The Association of Oxidative Stress in the Uvular Mucosa with Obstructive Sleep Apnea Syndrome: A Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.1.1. Inclusion and Exclusion Criteria
2.1.2. Sleep Study
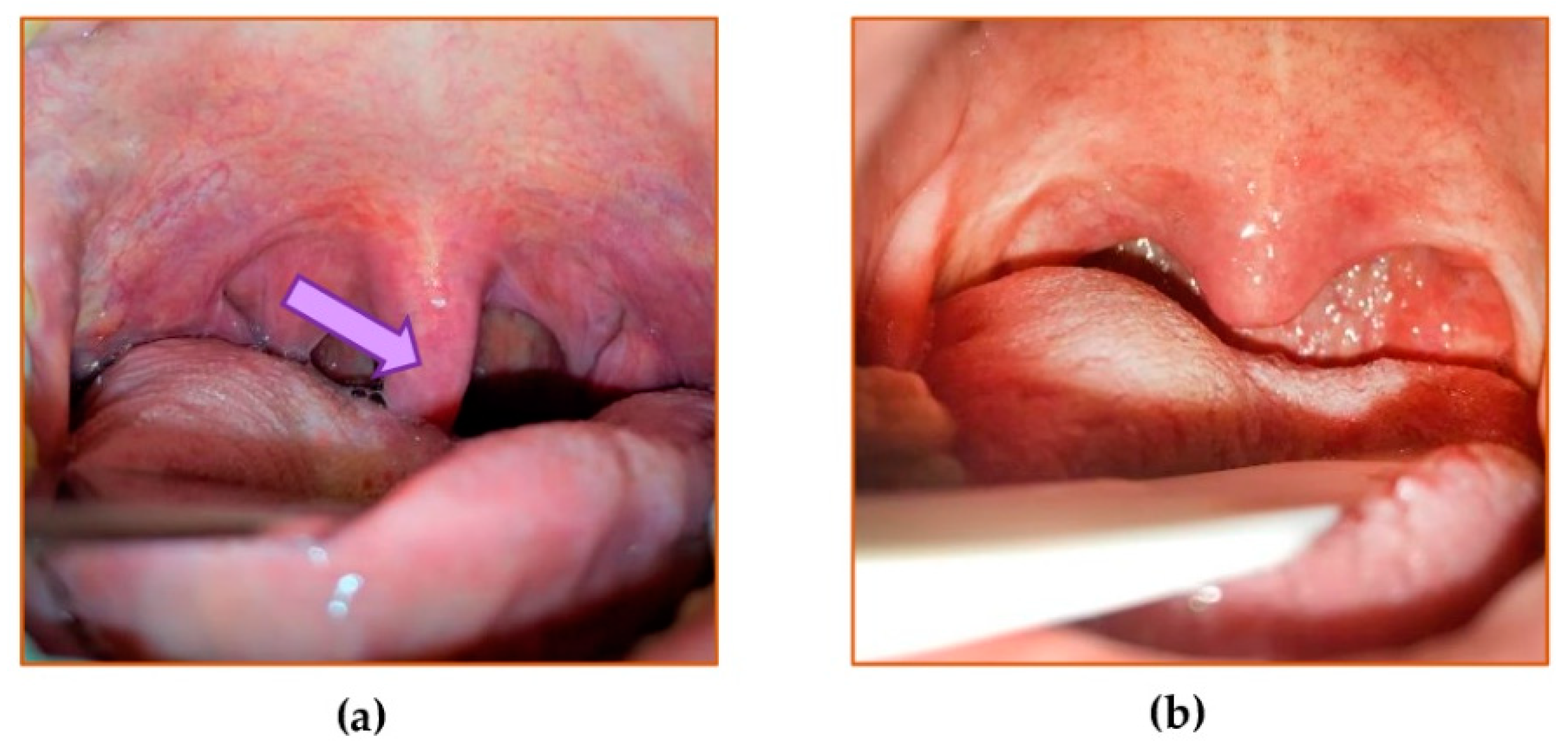
2.1.3. Surgical Procedures
2.2. Determination of Markers of Oxidative/Antioxidative Status
2.2.1. Preparation of the Aliquots of the Uvular Mucosa
2.2.2. Total Oxidative Status (TOS) and Total Antioxidative Status (TAS) Assay
2.2.3. Estimation of the Extent of Oxidative Stress
2.3. Statistical Analysis
3. Results
3.1. OSAS Severity
3.2. Type of Surgery
3.3. Mean Oxygen Saturation (MOS), Lowest Oxygen Saturation (LOS), and Oxygen Desaturation Index (ODI)
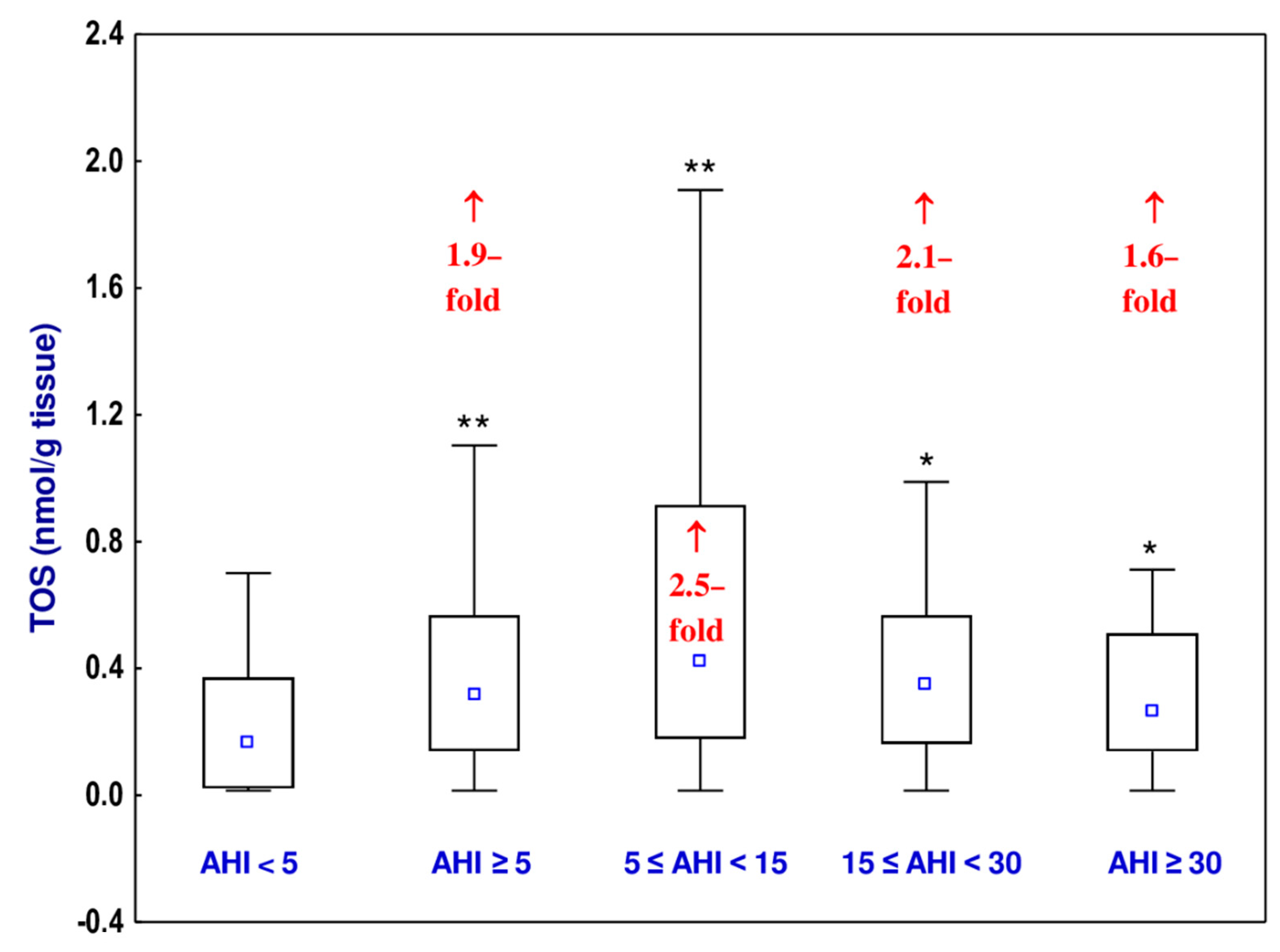
3.4. Total Oxidative Status (TOS)
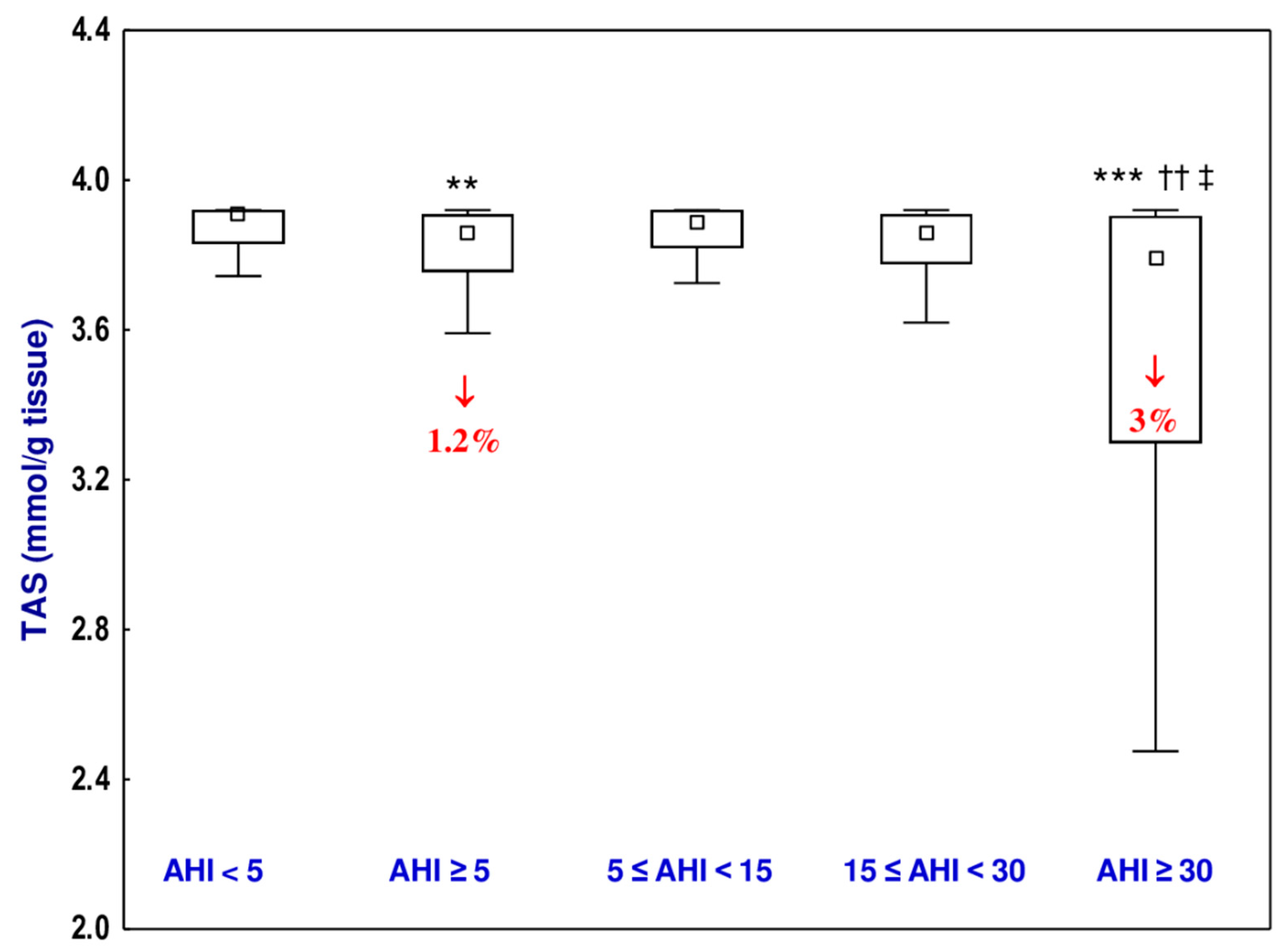
3.5. Total Antioxidative Status (TAS)
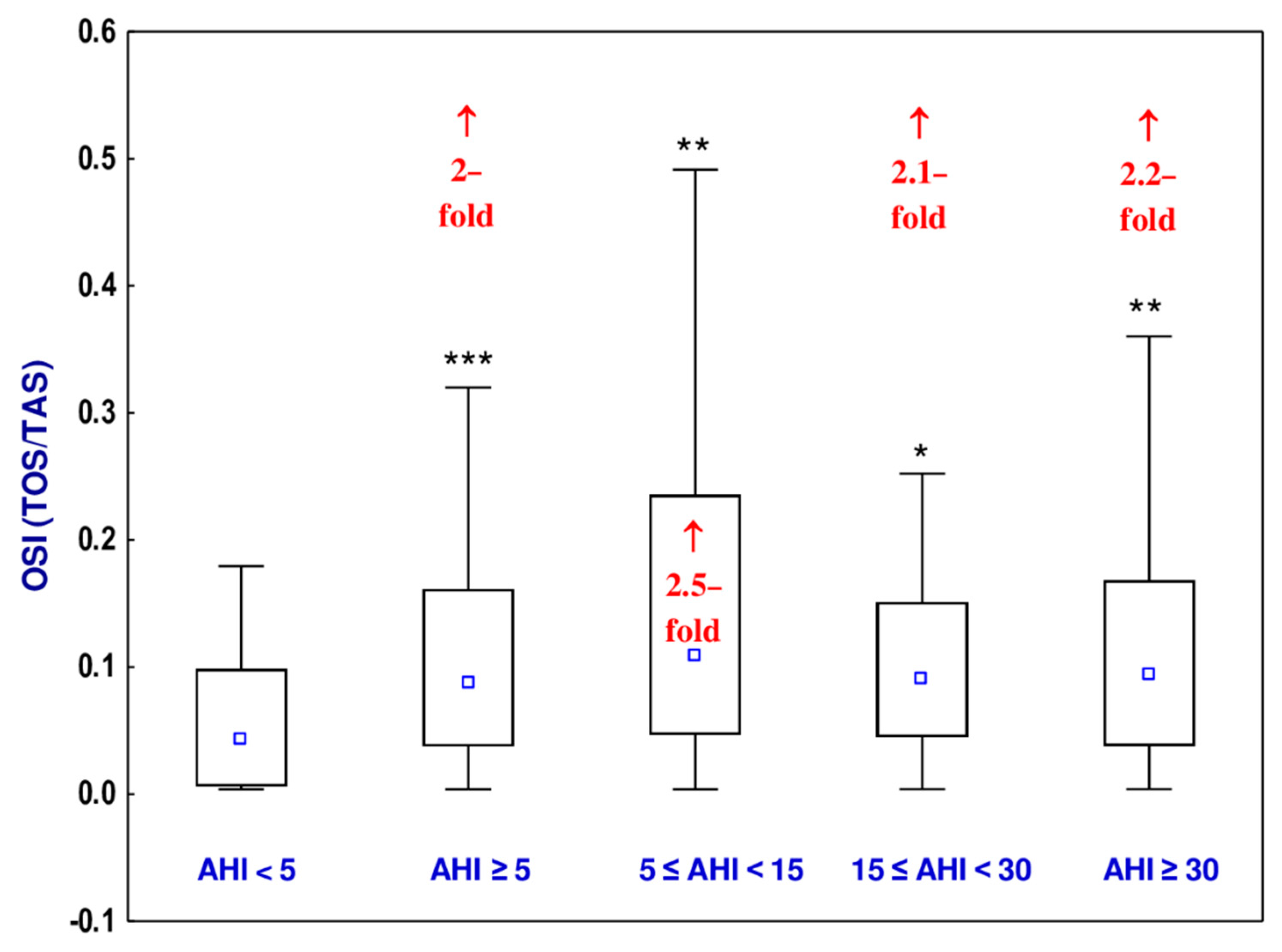
3.6. Oxidative Stress Index (OSI)
3.7. Mutual Dependences between the Investigated Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHI | apnea–hypopnea index |
| BMI | body mass index |
| CD | cluster of differentiation |
| CV | coefficient of variation |
| ELISA | enzyme-linked immunosorbent assay |
| ESS | Epworth Sleep Scale |
| LOS | lowest oxygen saturation |
| MOS | mean oxygen saturation |
| NO | nitric oxide |
| ODI | oxygen desaturation index |
| OSAS | obstructive sleep apnea syndrome |
| OSI | oxidative stress index |
| p | level of statistical significance |
| PSG | polysomnography |
| r | correlation coefficient |
| ROS | reactive oxygen species |
| SDB | sleep-disordered breathing |
| TAS | total antioxidative status |
| TOS | total oxidative status |
| VAS | visual analogue scale for snoring |
References
- Olszewska, E.; Woodson, B.T. Palatal anatomy for sleep apnea surgery. Laryngoscope 2019, 4, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Wang, F.; Xiong, X.; Xu, H.; Huang, H.; Shi, Y.; Li, X.; Qian, Y.; Zou, J.; Yi, H.; Guan, J.; et al. The association between obstructive sleep apnea syndrome and metabolic syndrome: A confirmatory factor analysis. Sleep Breath. 2019, 23, 1011–1019. [Google Scholar] [CrossRef]
- Borel, A.-L. Sleep Apnea and Sleep Habits: Relationships with Metabolic Syndrome. Nutrients 2019, 11, 2628. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, B.; Tjaden, A.H.; Temple, K.A.; Edelstein, S.L.; Sam, S.; Nadeau, K.J.; Hannon, T.S.; Manchanda, S.; Mather, K.J.; Kahn, S.E.; et al. Obstructive Sleep Apnea, Glucose Tolerance, and β-Cell Function in Adults with Prediabetes or Untreated Type 2 Diabetes in the Restoring Insulin Secretion (RISE) Study. Diabetes Care 2021, dc202127. [Google Scholar] [CrossRef]
- Peres, B.U.; Allen, A.J.H.; Kendzerska, T.; Shah, A.; Fox, N.; Laher, I.; Almeida, F.; Jen, R.; Sandford, A.J.; Van Eeden, S.F.; et al. Obstructive Sleep Apnea Severity, Body Mass Index, and Circulating Levels of Cellular Adhesion Molecules. Lung 2020, 198, 939–945. [Google Scholar] [CrossRef]
- Cofta, S.; Wysocka, E.; Michalak, S.; Piorunek, T.; Batura-Gabryel, H.; Torlinski, L. Endothelium-derived markers and antioxidant status in the blood of obstructive sleep apnea males. Eur. J. Med Res. 2009, 14, 49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, J.; Zhang, D.; Chen, B. Endothelial mechanisms of endothelial dysfunction in patients with obstructive sleep apnea. Sleep Breath. 2011, 16, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109 (Suppl. 1), III-27. [Google Scholar] [CrossRef]
- Mo, L.; Gupta, V.; Modi, R.; Munnur, K.; Cameron, J.D.; Seneviratne, S.; Edwards, B.A.; Landry, S.A.; Joosten, S.A.; Hamilton, G.S.; et al. Severe obstructive sleep apnea is associated with significant coronary artery plaque burden independent of traditional cardiovascular risk factors. Int. J. Cardiovasc. Imaging 2019, 36, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Destors, M.; Tamisier, R.; Baguet, J.-P.; Levy, P.; Pepin, J.-L. Morbidité cardiovasculaire associée au syndrome d’apnée obstructive du sommeil. Rev. Mal. Respir. 2014, 31, 375–385. [Google Scholar] [CrossRef]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep Apnea and Cardiovascular Disease. J. Am. Coll. Cardiol. 2008, 52, 686–717. [Google Scholar] [CrossRef]
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J. Am. Hear. Assoc. 2019, 8, e010440. [Google Scholar] [CrossRef]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O’Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Bahmani, D.S.; Brand, S. Evaluation of Serum and Plasma Interleukin-6 Levels in Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Immunol. 2020, 11, 1343. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.H.; Park, C.S.; Kim, B.G.; Kim, S.W.; Cho, J.H. Plasma Levels of MCP-1 and Adiponectin in Obstructive Sleep Apnea Syndrome. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 896–899. [Google Scholar] [CrossRef][Green Version]
- Hensley, K.; Robinson, K.A.; Gabbita, S.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free. Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. Reliability and Factor Analysis of the Epworth Sleepiness Scale. Sleep 1992, 15, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef]
- Friedman, M. Sleep Apnea and Snoring. Surgical and Non-Surgical Therapy, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; ISBN 978-1-4160-3112-3. [Google Scholar]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Badran, M.; Ayas, N.; Laher, I. Cardiovascular Complications of Sleep Apnea: Role of Oxidative Stress. Oxidative Med. Cell. Longev. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, H.; Neyal, A.; Geyik, S.; Taysi, S.; Anarat, R.; Bulut, M.; Neyal, A.M. Investigation of the Plasma Nitrite Levels and Oxidant–Antioxidant Status in Obstructive Sleep Apnea Syndrome. Arch. Neuropsychiatry 2015, 52, 221–225. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front. Biosci. 2012, 4, 1391–1403. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kimura, H. Oxidative Stress in Obstructive Sleep Apnea: Putative Pathways to the Cardiovascular Complications. Antioxidants Redox Signal. 2008, 10, 755–768. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Lavie, P.; Lavie, L. Increased Adhesion Molecules Expression and Production of Reactive Oxygen Species in Leukocytes of Sleep Apnea Patients. Am. J. Respir. Crit. Care Med. 2002, 165, 934–939. [Google Scholar] [CrossRef]
- Yamauchi, M.; Nakano, H.; Maekawa, J.; Okamoto, Y.; Ohnishi, Y.; Suzuki, T.; Kimura, H. Oxidative Stress in Obstructive Sleep Apnea. Chest 2005, 127, 1674–1679. [Google Scholar] [CrossRef]
- Harkness, J.H.; Bushana, P.N.; Todd, R.P.; Clegern, W.C.; Sorg, B.A.; Wisor, J.P. Sleep disruption elevates oxidative stress in parvalbumin-positive cells of the rat cerebral cortex. Sleep 2019, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fiedorczuk, P.; Stróżyński, A.; Olszewska, E. Is the Oxidative Stress in Obstructive Sleep Apnea Associated With Cardiovascular Complications?—Systematic Review. J. Clin. Med. 2020, 9, 3734. [Google Scholar] [CrossRef]
- Stål, P.S.; Johansson, B. Abnormal Mitochondria Organization and Oxidative Activity in the Palate Muscles of Long-Term Snorers with Obstructive Sleep Apnea. Respiration 2012, 83, 407–417. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Barcelò, A.; Barbé, F.; De La Peña, M.; Vila, M.; Perez, G.; Piérola, J.; Duran, J.; Agustí, A.G.N. Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. Eur. Respir. J. 2006, 27, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L.; Hefetz, A.; Luboshitzky, R.; Lavie, P. Plasma Levels of Nitric Oxide and L-Arginine in Sleep Apnea Patients: Effects of nCPAP Treatment. J. Mol. Neurosci. 2003, 21, 57–64. [Google Scholar] [CrossRef]
- Carlson, J.T.; Rångemark, C.; Hedner, J.A. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J. Hypertens. 1996, 14, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Roberts-Thomson, P.; Phillips, B.G.; Haynes, W.G.; Winnicki, M.; Accurso, V.; Somers, V.K. Impairment of Endothelium-Dependent Vasodilation of Resistance Vessels in Patients with Obstructive Sleep Apnea. Circulation 2000, 102, 2607–2610. [Google Scholar] [CrossRef]
- Woodson, B.T.; Toohill, R.J.; Garancis, J.C. Histopathologic changes in snoring and obstructive sleep apnea syndrome. Laryngoscope 1991, 101, 1318–1322. [Google Scholar] [CrossRef]
- Sekosan, M.; Zakkar, M.; Wenig, B.L.; Olopade, C.O.; Rubinstein, I. Inflammation in the Uvula Mucosa of Patients With Obstructive Sleep Apnea. Laryngoscope 1996, 106, 1018–1020. [Google Scholar] [CrossRef]
- Paulsen, F.P.; Steven, P.; Tsokos, M.; Jungmann, K.; Mueller, A.; Verse, T.; Pirsig, W. Upper Airway Epithelial Structural Changes in Obstructive Sleep-disordered Breathing. Am. J. Respir. Crit. Care Med. 2002, 166, 501–509. [Google Scholar] [CrossRef]
- Allaouchiche, B.; DeBon, R.; Goudable, J.; Chassard, D.; Duflo, F. Oxidative Stress Status During Exposure to Propofol, Sevoflurane and Desflurane. Anesthesia Analg. 2001, 93, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Erbas, M.; Demiraran, Y.; Yildirim, H.A.; Sezen, G.; Iskender, A.; Karagöz, I.; Kandis, H. Comparação dos efeitos da perfusão de sevoflurano, desflurano e propofol sobre o sistema oxidante/antioxidante durante anestesia geral. Braz. J. Anesthesiol. 2015, 65, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Nilius, G.; Domanski, U.; Schroeder, M.; Franke, K.-J.; Hogrebe, A.; Margarit, L.; Stoica, M.; D’Ortho, M.-P. A randomized controlled trial to validate the Alice PDX ambulatory device. Nat. Sci. Sleep 2017, 9, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Chesson, A.L., Jr.; Berry, R.B.; Pack, A. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep 2003, 26, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Force, U.P.S.T.; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.R.; Herzstein, J.; Kemper, A.R.; Krist, A.H.; et al. Screening for Obstructive Sleep Apnea in Adults. JAMA 2017, 317, 407–414. [Google Scholar] [CrossRef]
- Lavie, L.; Kraiczi, H.; Hefetz, A.; Ghandour, H.; Perelman, A.; Hedner, J.; Lavie, P. Plasma Vascular Endothelial Growth Factor in Sleep Apnea Syndrome. Am. J. Respir. Crit. Care Med. 2002, 165, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
| Group | Parameter | Male | Female | ||
|---|---|---|---|---|---|
| Median | Min.–Max. | Median | Min.–Max. | ||
| Non-OSAS (AHI < 5); n = 40 | age | 38.0 | 21.0–73.0 | 53.0 | 51.0–55.0 |
| Male = 38; Female = 2 | BMI | 29.9 | 29.0–34.8 | 27.1 | 21.5–32.7 |
| OSAS participants (AHI ≥ 5); n = 88 | age | 46.0 | 26.0–78.0 | 44.5 | 32.0–61.0 |
| Male = 84; Female = 4 | BMI | 30.9 | 25.1–34.9 | 30.8 | 29.4–34.6 |
| Group | n | AHI | |
|---|---|---|---|
| Median | Min.–Max. | ||
| Non-OSAS (AHI < 5) | 40 | 4.00 | 0.10–4.90 |
| OSAS participants (AHI ≥ 5) | 88 | 20.75 *** | 5.30–70.6 |
| Mild OSAS (5 ≤ AHI < 15) | 32 | 11.25 *** | 5.30–14.5 |
| Moderate OSAS (15 ≤ AHI < 30) | 25 | 21.00 ***,††† | 15.2–29.7 |
| Severe OSAS (AHI ≥ 30) | 31 | 46.00 ***,†††,‡‡‡ | 30.1–70.6 |
| Group | Total Number of Participants Subjected to Surgery | Number of Participants Subjected to Surgery under Local Anesthesia (% of All Patients) | Number of Participants Subjected to Surgery under General Anesthesia (% of All Patients) |
|---|---|---|---|
| Non-OSAS (AHI < 5) | 40 | 40 (100%) | 0 |
| OSAS participants (AHI ≥ 5) | 88 | 35 (40%) | 53 (60%) |
| Mild OSAS (5 ≤ AHI < 15) | 32 | 22 (69%) | 10 (31%) |
| Moderate OSAS (15 ≤ AHI < 30) | 25 | 9 (36%) | 16 (64%) |
| Severe OSAS (AHI ≥ 30) | 31 | 4 (13%) | 27 (87%) |
| Group | n | MOS [%] Median (Min.–Max.) | LOS [%] Median (Min.–Max.) | ODI [%] Median (Min.–Max.) |
|---|---|---|---|---|
| Non-OSAS (AHI < 5) | 40 | 94.10 (85.3–96.0) | 86.00 (79.0–94.0) | 4.0 (0.5–5.0) |
| OSAS participants (AHI ≥ 5) | 8 | 93.00 (77.0–96.0) | 80.00 * (50.0–91.0) | 30.8 *** (10.1–67.8) |
| Mild OSAS (5 ≤ AHI < 15) | 32 | 93.80 (90.1–96.0) | 83.00 (69.0–91.0) | 12.8 * (10.1–20.0) |
| Moderate OSAS (15 ≤ AHI < 30) | 25 | 93.05 (88.0–96.0) | 79.00 **,†† (50.0–84.0) | 27.8 *** (23.4–37.9) |
| Severe OSAS (AHI ≥ 30) | 31 | 92.00 *,††,# (77.0–96.0) | 78.00 **,†† (51.0–89.0) | 40.1 *** (24.3–67.8) |
| Group | n | Hedges’ g | ||
|---|---|---|---|---|
| TOS | TAS | OSI | ||
| OSAS participants (AHI ≥ 5) | 88 | 0.430 *** | 0.349 *** | 0.436 *** |
| Mild OSAS (5 ≤ AHI < 15) | 32 | 0.840 *** | 0.016 NS | 0.840 *** |
| Moderate OSAS (15 ≤ AHI < 30) | 25 | 0.655 *** | 0.052 NS | 0.640 *** |
| Severe OSAS (AHI ≥ 30) | 31 | 0.435 *** | 0.829 *** | 0.543 *** |
| Variable | Age | BMI | AHI | ESS | VAS | MOS | LOS | ODI | TOS | TAS |
|---|---|---|---|---|---|---|---|---|---|---|
| BMI | NS | – | ||||||||
| AHI | 0.207 * | NS | – | |||||||
| ESS | NS | NS | NS | – | ||||||
| VAS | NS | 0.527 ‡ | NS | NS | – | |||||
| MOS | –0.279 * | –0.378 * | –0.366 ‡ | –0.310 * | NS | – | ||||
| LOS | NS | NS | –0.366 † | NS | NS | 0.584 ‡ | – | |||
| ODI | NS | NS | 0.932 ‡ | NS | NS | NS | –0.428 # | – | ||
| TOS | NS | NS | NS | NS | 0.266 # | NS | NS | 0.342 † | – | |
| TAS | NS | NS | –0.286 † | –0.343 * | NS | 0.515 ‡ | 0.259 * | –0.220 # | NS | – |
| OSI | NS | NS | NS | NS | 0.267 # | NS | NS | 0.395 ‡ | 0.982 ‡ | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska, E.; Rogalska, J.; Brzóska, M.M. The Association of Oxidative Stress in the Uvular Mucosa with Obstructive Sleep Apnea Syndrome: A Clinical Study. J. Clin. Med. 2021, 10, 1132. https://doi.org/10.3390/jcm10051132
Olszewska E, Rogalska J, Brzóska MM. The Association of Oxidative Stress in the Uvular Mucosa with Obstructive Sleep Apnea Syndrome: A Clinical Study. Journal of Clinical Medicine. 2021; 10(5):1132. https://doi.org/10.3390/jcm10051132
Chicago/Turabian StyleOlszewska, Ewa, Joanna Rogalska, and Małgorzata M. Brzóska. 2021. "The Association of Oxidative Stress in the Uvular Mucosa with Obstructive Sleep Apnea Syndrome: A Clinical Study" Journal of Clinical Medicine 10, no. 5: 1132. https://doi.org/10.3390/jcm10051132
APA StyleOlszewska, E., Rogalska, J., & Brzóska, M. M. (2021). The Association of Oxidative Stress in the Uvular Mucosa with Obstructive Sleep Apnea Syndrome: A Clinical Study. Journal of Clinical Medicine, 10(5), 1132. https://doi.org/10.3390/jcm10051132






