The Accuracy of Emergency Physicians’ Suspicions of Active Pulmonary Tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
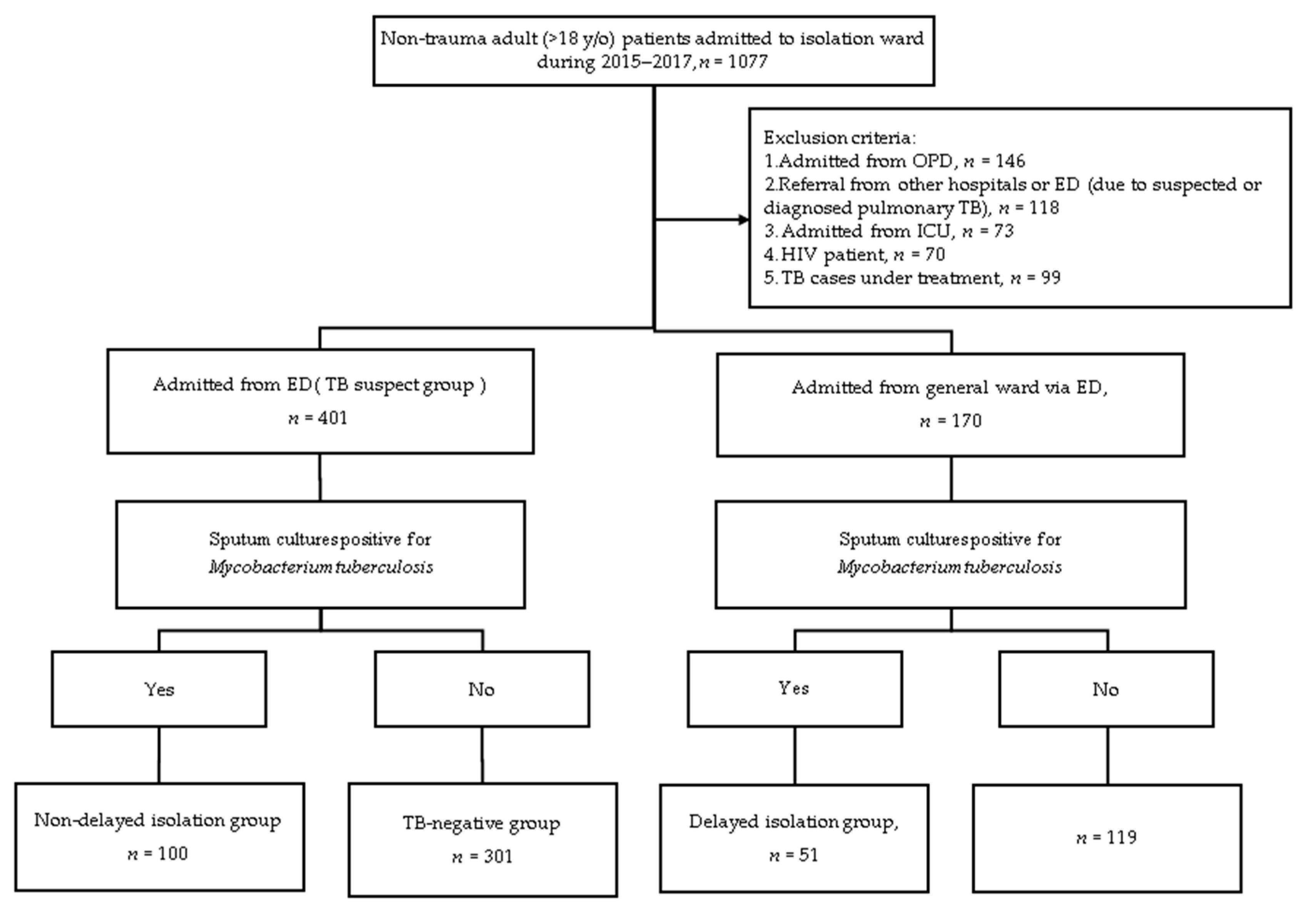
2.2. Definition of the Study Population
2.3. Data Collection and Definitions
2.4. Statistical Analysis
3. Results
3.1. Factors Associated with the Recognition of Pulmonary Tuberculosis (PTB) in the Emergency Department (ED)
3.2. Factors Associated with Delayed Isolation of PTB in the ED
3.3. Analysis of Delayed Isolation Subgroup
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Tuberculosis Report 2020; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020.
- Sokolove, P.E.; Rossman, L.; Cohen, S.H. The emergency department presentation of patients with active pulmonary tuberculosis. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2000, 7, 1056–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleton, S.C.; Connell, D.W.; Singanayagam, A.; Bradley, P.; Pan, D.; Sanderson, F.; Cleaver, B.; Rahman, A.; Kon, M.O. Evaluation of prediagnosis emergency department presentations in patients with active tuberculosis: The role of chest radiography, risk factors and symptoms. BMJ Open Respir. Res. 2017, 4, e000154. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.J.; McCabe, F.; Morgan, M.T.; Talan, D.A. Delayed recognition and infection control for tuberculosis patients in the emergency department. Ann. Emerg. Med. 1995, 26, 290–295. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Liang, H.-W.; Chiang, P.-C.; Hsiung, T.-C.; Huang, C.-C.; Chen, N.-H.; Hu, H.-C.; Tsai, Y.-H. Delayed suspicion, treatment and isolation of tuberculosis patients in pulmonology/infectious diseases and non-pulmonology/infectious diseases wards. J. Formos. Med. Assoc. Taiwan Yi Zhi 2009, 108, 202–209. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Definitions and Reporting Framework for Tuberculosis—2013 Revision. Updated December 2014 and January 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/79199/9789241505345_eng.pdf?sequence=1 (accessed on 27 December 2020).
- Davis, J.L.; Worodria, W.; Kisembo, H.; Metcalfe, J.Z.; Cattamanchi, A.; Kawooya, M.; Kyeyune, R.; Boon, S.D.; Powell, K.; Okello, R.; et al. Clinical and radiographic factors do not accurately diagnose smear-negative tuberculosis in HIV-infected inpatients in Uganda: A cross-sectional study. PLoS ONE 2010, 5, e9859. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.N.; Al-Jahdali, H.; Al-Ghanem, S.; Gouda, A. Reading chest radiographs in the critically ill (Part I): Normal chest radiographic appearance, instrumentation and complications from instrumentation. Ann. Thorac. Med. 2009, 4, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Chen, H.C.; Chong, I.W.; Hwang, J.J.; Huang, M.S. Predictors for identifying the most infectious pulmonary tuberculosis patient. J. Formosan Med Assoc. Taiwan Yi Zhi 2008, 107, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.G.; Asch, S.M.; Yu, E.I.; Knowles, L.; Gelberg, L.; Davidson, P. A population-based survey of tuberculosis symptoms: How atypical are atypical presentations? Clin. Infect. Dis. An Off. Publ. Infect. Dis. Soc. Am. 2000, 30, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K. Numerical and functional deficiency in T helper cells in protein energy malnutrition. Clin. Exp. Immunol. 1983, 51, 126–132. [Google Scholar]
- Leutscher, P.; Madsen, G.; Erlandsen, M.; Veirum, J.; Ladefoged, K.; Thomsen, V.; Wejse, C.; Hilberg, O. Demographic and clinical characteristics in relation to patient and health system delays in a tuberculosis low-incidence country. Scand. J. Infect. Dis. 2012, 44, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Tomiyama, S.; Kitagawa, I.; Tokuda, Y. Delayed isolation of smear-positive pulmonary tuberculosis patients in a Japanese acute care hospital. BMC Pulm. Med. 2018, 18, 94. [Google Scholar] [CrossRef]
- Yen, Y.L.; Chen, I.C.; Wu, C.H.; Li, W.C.; Wang, C.H.; Tsai, T.C. Factors associated with delayed recognition of pulmonary tuberculosis in emergency departments in Taiwan. Heart Lung J. Crit. Care 2015, 44, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.J.; Neoh, C.A.; Chen, C.R.; Chou, C.Y.; Wu, M.T. A high resolution computer tomography scoring system to predict culture-positive pulmonary tuberculosis in the emergency department. PLoS ONE 2014, 9, e93847. [Google Scholar] [CrossRef]
- Han, J.; Da Nam, B.; Park, S.Y.; Park, J.; Lee, E.; Lee, E.J.; Hwang, J.H.; Kim, T.H. Risk Factors for Delayed Isolation of Patients with Active Pulmonary Tuberculosis in an Acute-care Hospital. Sci. Rep. 2019, 9, 4849. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.D.; Hwang, J.H.; Park, S.Y.; Kim, T.H.; Oh, E.; Lee, E.J. Delayed Isolation of Active Pulmonary Tuberculosis in Hospitalized Patients: A Pivotal Role of Radiologic Evaluation. AJR Am. J. Roentgenol. 2020, 215, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.P.; Chakra, C.N.A.; Yansouni, C.P.; Cnossen, S.; Shrier, I.; Menzies, D.; Greenaway, C. Risk of Active Tuberculosis in Patients with Cancer: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. An Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.C.; Liao, K.M.; Chen, Y.C.; Wang, J.J.; Ho, C.H. The burdens of tuberculosis on patients with malignancy: Incidence, mortality and relapse. Sci. Rep. 2019, 9, 11901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasper, A.; Gibikote, S.; Kirupakaran, H.; Christopher, D.J.; Mathews, P. Is routine pre-entry chest radiograph necessary in a high tuberculosis prevalence country? J. Postgrad. Med. 2020, 66, 90–93. [Google Scholar] [CrossRef]
- Waitt, C.J.; Joekes, E.C.; Jesudason, N.; Waitt, P.I.; Goodson, P.; Likumbo, G.; Kampondeni, S.; Faragher, E.B.; Squire, S.B. The effect of a tuberculosis chest X-ray image reference set on non-expert reader performance. Eur. Radiol. 2013, 23, 2459–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peri, A.M.; Bernasconi, D.P.; Galizzi, N.; Matteelli, A.; Codecasa, L.; Giorgio, V.; Di Biagio, A.; Franzetti, F.; Cingolani, A.; Gori, A.; et al. Determinants of patient and health care services delays for tuberculosis diagnosis in Italy: A cross-sectional observational study. BMC Infect. Dis. 2018, 18, 690. [Google Scholar] [CrossRef]
- Kim, C.-J.; Kim, Y.; Bae, J.; Kim, A.; Kim, J.; Son, H.; Choi, H. Risk factors of delayed isolation of patients with pulmonary tuberculosis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Sosa, L.E.; Njie, G.J.; Lobato, M.N.; Morris, S.B.; Buchta, W.; Casey, M.L.; Goswami, N.D.; Gruden, M.; Hurst, B.J.; Khan, A.R.; et al. Tuberculosis Screening, Testing, and Treatment of U.S. Health Care Personnel: Recommendations from the National Tuberculosis Controllers Association and CDC, 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 439–443. [Google Scholar] [CrossRef] [PubMed]
- San, K.E.; Muhamad, M. Pulmonary Tuberculosis in HIV Infection: The Relationship of the Radiographic Appearance to CD4 T-Lymphocytes Count. Malays. J. Med Sci. MJMS 2001, 8, 34–40. [Google Scholar] [PubMed]
| Characteristics | Total (n = 401) | TB Isolated (n = 100) | non-TB Isolated (n = 301) | p-Value |
|---|---|---|---|---|
| Demographic | ||||
| Age a (years) | 66.24 ± 17.89 | 66.0 ± 17.9 | 66.3 ± 17.9 | 0.960 c |
| Male sex b | 286 (71.3) | 80 (80.0) | 206 (68.4) | 0.027 c |
| Comorbidities | ||||
| Smoker b | 192 (47.9) | 49 (49.0) | 143 (47.5) | 0.796 c |
| DM b | 143 (35.7) | 22 (22.0) | 82 (27.3) | 0.293 c |
| CKD b | 46 (11.5) | 7 (7.0) | 39 (13.0) | 0.106 c |
| ESRD b | 13 (3.2) | 2 (2.0) | 11 (3.7) | 0.532 d |
| Lung cancer b | 9 (2.2) | 2 (2.0) | 7 (2.3) | 1.000 d |
| Malignancy b | 47 (11.7) | 8 (8.0) | 39 (13.0) | 0.182 c |
| History of PTB b | 88 (21.9) | 23 (23.0) | 65 (21.6) | 0.769 c |
| Clinical symptoms | ||||
| Cough b | 296 (73.8) | 66 (66.0) | 230 (76.4) | 0.040 c |
| Expectoration b | 233 (58.1) | 50 (50.0) | 183 (60.8) | 0.058 c |
| Dyspnea b | 146 (36.4) | 24 (24.0) | 122 (40.5) | 0.003 c |
| Fever b | 180 (44.9) | 42 (42.0) | 138 (45.8) | 0.503 c |
| Chillness b | 55 (13.7) | 14 (14.0) | 41 (13.6) | 0.924 c |
| Hemoptysis b | 50 (12.5) | 8 (8.0) | 42 (14.0) | 0.119 c |
| Weight loss b | 84 (20.9) | 29 (29.0) | 55 (18.3) | 0.023 c |
| Chest pain b | 58 (14.5) | 19 (19.0) | 39 (13.0) | 0.137 c |
| Night sweats b | 5 (1.2) | 1 (1.0) | 4 (1.3) | 1.000 d |
| No symptom b | 32 (8.0) | 12 (12.0) | 20 (6.6) | 0.087 c |
| The involved field on chest X-ray (CXR) | ||||
| Right upper field b | 300 (74.8) | 68 (68.0) | 232 (77.1) | 0.070 c |
| Right middle field b | 108 (26.9) | 25 (25.0) | 83 (27.6) | 0.616 c |
| Right lower field b | 120 (29.9) | 24 (24.0) | 96 (31.9) | 0.136 c |
| Left upper field b | 194 (48.4) | 59 (59.0) | 135 (44.9) | 0.014 c |
| Left lower field b | 116 (28.9) | 31 (31.0) | 85 (28.2) | 0.598 c |
| Others | ||||
| Cavity on CXR b | 38 (9.5) | 10 (10.0) | 28 (9.3) | 0.837 c |
| Chest CT in ED b | 118 (29.4) | 26 (26.0) | 92 (30.6) | 0.386 |
| Laboratory findings | ||||
| Leukocytosis e | 176 (43.9) | 35 (35.0) | 141 (46.8) | 0.039 c |
| Elevated CRP f | 206 (51.4) | 49 (49.0) | 157 (52.2) | 0.584 c |
| Anemia g | 90 (22.4) | 19 (19.0) | 71(23.6) | 0.341 c |
| Characteristics | Adjusted OR | 95% CI for OR | p-Value |
|---|---|---|---|
| Demographic | |||
| Age | |||
| ≥65 years vs. ≤65 years | 0.85 | 0.52–1.36 | 0.472 |
| Sex | |||
| male vs. female | 1.70 | 0.96–3.01 | 0.069 |
| Clinical symptoms | |||
| Cough | |||
| yes vs. no | 0.61 | 0.36–1.02 | 0.061 |
| Dyspnea | |||
| yes vs. no | 0.52 | 0.30–0.89 | 0.017 |
| Weight loss | |||
| yes vs. no | 1.89 | 1.09–3.27 | 0.023 |
| The involved field on CXR | |||
| Left upper field | |||
| yes vs. no | 1.77 | 1.10–2.86 | 0.019 |
| Laboratory findings | |||
| Leukocytosis | |||
| yes vs. no | 0.65 | 0.40–1.06 | 0.083 |
| Characteristics | Total (n = 151) | TB Isolated (n = 100) | Delayed Isolation (n = 51) | p-Value |
|---|---|---|---|---|
| Demographic | ||||
| Age a (years) | 71 ± 17.6 | 66.0 ± 17.9 | 76.4 ± 14.6 | <0.001 c |
| Male sex b | 119 (78.8) | 80 (80.0) | 39 (76.5) | 0.616 c |
| Comorbidities | ||||
| Smoker b | 66 (43.7) | 49 (49.0) | 17 (33.3) | 0.067 c |
| DM b | 40 (26.5) | 22 (22.0) | 18 (35.3) | 0.081 c |
| CKD b | 17 (11.3) | 7 (7.0) | 10 (19.6) | 0.020 c |
| ESRD b | 5 (3.3) | 2 (2.0) | 3 (5.9) | 0.336 d |
| Lung cancer b | 3 (2.0) | 2 (2.0) | 1 (2.0) | 1.000 d |
| Malignancy b | 19 (12.6) | 8 (8.0) | 11 (21.6) | 0.017 c |
| History of PTB b | 27 (17.9) | 23 (23.0) | 4 (7.8) | 0.025 d |
| Clinical symptoms | ||||
| Cough b | 89 (58.9) | 66 (66.0) | 23 (45.1) | 0.013 c |
| Expectoration b | 70 (46.3) | 50 (50.0) | 20 (39.2) | 0.210 c |
| Dyspnea b | 44 (29.1) | 24 (24.0) | 20 (39.2) | 0.052 c |
| Fever b | 69 (45.7) | 42 (42.0) | 27 (52.9) | 0.203 c |
| Chillness b | 22 (14.6) | 14 (14.0) | 8 (15.7) | 0.781 c |
| Hemoptysis b | 11 (7.2) | 8 (8.0) | 3 (5.9) | 0.751 d |
| Weight loss b | 36 (23.8) | 29 (29.0) | 7 (13.7) | 0.037 c |
| Chest pain b | 27 (17.9) | 19 (19.0) | 8 (15.7) | 0.616 c |
| Night sweats b | 1 (0.7) | 1 (1.0) | 0 (0.0) | 1.000 d |
| No symptom b | 20 (13.2) | 12 (12.0) | 8 (15.7) | 0.528 c |
| Involved field on CXR | ||||
| Right upper field b | 88 (58.3) | 68 (68.0) | 20 (39.2) | <0.001 c |
| Right middle field b | 46 (30.5) | 25 (25.0) | 21 (41.2) | 0.041 c |
| Right lower field b | 51 (33.8) | 24 (24.0) | 27 (52.9) | <0.001 c |
| Left upper field b | 75 (49.7) | 59 (59.0) | 16 (31.4) | 0.001 c |
| Left lower field b | 48 (31.8) | 31 (31.0) | 17 (33.3) | 0.771 c |
| Others | ||||
| Cavity on CXR b | 10 (6.6) | 10 (10.0) | 0 (0.0) | 0.017 d |
| Chest CT in ED b | 33 (21.9) | 26 (26.0) | 7 (13.7) | 0.085 c |
| Laboratory findings | ||||
| Leukocytosis e | 53 (35.1) | 35 (35.0) | 18 (35.3) | 0.971 c |
| Elevated CRP f | 79 (52.3) | 49 (49.0) | 30 (58.8) | 0.254 c |
| Anemia g | 31 (20.5) | 19 (19.0) | 12 (23.5) | 0.516 c |
| Characteristics | Adjusted OR | 95% CI for OR | p-Value |
|---|---|---|---|
| Demographics | |||
| Age | |||
| ≥65 years vs. ≤65 years | 1.84 | 0.67–5.01 | 0.236 |
| Sex | |||
| male vs. female | 0.93 | 0.33–2.61 | 0.897 |
| Comorbidities | |||
| CKD | |||
| yes vs. no | 4.68 | 0.98–22.23 | 0.053 |
| Malignancy | |||
| yes vs. no | 4.49 | 1.33–15.18 | 0.016 |
| History of PTB | |||
| yes vs. no | 0.18 | 0.05–0.65 | 0.009 |
| Clinical symptoms | |||
| Cough | |||
| yes vs. no | 0.54 | 0.22–1.35 | 0.188 |
| Weight loss | |||
| yes vs. no | 0.36 | 0.12–1.12 | 0.078 |
| Involved field on CXR | |||
| Right upper field | |||
| yes vs. no | 0.19 | 0.07–0.52 | 0.001 |
| Right middle field | |||
| yes vs. no | 3.13 | 0.76–12.88 | 0.11 |
| Right lower field | |||
| yes vs. no | 1.70 | 0.47–6.19 | 0.421 |
| Left upper field | |||
| yes vs. no | 0.35 | 0.14–0.88 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-J.; Lin, C.-Y.; Huang, T.-L.; Hsu, Y.-C.; Liu, K.-T. The Accuracy of Emergency Physicians’ Suspicions of Active Pulmonary Tuberculosis. J. Clin. Med. 2021, 10, 860. https://doi.org/10.3390/jcm10040860
Chen S-J, Lin C-Y, Huang T-L, Hsu Y-C, Liu K-T. The Accuracy of Emergency Physicians’ Suspicions of Active Pulmonary Tuberculosis. Journal of Clinical Medicine. 2021; 10(4):860. https://doi.org/10.3390/jcm10040860
Chicago/Turabian StyleChen, Shiang-Jin, Chun-Yu Lin, Tzu-Ling Huang, Ying-Chi Hsu, and Kuan-Ting Liu. 2021. "The Accuracy of Emergency Physicians’ Suspicions of Active Pulmonary Tuberculosis" Journal of Clinical Medicine 10, no. 4: 860. https://doi.org/10.3390/jcm10040860
APA StyleChen, S.-J., Lin, C.-Y., Huang, T.-L., Hsu, Y.-C., & Liu, K.-T. (2021). The Accuracy of Emergency Physicians’ Suspicions of Active Pulmonary Tuberculosis. Journal of Clinical Medicine, 10(4), 860. https://doi.org/10.3390/jcm10040860






