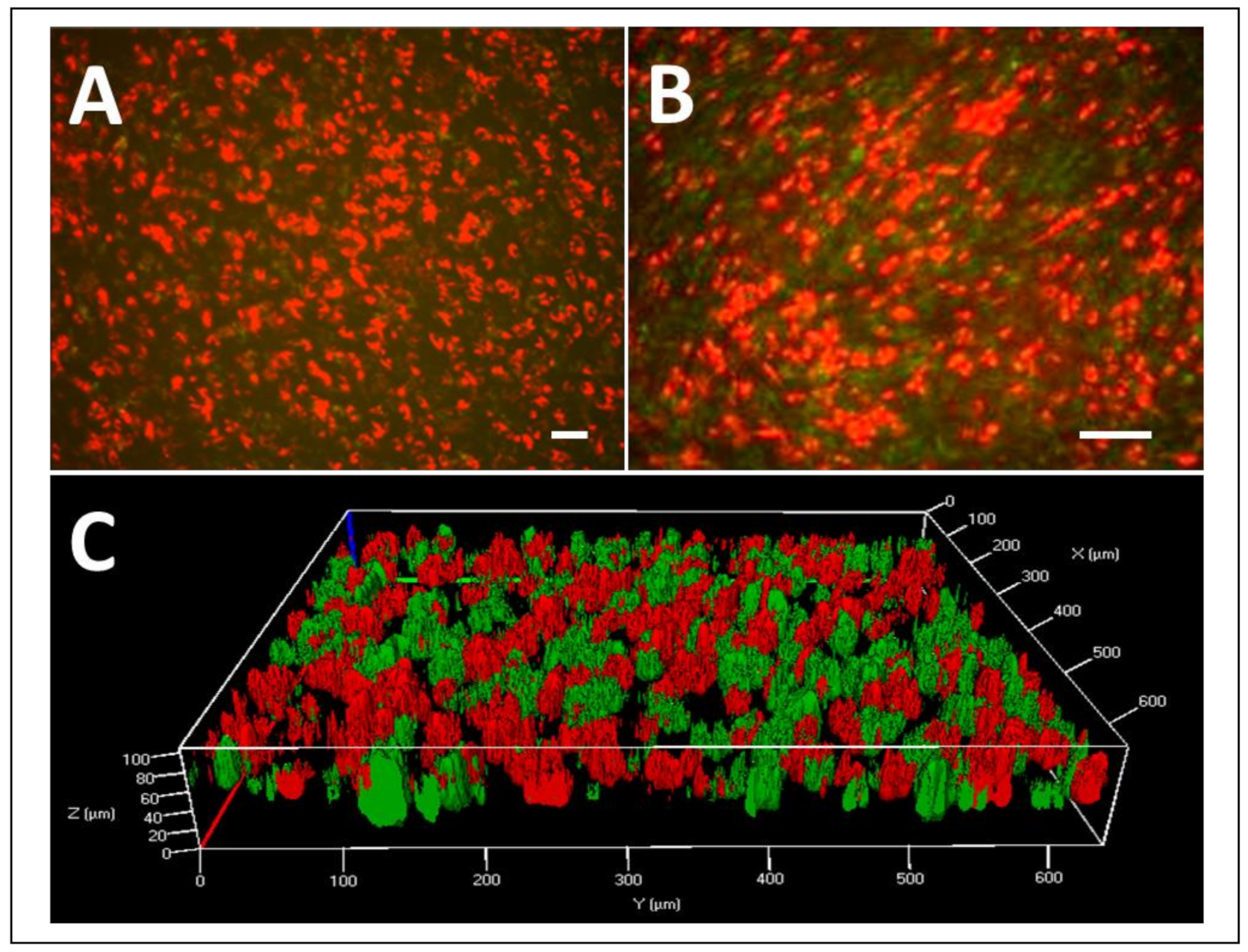
2.7. Production of MSCORS-SM-Sheet
2.8. MSCORS-Derived Endothelial Cells Attachment to MSCORS-SM-Sheet
2.9. Statistical Analysis
3. Results
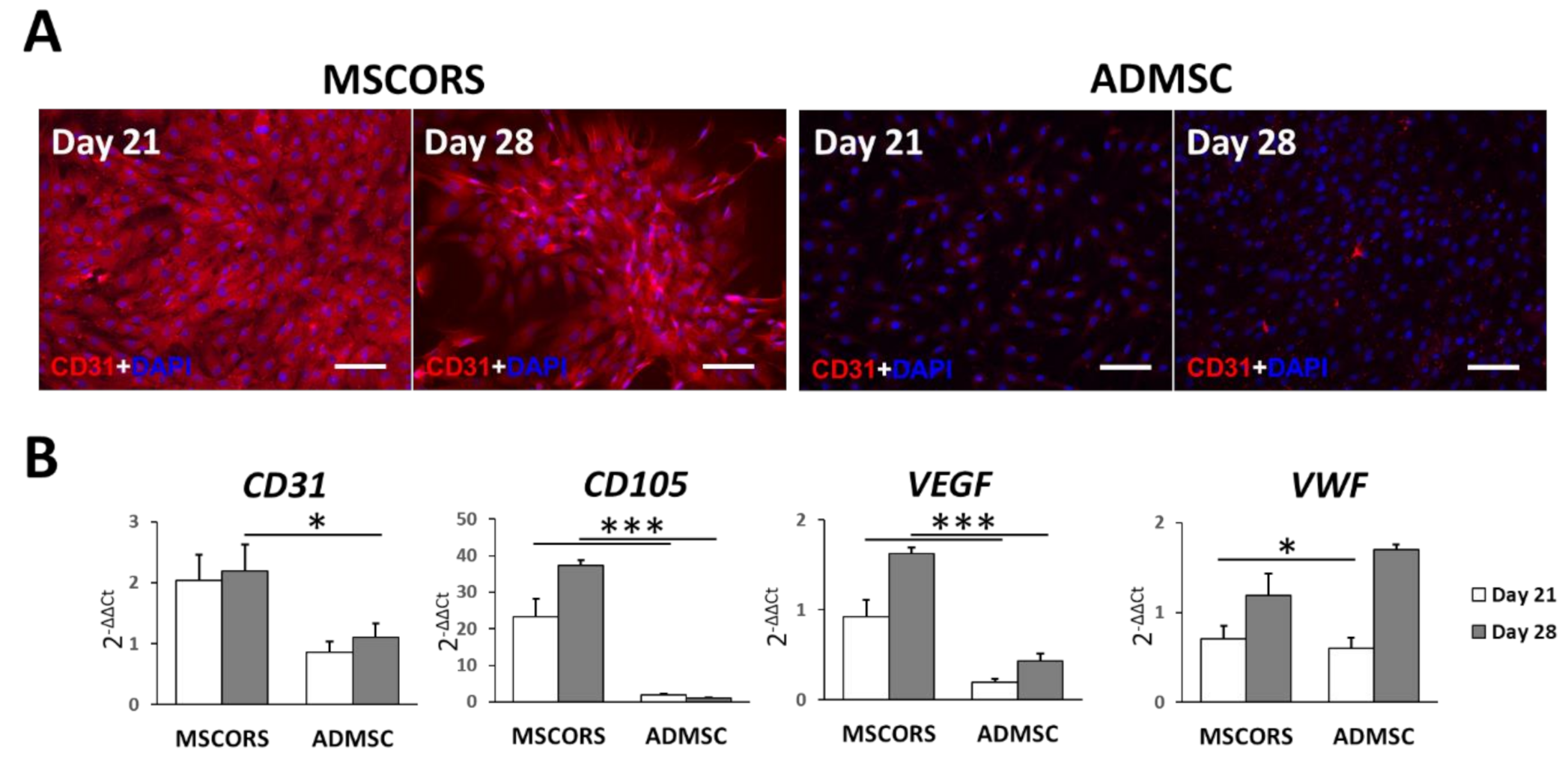
3.1. Endothelial Differentiation of MSCs in 2D
3.2. Angiogenic Potential of Differentiated MSCORS and ADMSCs
3.3. Smooth Muscle Differentiation of MSCs in 2D
3.4. MSCORS-Derived Endothelial Cells to Smooth Muscle Cell Sheet
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darland, D.C.; D’Amore, P.A. Blood vessel maturation: Vascular development comes of age. J. Clin. Investig. 1999, 103, 157–158. [Google Scholar] [CrossRef]
- García, J.R.; García, A.J. Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Deliv. Transl. Res. 2016, 6, 77–95. [Google Scholar] [CrossRef]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 83, 197–209. [Google Scholar] [CrossRef]
- Weinberg, C.B.; Bell, E. A blood vessel model constructed from collagen and cultured vascular cells. Science 1986, 231, 397–400. [Google Scholar] [CrossRef]
- Niklason, L.E.; Gao, J.; Abbott, W.M.; Hirschi, K.K.; Houser, S.; Marini, R.; Langer, R. Functional arteries grown in vitro. Science 1999, 284, 489–493. [Google Scholar] [CrossRef]
- L’Heureux, N.; Dusserre, N.; Konig, G.; Victor, B.; Keire, P.; Wight, T.N.; Chronos, N.A.; Kyles, A.E.; Gregory, C.R.; Hoyt, G.; et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 2006, 12, 361–365. [Google Scholar] [CrossRef]
- Jung, Y.; Ji, H.; Chen, Z.; Fai Chan, H.; Atchison, L.; Klitzman, B.; Truskey, G.; Leong, K.W. Scaffold-free, Human Mesenchymal Stem Cell-Based Tissue Engineered Blood Vessels. Sci. Rep. 2015, 5, 15116. [Google Scholar] [CrossRef]
- Gui, L.; Dash, B.C.; Luo, J.; Qin, L.; Zhao, L.; Yamamoto, K.; Hashimoto, T.; Wu, H.; Dardik, A.; Tellides, G.; et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 2016, 102, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef]
- Gong, Z.; Niklason, L.E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 1635–1648. [Google Scholar] [CrossRef]
- Savkovic, V.; Li, H.; Seon, J.-K.; Hacker, M.; Franz, S.; Simon, J.-C. Mesenchymal stem cells in cartilage regeneration. Curr. Stem Cell Res. Ther. 2014, 9, 469–488. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.E.; Gutowski, K.A.; Hematti, P. Clinical applications of mesenchymal stem cells in soft tissue augmentation. Aesthet. Surg. J. 2010, 30, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.I.; Reis, R.L. Vascularization in bone tissue engineering: Physiology, current strategies, major hurdles and future challenges. Macromol Biosci. 2010, 10, 12–27. [Google Scholar] [CrossRef]
- Aldridge, J.M., 3rd; Urbaniak, J.R. Avascular necrosis of the femoral head: Role of vascularized bone grafts. Orthop. Clin. North Am. 2007, 38, 13–22. [Google Scholar] [CrossRef]
- Logeart-Avramoglou, D.; Anagnostou, F.; Bizios, R.; Petite, H. Engineering bone: Challenges and obstacles. J. Cell Mol. Med. 2005, 9, 72–84. [Google Scholar]
- Aghaloo, T.L.; Misch, C.; Lin, G.H.; Iacono, V.J.; Wang, H.L. Bone Augmentation of the Edentulous Maxilla for Implant Placement: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2016, 31, s19–s30. [Google Scholar] [CrossRef]
- De Groot, R.J.; Oomens, M.; Forouzanfar, T.; Schulten, E. Bone augmentation followed by implant surgery in the edentulous mandible: A systematic review. J. Oral Rehabil. 2018, 45, 334–343. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Carrillo de Albornoz, A.; Figuero, E.; Schwarz, F.; Jung, R.; Sanz, M.; Thoma, D. Effects of lateral bone augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl S15), 18–31. [Google Scholar] [CrossRef]
- Li, P.; Liu, F.; Wu, C.; Jiang, W.; Zhao, G.; Liu, L.; Bai, T.; Wang, L.; Jiang, Y.; Guo, L.; et al. Feasibility of human hair follicle-derived mesenchymal stem cells/CultiSpher(®)-G constructs in regenerative medicine. Cell Tissue Res. 2015, 362, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Tan, X.; Li, G.; Gao, Y.; Liu, X.; Zhang, L.; Li, Y. Induced pluripotent stem cells from human hair follicle mesenchymal stem cells. Stem Cell Rev. Rep. 2013, 9, 451–460. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gao, Y.; Liu, X.; Bai, T.; Li, M.; Li, L.; Chi, G.; Xu, H.; Liu, F.; et al. Maintenance of high proliferation and multipotent potential of human hair follicle-derived mesenchymal stem cells by growth factors. Int. J. Mol. Med. 2013, 31, 913–921. [Google Scholar] [CrossRef]
- Li, H.; Masieri, F.F.; Schneider, M.; Bartella, A.; Gaus, S.; Hahnel, S.; Zimmerer, R.; Sack, U.; Maksimovic-Ivanic, D.; Mijatovic, S.; et al. The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers. Biomolecules 2021, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Masieri, F.F.; Schneider, M.; Kottek, T.; Hahnel, S.; Yamauchi, K.; Obradović, D.; Seon, J.-K.; Yun, S.J.; Ferrer, R.A.; et al. Autologous, Non-Invasively Available Mesenchymal Stem Cells from the Outer Root Sheath of Hair Follicle Are Obtainable by Migration from Plucked Hair Follicles and Expandable in Scalable Amounts. Cells 2020, 9, 2069. [Google Scholar] [CrossRef] [PubMed]
- In Proceedings of the 4th ImageJ User and Developer Conference. In Proceedings of the 4th ImageJ User and Developer Conference, Mondorf-les-Bains, Luxembourg, 24–26 October 2012; pp. 198–201.
- Ning, H.; Liu, G.; Lin, G.; Yang, R.; Lue, T.F.; Lin, C.S. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J. Sex. Med. 2009, 6, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Kinner, B.; Zaleskas, J.M.; Spector, M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp. Cell Res. 2002, 278, 72–83. [Google Scholar] [CrossRef]
- Noden, D.M. Origins and assembly of avian embryonic blood vessels. Ann. N. Y. Acad. Sci. 1990, 588, 236–249. [Google Scholar] [CrossRef]
- Vodyanik, M.A.; Yu, J.; Zhang, X.; Tian, S.; Stewart, R.; Thomson, J.A.; Slukvin, I.I. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 2010, 7, 718–729. [Google Scholar] [CrossRef]
- Era, T.; Izumi, N.; Hayashi, M.; Tada, S.; Nishikawa, S.; Nishikawa, S. Multiple mesoderm subsets give rise to endothelial cells, whereas hematopoietic cells are differentiated only from a restricted subset in embryonic stem cell differentiation culture. Stem Cells 2008, 26, 401–411. [Google Scholar] [CrossRef]
- Shang, T.; Li, S.; Zhang, Y.; Lu, L.; Cui, L.; Guo, F.F. Hypoxia promotes differentiation of adipose-derived stem cells into endothelial cells through demethylation of ephrinB2. Stem Cell Res. Ther. 2019, 10, 133. [Google Scholar] [CrossRef]
- Igarashi, Y.; Chosa, N.; Sawada, S.; Kondo, H.; Yaegashi, T.; Ishisaki, A. VEGF-C and TGF-β reciprocally regulate mesenchymal stem cell commitment to differentiation into lymphatic endothelial or osteoblastic phenotypes. Int. J. Mol. Med. 2016, 37, 1005–1013. [Google Scholar] [CrossRef]
- Kudo, F.A.; Nishibe, T.; Nishibe, M.; Yasuda, K. Autologous transplantation of peripheral blood endothelial progenitor cells (CD34+) for therapeutic angiogenesis in patients with critical limb ischemia. Int. Angiol. J. Int. Union Angiol. 2003, 22, 344–348. [Google Scholar]
- Janeczek Portalska, K.; Leferink, A.; Groen, N.; Fernandes, H.; Moroni, L.; van Blitterswijk, C.; de Boer, J. Endothelial differentiation of mesenchymal stromal cells. PLoS ONE 2012, 7, e46842. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Bernardo, A.S.; Trotter, M.W.; Pedersen, R.A.; Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol. 2012, 30, 165–173. [Google Scholar] [CrossRef]
- Wang, A.; Tang, Z.; Li, X.; Jiang, Y.; Tsou, D.A.; Li, S. Derivation of smooth muscle cells with neural crest origin from human induced pluripotent stem cells. Cells Tissues Organs 2011, 195, 5–14. [Google Scholar] [CrossRef]
- Kumar, A.; D’Souza, S.S.; Moskvin, O.V.; Toh, H.; Wang, B.; Zhang, J.; Swanson, S.; Guo, L.W.; Thomson, J.A.; Slukvin, I.I. Specification and Diversification of Pericytes and Smooth Muscle Cells from Mesenchymoangioblasts. Cell Rep. 2017, 19, 1902–1916. [Google Scholar] [CrossRef]
- Wang, C.; Yin, S.; Cen, L.; Liu, Q.; Liu, W.; Cao, Y.; Cui, L. Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-beta1 and bone morphogenetic protein-4. Tissue Eng. Part A 2010, 16, 1201–1213. [Google Scholar] [CrossRef]
- Kim, M.R.; Jeon, E.S.; Kim, Y.M.; Lee, J.S.; Kim, J.H. Thromboxane a(2) induces differentiation of human mesenchymal stem cells to smooth muscle-like cells. Stem Cells 2009, 27, 191–199. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar]
- Tanaka, K.; Sata, M.; Hirata, Y.; Nagai, R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ. Res. 2003, 93, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, M.A.; Pippenger, B.E.; Sack, R.; Zingg, D.; Ferralli, J.; Schenk, S.; Martin, I.; Chiquet-Ehrismann, R. TGF-β-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J. Cell Sci. 2014, 127, 1079–1091. [Google Scholar] [CrossRef]
- Bowers, D.T.; Song, W.; Wang, L.-H.; Ma, M. Engineering the vasculature for islet transplantation. Acta Biomater. 2019, 95, 131–151. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savkovic, V.; Li, H.; Obradovic, D.; Masieri, F.F.; Bartella, A.K.; Zimmerer, R.; Simon, J.-C.; Etz, C.; Lethaus, B. The Angiogenic Potential of Mesenchymal Stem Cells from the Hair Follicle Outer Root Sheath. J. Clin. Med. 2021, 10, 911. https://doi.org/10.3390/jcm10050911
Savkovic V, Li H, Obradovic D, Masieri FF, Bartella AK, Zimmerer R, Simon J-C, Etz C, Lethaus B. The Angiogenic Potential of Mesenchymal Stem Cells from the Hair Follicle Outer Root Sheath. Journal of Clinical Medicine. 2021; 10(5):911. https://doi.org/10.3390/jcm10050911
Chicago/Turabian StyleSavkovic, Vuk, Hanluo Li, Danilo Obradovic, Federica Francesca Masieri, Alexander K. Bartella, Rüdiger Zimmerer, Jan-Christoph Simon, Christian Etz, and Bernd Lethaus. 2021. "The Angiogenic Potential of Mesenchymal Stem Cells from the Hair Follicle Outer Root Sheath" Journal of Clinical Medicine 10, no. 5: 911. https://doi.org/10.3390/jcm10050911
APA StyleSavkovic, V., Li, H., Obradovic, D., Masieri, F. F., Bartella, A. K., Zimmerer, R., Simon, J.-C., Etz, C., & Lethaus, B. (2021). The Angiogenic Potential of Mesenchymal Stem Cells from the Hair Follicle Outer Root Sheath. Journal of Clinical Medicine, 10(5), 911. https://doi.org/10.3390/jcm10050911







