Early Diagnostics and Early Intervention in Neurodevelopmental Disorders—Age-Dependent Challenges and Opportunities
Abstract
1. Introduction
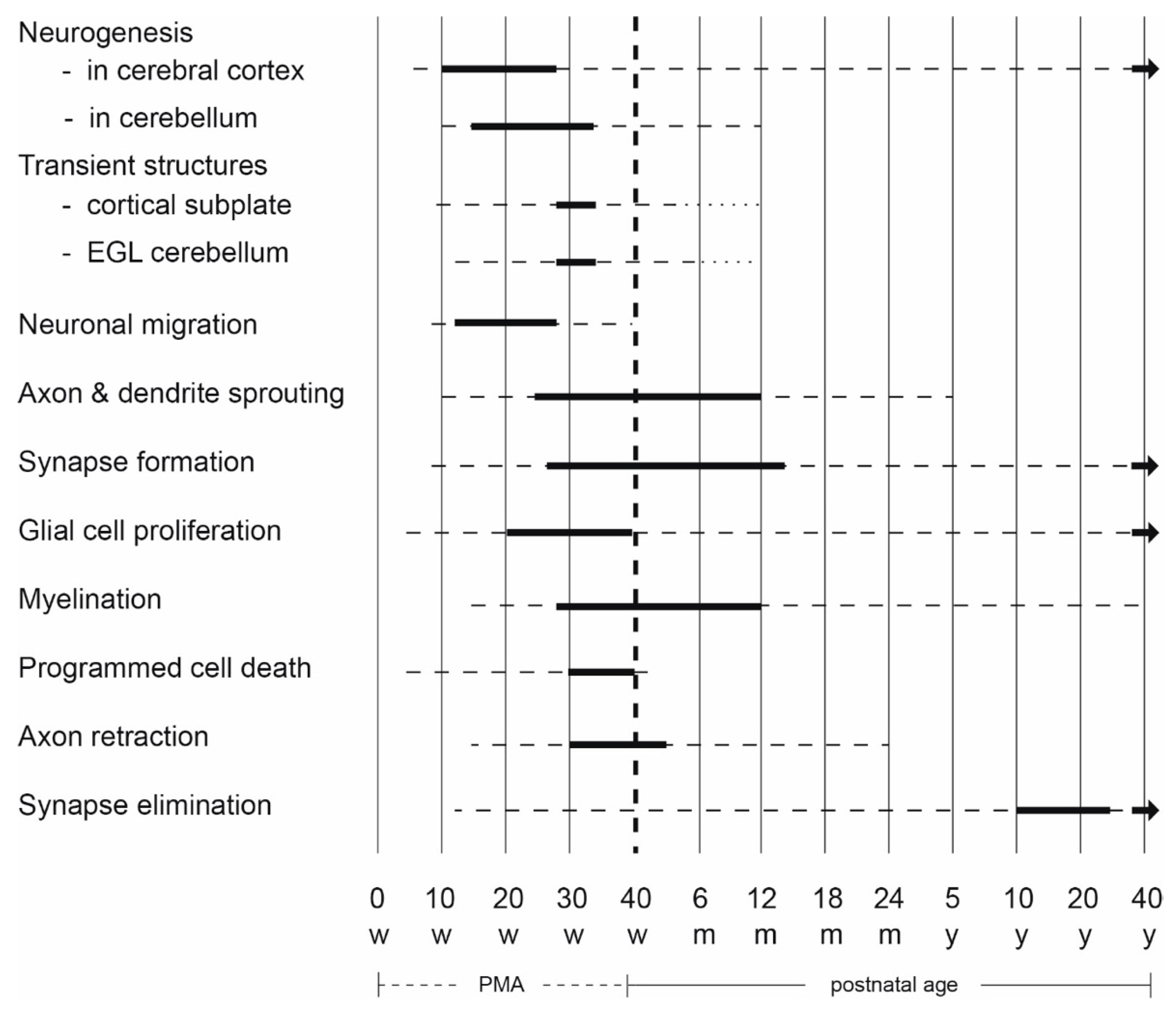
2. Early Human Brain Development
3. Early Detection of Developmental Disorders
3.1. Diagnostic Implications of the Developing Brain
3.2. Neuroimaging
3.3. Clinical Assessments
3.3.1. Neurological Assessments
3.3.2. Motor Assessments
3.3.3. Developmental Assessments
3.3.4. Assessments Aiming at the Early Detection of ASD
3.4. Summary: Early Diagnostics and the Developing Brain
4. Early Intervention
4.1. Intervention in Infants Admitted to the Neonatal Intensive Care Unit
4.2. Early Intervention in Infants at Low to Moderate Risk of CP and Intellectual Disability
4.3. Early Intervention in Infants with or at (Very) High Risk of CP and Intellectual Disability
4.4. Early Intervention in Infants with or at High Risk of ASD
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sellier, E.; McIntyre, S.; Smithers-Sheedy, H.; Platt, M.J.; SCPE and ACPR Groups. European and Australian cerebral palsy surveillance networks working together for collaborative research. Neuropediatrics 2020, 51, 105–112. [Google Scholar] [CrossRef]
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics 2019, 144, e20190811. [Google Scholar] [CrossRef]
- McGuire, D.O.; Tian, L.H.; Yeargin-Allsopp, M.; Dowling, N.F.; Christensen, D.L. Prevalence of cerebral palsy, intellectual disability, hearing loss, and blindness, National Health Interview Survey, 2009–2016. Disabil. Health J. 2019, 12, 443–451. [Google Scholar] [CrossRef]
- Maulik, P.K.; Mascarenhas, M.N.; Mathers, C.D.; Dua, T.; Saxena, S. Prevalence of intellectual disability: A meta-analysis of population-based studies. Res. Dev. Disabil. 2011, 32, 419–436. [Google Scholar] [CrossRef]
- Delobel-Ayoub, M.; Saemundsen, E.; Gissler, M.; Ego, A.; Moilanen, I.; Ebeling, H.; Rafnsson, V.; Klapouszczak, D.; Thorsteinsson, E.; Arnaldsdóttir, K.M.; et al. prevalence of Autism Spectrum Disorder in 7–9-year-old children in Denmark, Finland, France and Iceland: A population-based registries approach within the ASDEU Project. J. Autism. Dev. Disord. 2020, 50, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.C.; Tseng, Y.C.; Guo, H.R. Trends in the prevalence of childhood disability: Analysis of data from the national disability registry of Taiwan, 2000–2011. Res. Dev. Disabil. 2013, 34, 3766–3772. [Google Scholar] [CrossRef]
- Global Burden of Disease Child and Adolescent Health Collaboration; Kassebaum, N.; Kyu, H.H.; Zoeckler, L.; Olsen, H.E.; Thomas, K.; Pinho, C.; Bhutta, Z.A.; Dandona, L.; Ferrari, A.; et al. Child and adolescent health from 1990- to 2015: Findings from the global burden of diseases, injuries and risk factors 2015 study. JAMA Pediatr. 2017, 171, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Kakooza-Mwesige, A.; Andrews, C.; Peterson, S.; Wabwire Mangen, F.; Eliasson, A.C.; Forssberg, H. Prevalence of cerebral palsy in Uganda: A population-based study. Lancet Glob. Health 2017, 5, e1275–e1282. [Google Scholar] [CrossRef]
- Khandaker, G.; Muhitm, M.; Karim, T.; Smithers-Sheedy, H.; Novak, I.; Jones, C.; Badawi, N. Epidemiology of cerebral palsy in Bangladesh: A population-based surveillance study. Dev. Med. Child. Neurol. 2019, 61, 601–609. [Google Scholar] [CrossRef]
- India State-Level Disease Burden Initiative Mental Disorders Collaborators. The burden of mental disorders across the states of India: The Global Burden of Disease Study 1990–2017. Lancet Psychiatry 2020, 7, 148–161. [Google Scholar] [CrossRef]
- Franki, I.; Mailleux, L.; Emsell, L.; Peedima, M.L.; Fehrenbach, A.; Feys, H.; Ortibus, E. The relationship between neuroimaging and motor outcome in children with cerebral palsy: A systematic review–Part A, Structural imaging. Res. Dev. Disabil. 2020, 100, 103606. [Google Scholar] [CrossRef]
- Key, P.F.; Thornton-Wells, T.A. Brain-based methods in the study of developmental disabilities: Examples from event-related potentials and magnetic resonance imaging research. In The Oxford Handbook of Intellectual Disability and Development; Burack, J.A., Hodapp, R.M., Iarocci, G., Zigler, E., Eds.; Oxford Handbooks Online: Oxford, UK, 2011. [Google Scholar] [CrossRef]
- Ecker, C. The neuroanatomy of autism spectrum disorder. An overview of structural neuroimaging findings and their translatability to the clinical setting. Autism 2017, 21, 18–28. [Google Scholar] [CrossRef]
- Mailleux, L.; Franki, I.; Emsell, L.; Peedima, M.L.; Fehrenbach, A.; Feys, H.; Ortibus, E. The relationship between neuroimaging and motor outcome in children with cerebral palsy: A systematic review–Part B, Diffusion imaging and tractography. Res. Dev. Disabil. 2020, 97, 103569. [Google Scholar] [CrossRef]
- Sato, W.; Uono, S. The atypical social brain network in autism: Advances in structural and functional MRI studies. Curr. Opin. Neurol. 2019, 32, 617–621. [Google Scholar] [CrossRef]
- Hadders-Algra, M. (Ed.) Early Detection and Early Intervention in Developmental Motor Disorders–from Neuroscience to Participation; Series: Clinics in Developmental Medicine; Mac Keith Press: London, UK, 2021; in press. [Google Scholar]
- Hadders-Algra, M. Early human brain development: Starring the subplate. Neurosci. Biobehav. Rev. 2018, 92, 276–290. [Google Scholar] [CrossRef]
- Bystron, I.; Blakemore, C.; Rakic, P. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 2008, 9, 110–122. [Google Scholar] [CrossRef]
- Lui, J.H.; Hansen, D.V.; Kriegstein, A.R. Development and evolution of the human neocortex. Cell 2011, 146, 18–36. [Google Scholar] [CrossRef]
- Kostović, I.; Sedmak, G.; Vukšić, M.; Judaš, M. The relevance of human fetal subplate zone for developmental neuropathology of neuronal migration disorders and cortical dysplasia. CNS Neurosci. Ther. 2015, 21, 74–82. [Google Scholar] [CrossRef]
- Hoerder-Suabedissen, A.; Molnár, Z. Development, evolution and pathology of neocortical subplate neurons. Nat. Rev. Neurosci. 2015, 16, 133–146. [Google Scholar] [CrossRef]
- Trivedi, R.; Gupta, R.K.; Husain, N.; Rathore, R.K.; Saksena, S.; Srivastava, S.; Malik, G.K.; Das, V.; Pradhan, M.; Sarma, M.K.; et al. Region-specific maturation of cerebral cortex in human fetal brain: Diffusion tensor imaging and histology. Neuroradiology 2009, 51, 567–576. [Google Scholar] [CrossRef]
- Molnár, Z.; Clowry, G.J.; Šestan, N.; Alzu’bi, A.; Bakken, T.; Hevner, R.F.; Hüppi, P.S.; Kostović, I.; Rakic, P.; Anton, E.S.; et al. New insights into the development of the humen cerebral cortex. J. Anat. 2019, 235, 432–451. [Google Scholar] [CrossRef]
- Judaš, M.; Sedmak, G.; Kostović, I. The significance of the subplate for evolution and developmental plasticity of the human brain. Front. Hum. Neurosci. 2013, 7, 423. [Google Scholar] [CrossRef]
- Kostović, I.; Sedmak, G.; Judaš, M. Neural histology and neurogenesis of the human fetal and infant brain. Neuroimage 2019, 188, 743–773. [Google Scholar] [CrossRef] [PubMed]
- Kostović, I.; Kostović-Srzentić, M.; Benjak, V.; Jovanov-Milošević, N.; Radoš, M. Developmental dynamics of radial vulnerability in the cerebral compartments in preterm infants and neonates. Front. Neurol. 2014, 295, 139. [Google Scholar] [CrossRef]
- Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Yakovlev, P.L.; Lecours, A.R. The myelogenetic cycles of regional maturation of the brain. In Regional Development of the Brain in Early Life; Minkowski, A., Ed.; Blackwell: Oxford, UK, 1967; pp. 3–70. [Google Scholar]
- Haynes, R.L.; Borenstein, N.S.; Desilva, T.M.; Folkert, R.D.; Liu, L.G.; Volpe, J.J.; Kinney, H.C. Axoaml development in the cerebral white matter of the human fetus and infant. J. Comp. Neurol. 2005, 484, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Walker, L.; Leemans, A.; Phillips, L.; Beaulieu, C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 2008, 40, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Lossi, L.; Merighi, A. In vivo cellular and molecular mechanisms of neuronal apoptosis in the mammalian CNS. Prog. Neurobiol. 2003, 69, 287–312. [Google Scholar] [CrossRef]
- Kostović, I.; Jovanov-Milosević, N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin. Fetal Neonatal. Med. 2006, 11, 415–422. [Google Scholar] [CrossRef]
- Eyre, J.A. Corticospinal tract development and its plasticity after perinatal injury. Neurosci. Biobehav. Rev. 2007, 31, 1136–1149. [Google Scholar] [CrossRef]
- Williams, P.T.J.A.; Jiang, Y.-Q.; Martin, J.H. Motor system plasticity after unilateral injury in the developing brain. Dev. Med. Child. Neurol. 2017, 59, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Petanjek, Z.; Judaš, M.; Šimic, G.; Rasin, M.R.; Uylings, H.B.; Rakic, P.; Kostovic, I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 13281–13286. [Google Scholar] [CrossRef]
- Herlenius, E.; Lagercrantz, H. Neurotransmitters and neuromodulators, In The Newborn Brain. Neuroscience and Clinical Applications, 2nd ed.; Lagercrantz, H., Hanson, M.A., Ment, L.R., Peebles, D.M., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 99–117. [Google Scholar]
- Ben-Ari, Y.; Spitzer, N.C. Phenotypic checkpoints regulate neuronal development. Trends Neurosci. 2010, 33, 485–492. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Neural substrate and clinical significance of general movements: An update. Dev. Med. Child Neurol. 2018, 60, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P.; Sidman, R.L. Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J. Comp. Neurol. 1970, 139, 473–500. [Google Scholar] [CrossRef]
- Volpe, J.J. Cerebellum of the premature infant: Rapidly developing, vulnerable, clinically important. J. Child Neurol. 2009, 24, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Q.; Wei, H.; Dong, W.; Fan, Y.; Hua, Z. Prognostic value of clinical tests in neonates with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia: A systematic review and meta-analysis. Front. Neurol. 2020, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, S.; Smidt, L.C.A.; Dudink, J.; Benders, M.J.N.L.; de Vries, L.S.; Groenendaal, F.; van der Aa, N.E. Predictors of outcomes in hypoxic-ischemic encephalopathy following hypothermia: A meta-analysis. Neonatology 2020, 117, 422–427. [Google Scholar] [CrossRef]
- Niutanen, U.; Harra, T.; Lano, A.; Metsäranta, M. Systematic review of sensory processing in preterm children reveals abnormal sensory modulation, somatosensory processing and sensory-based motor processing. Acta Paediatr. 2020, 109, 45–55. [Google Scholar] [CrossRef]
- Eeles, A.L.; Spittle, A.J.; Anderson, P.J.; Brown, N.; Lee, K.J.; Boyd, R.N.; Doyle, L.W. Assessments of sensory processing in infants: A systematic revies. Dev. Med. Child Neurol. 2013, 55, 314–326. [Google Scholar] [CrossRef]
- Dubowitz, L.; Dubowitz, V.; Mercuri, E. The Neurological Assessment of the Preterm and Full Term Infant, 2nd ed.; Mac Keith Press: London, UK, 1999; pp. 1–167. [Google Scholar]
- Romeo, D.M.; Ricci, D.; Brogna, C.; Mercuri, E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: A critical review of the literature. Dev. Med. Child Neurol. 2016, 58, 240–245. [Google Scholar] [CrossRef]
- Richards, J.E.; Xie, W. Brains for all the ages: Structural neurodevelopment in infants and children from a life-span perspective. Adv. Child Dev. Behav. 2015, 48, 1–52. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Two distinct forms of minor neurological dysfunction: Perspectives emerging from a review of data of the Groningen Perinatal Project. Dev. Med. Child Neurol. 2002, 44, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, H.; Dijk-Stigter, G.R.; Grooten, H.M.; Janssen-Plas, F.E.; Koopmans, A.J.; Mulder, C.D.; van Belle, A.; Hadders-Algra, M. Predictive value of definitely abnormal general movements in the general population. Dev. Med. Child Neurol. 2010, 52, 456–461. [Google Scholar] [CrossRef]
- Ozonoff, S.; Iosif, A.M.; Baguio, F.; Cook, I.C.; Hill, M.M.; Hutman, T.; Rogers, S.J.; Rozga, A.; Sangha, S.; Sigman, M.; et al. A prospective study of the emergence of early behavioral signs of autism. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, e1–e2. [Google Scholar]
- Bosanquet, M.; Copeland, L.; Ware, R.; Boyd, R. A systematic review of tests to predict cerebral palsy in young children. Dev. Med. Child Neurol. 2013, 55, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Hielkema, T.; Hadders-Algra, M. Motor and cognitive outcome after specific early lesions of the brain—A systematic review. Dev. Med. Child Neurol. 2016, 58 (Suppl. 4), 46–52. [Google Scholar] [CrossRef]
- Fluss, J.; Dinomais, M.; Chabrier, S. Perinatal stroke syndromes: Similarities and diversities in aetiology, outcome and management. Eur. J. Paediatr. Neurol. 2019, 23, 368–383. [Google Scholar] [CrossRef]
- Cioni, G.; Bos, A.F.; Einspieler, C.; Ferrari, F.; Martijn, A.; Paolicelli, P.B.; Rapisardi, G.; Roversi, M.F.; Prechtl, H.F. Early neurological signs in preterm infants with unilateral introparenchymal echodensity. Neuropediatrics 2000, 31, 240–251. [Google Scholar] [CrossRef]
- Sakzewski, L.; Sicola, E.; Verhage, C.H.; Sgandurra, G.; Eliasson, A.C. Development of hand function during the first year of life in children with unilateral cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.J.; Hadders-Algra, M. Assessments in the neonatal period and early infancy. In Early Detection and Early Intervention in Developmental Disorders–From Neuroscience to Participation; Hadders-Algra, M., Ed.; Mac Keith Press: London, UK, 2021; pp. 124–143. [Google Scholar]
- Benders, M.J.N.L.; Kersbergen, K.J.; de Vries, L.S. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. Clin. Perinatol. 2014, 41, 69–82. [Google Scholar] [CrossRef]
- Kwon, S.H.; Vasung, L.; Ment, L.R.; Huppi, P.S. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin. Perinatol. 2014, 41, 257–283. [Google Scholar] [CrossRef]
- Annink, K.V.; de Vries, L.S.; Groenendaal, F.; Vijlbrief, D.C.; Weeke, L.C.; Roehr, C.C.; Lequin, M.; Reiss, I.; Govaert, P.; Benders, M.J.N.L.; et al. The development and validation of a cerebral ultrasound scoring system for infants with hypoxic-ischaemic encephalopathy. Pediatr. Res. 2020, 87 (Suppl. 1), 59–66. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.S.; Benders, M.J.; Groenendaal, F. Imaging the premature brain: Ultrasound of MRI? Neuroradiology 2013, 55 (Suppl. 2), 13–22. [Google Scholar] [CrossRef]
- Thayyil, S.; Chandrasekaran, M.; Taylor, A.; Bainbridge, A.; Cady, E.B.; Chong, W.K.; Murad, S.; Omar, R.Z.; Robertson, N.J. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: A meta-analysis. Pediatrics 2010, 125, e382–e395. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Fernández, I.; Morales-Quezada, J.L.; Law, S.; Kim, P. Prognostic value of brain magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: A meta-analysis. J. Child Neurol. 2017, 32, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hooft, J.; van der Lee, J.H.; Opmeer, B.C.; Aarnoudse-Moens, C.S.; Leenders, A.G.; Mol, B.W.; de Haan, T.R. Predicting developmental outcomes in premature infants by term equivalent MRI: Systematic review and meta-analysis. Syst. Rev. 2015, 4, 71. [Google Scholar] [CrossRef]
- Rath, C.P.; Desai, S.; Rao, S.C.; Patole, S. Diffuse excessive high signal intensity on term equivalent MRI does not predict disability: A systematic review and meta-analysis. Arch. Dis. Child Fetal. Neonatal. Ed. 2021, 106, 9–16. [Google Scholar] [CrossRef]
- George, J.M.; Pannek, K.; Rose, S.E.; Ware, R.S.; Colditz, P.B.; Boyd, R.N. Diagnostic accuracy of early magnetic resonance imaging to determine motor outcomes in infants born preterm: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Alison, M.; Counsell, S.J.; Hertz-Pannier, L.; Hüppi, P.S.; Benders, M.J.N.L. MRI of the neonatal brain: A review of methodological challenges and neuroscientific advances. J. Magn. Reson. Imaging 2020. [Google Scholar] [CrossRef]
- Parikh, N.A. Advanced neuroimaging and its role in predicting neurodevelopmental outcomes in very preterm infants. Semin. Perinatol. 2016, 40, 530–541. [Google Scholar] [CrossRef]
- Parikh, N.A.; Hershey, A.; Altaye, M. Early detection of cerebral palsy using sensorimotor tract biomarkers in very preterm infants. Pediatr. Neurol. 2019, 98, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Biarge, M.; Groenendaal, F.; Kersbergen, K.J.; Benders, M.J.; Foti, F.; Cowan, F.M.; de Vries, L.S. MRI based preterm white matter injury classification: The importance of sequential imaging in determining severity of injury. PLoS ONE 2016, 11, e0156245. [Google Scholar] [CrossRef] [PubMed]
- Prechtl, H.F.R. The Neurological Examination of the Full Term Newborn, 2nd ed.; Spastic Society with Heinemann Medical: London, UK, 1977. [Google Scholar]
- Gosselin, J.; Amiel-Tison, C. Neurological Development from Birth to 6, 2nd ed.; Éditions du CHU Sainte-Justine: Montreal, QC, Canada, 2011. [Google Scholar]
- Raichle, M.E. The restless brain: How intrinsic activity organizes brain function, Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140172. [Google Scholar] [CrossRef]
- Prechtl, H.F. Qualitative changes of spontaneous movements in fetus and preterm infant are a marker of neurological dysfunction. Early Hum. Dev. 1990, 23, 151–158. [Google Scholar] [CrossRef]
- Touwen, B.C. Variability and stereotypy of spontaneous motility as a predictor of neurological development in preterm infants. Dev. Med. Child Neurol. 1990, 32, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hadders-Algra, M.; Tacke, U.; Pietz, J.; Rupp, A.; Philippi, H. Reliability and predictive validity of the Standardized Infant NeuroDevelopmental Assessment neurological scale. Dev. Med. Child Neurol. 2019, 61, 654–660. [Google Scholar] [CrossRef]
- Spittle, A.J.; Walsh, J.M.; Potter, C.; Mcinnes, E.; Olsen, J.E.; Lee, K.J.; Anderson, P.J.; Doyle, L.W.; Cheong, J.L. Neurobehaviour at term-equivalent age and neurodevelopmental outcomes at 2 years in infants born moderate-to-late preterm. Dev. Med. Child Neurol. 2017, 59, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lawford, H.L.S.; Nuamah, M.A.; Liley, H.G.; Lee, A.C.; Kumar, S.; Adjei, A.A.; Bora, S. IMPRINT Study Group. Neonatal neurological examination in a resource-limited setting: What defines normal? Eur. J. Paediatr. Neurol. 2020, 29, 71–80. [Google Scholar] [CrossRef]
- Venkata, S.K.R.G.; Pournami, F.; Prabhakar, J.; Nandakumar, A.; Jain, N. Disability prediction by early Hammersmith Neonatal Neurological Examination: A diagnostic study. J. Child Neurol. 2020, 35, 731–736. [Google Scholar] [CrossRef]
- Setänen, S.; Lahti, K.; Lehtonen, L.; Parkkola, R.; Maunu, J.; Saarinen, K.; Haataja, L. Neurological examination combined with brain MRI or cranial US improves prediction of neurological outcome in preterm infants. Early Hum. Dev. 2014, 90, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Guzzetta, A.; Haataja, L.; Cowan, F.; Rutherford, M.; Counsell, S.; Papadimitriou, M.; Cioni, G.; Dubowitz, L. Neonatal neurological examination in infants with hypoxic ischaemic encephalopathy: Correlation with MRI findings. Neuropediatrics 1999, 30, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Majnemer, A.; Snider, L.; Hadders-Algra, M. Assessments of infants and toddlers. In Early Detection and Early Intervention in Developmental Disorders–from Neuroscience to Participation; Hadders-Algra, M., Ed.; Mac Keith Press: London, UK, 2021; pp. 144–170. [Google Scholar]
- Roth, S.; Wyatt, J.; Baudin, J.; Townsend, J.; Rifkin, L.; Rushe, T.; Amiel-Tison, C.; Stewart, A.L. Neurodevelopmental status at 1 year predicts neuropsychiatric oucome at 14-15 years of age in very preterm infants. Early Hum. Dev. 2001, 65, 81–89. [Google Scholar] [CrossRef]
- Kodric, J.; Sustersic, B.; Paro-Panjan, D. Relationship between neurological assessment of preterm infants in the first 2 years and cognitive outcome at school age. Pediatr. Neurol. 2014, 51, 681–687. [Google Scholar] [CrossRef]
- Harmon, H.M.; Taylor, H.G.; Minich, N.; Wilson-Costello, D.; Hack, M. Early school outcomes for extremely preterm infants with transient neurological abnormalities. Dev. Med. Child Neurol. 2015, 57, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Romeo, D.M.; Cowan, F.M.; Haataja, L.; Ricci, D.; Pede, E.; Gallini, F.; Cota, F.; Brogna, C.; Vento, G.; Romeo, M.G.; et al. Hammersmith Infant Neurological Examination for infants born preterm: Predicting outcomes other than cerebral palsy. Dev. Med. Child Neurol. 2020. [Google Scholar] [CrossRef]
- Hay, K.; Nelinl, M.; Careyl, H.; Chornal, O.; Moore-Clingenpeel, M.; Mas, M.; Maitre, N. NCH Early Developmental Group. Hammersmit Infant Neurological Examination Asymmetry Score distinguishes cerebral palsy from typical development. Pediatr. Neurol. 2018, 87, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Hadders-Algra, M.; Tacke, U.; Pietz, J.; Rupp, A.; Philippi, H. Standardized Infant NeuroDevelopmental Assessment developmental and socio-emotional scales: Reliability and predictive value in an at-risk population. Dev. Med. Child Neurol. 2020, 62, 845–853. [Google Scholar] [CrossRef]
- Piper, M.C.; Darrah, J. Motor Assessment of the Developing Infant; W.B. Saunders Company: Philadelphia, PA, USA, 1994. [Google Scholar]
- Bayley, N. Bayley Scales of Infant and Toddler Development, 3rd Edition: Administration Manual and Technical Manual; Harcourt Assessment: San Antonio, TX, USA, 2006. [Google Scholar]
- Einspieler, C.; Prechtl, H.F.; Bos, A.F.; Ferrari, F.; Cioni, G. Prechtl’s Method on the Qualitative Assessment of General Movements in Preterm, Term and Young Infants; Mac Keith Press: London, UK, 2004. [Google Scholar]
- Campbell, S.K.; Kolobe, T.H.; Osten, E.T.; Lenke, M.; Girolami, G.L. Construct validity of the test of infant motor performance. Phys. Ther. 1995, 75, 585–596. [Google Scholar] [CrossRef]
- Hadders-Algra, M.; Heineman, K.R. The Infant Motor Profile; Routledge: Abingdon, UK, 2021. [Google Scholar]
- Hielkema, T.; Blauw-Hospers, C.H.; Dirks, T.; Drijver-Messelink, M.; Bos, A.F.; Hadders-Algra, M. Does physiotherapeutic intervention affect motor outcome in high-risk infants? An approach combining a randomized controlled trial and process evaluation. Dev. Med. Child Neurol. 2011, 53, e8–e15. [Google Scholar] [CrossRef]
- Sgandurra, G.; Bartalena, L.; Cecchi, F.; Cioni, G.; Giampietri, M.; Greisen, G.; Herskind, A.; Inguaggiato, E.; Lorentzen, J.; Nielsen, J.B.; et al. A pilot study on early home-based intervention through an intelligent baby gym (CareToy) in preterm infants. Res. Dev. Disabil. 2016, 53–54, 32–42. [Google Scholar] [CrossRef]
- Sgandurra, G.; Lorentzen, J.; Inguaggiato, E.; Bartalena, L.; Beani, E.; Cecchi, F.; Dario, P.; Giampietri, M.; Greisen, G.; Herskind, A.; et al. A randomized clinical trial in preterm infants on the effects of a home-based early intervention with the ‘Care-Toy System’. PLoS ONE 2017, 12, e0173521. [Google Scholar] [CrossRef]
- Akhbari Ziegler, S.; von Rhein, M.; Meichtry, A.; Wirz, M.; Hielkema, T.; Hadders-Algra, M.; Swiss Neonatal Network & Follow-Up Group. The Coping with and Caring for Infants with Special Needs intervention was associated with improved motor development in preterm infants. Acta Paediatr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Sigafoos, J.; Bölte, S.; Bratl-Pokorny, K.D.; Landa, R.; Marschik, P.B. Highlighting the first 5 months of life: General movements in infants later diagnosed with autism spectrum disorder or Rett Syndrome. Res. Autism. Spectr. Disord. 2014, 8, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Støen, R.; Songstad, N.T.; Silberg, I.E.; Fjørtoft, T.; Jensenius, A.R.; Adde, L. Computer-based video analysis identifies infants with absence of fidgety movements. Pediatr. Res. 2017, 82, 665–670. [Google Scholar] [CrossRef]
- Marschik, P.B.; Pokorny, F.B.; Peharz, R.; Zhang, D.; O’Muircheartaigh, J.; Roeyers, H.; Bölte, S.; Spittle, A.J.; Urlesberger, B.; Schuller, B.; et al. A novel way to measure and predict development: A heuristic approach to facilitate early detection of neurodevelopmental disorders. Curr. Neurol. Neurosci. Rep. 2017, 17, 43. [Google Scholar] [CrossRef]
- Marchi, V.; Hakala, A.; Knight, A.; D’Acunto, F.; Scattoni, M.L.; Guzzetta, A.; Vanhatalo, S. Automated pose estimation captures key aspects of General Movements at eight to 17 weeks from conventional videos. Acta Paediatr. 2019, 108, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.S.; Hesse, N.; Weinberger, R.; Tacke, U.; Gerstl, L.; Hilgendorff, A.; Heinen, F.; Arens, M.; Dijkstra, L.J.; Pujades Rocamora, S.; et al. General Movement Assessment from videos of computed 3D infant body models is equally effective compared to conventional RGB video rating. Early Hum. Dev. 2020. [Google Scholar] [CrossRef]
- Kim, S.A.; Lee, Y.J.; Lee, Y.G. Predictive value of Test of Infant Motor Performance for infants based on correlation between TIMP and Bayly Scales of Infant Development. Ann. Rehabil. Med. 2011, 35, 860–866. [Google Scholar] [CrossRef]
- Campbell, S.K.; Kolobe, T.H.; Wright, B.D.; Linacre, J.M. Validity of the Test of Infant Motor Performance for prediction of 6-, 9- and 12-months scores on the Albert Infant Motor Scale. Dev. Med. Child Neurol. 2002, 44, 263–272. [Google Scholar] [CrossRef]
- Nuysink, J.; van Haastert, I.C.; Eijsermans, M.J.; Koopman-Esseboom, C.; Helders, P.J.; de Vries, L.S.; van der Net, J. Prediction of gross motor development an independent walking in infants born very preterm using the Test of Infant Motor Performance and the Alberta Infant Motor Scale. Early Hum. Dev. 2013, 89, 693–697. [Google Scholar] [CrossRef]
- Peyton, C.; Schreiber, M.D.; Msall, M.E. The Test of Infant Motor Performance at 3 months predicts language, cognitive, and motor outcomes in infants born preterm at 2 years of age. Dev. Med. Child Neurol. 2018, 60, 1239–1243. [Google Scholar] [CrossRef]
- Kolobe, T.H.; Bulanda, M.; Susman, L. Predicting motor outcome at preschool age for infants tested at 7, 30, 60, and 90 days after term age using the Test of Infant Motor Performance. Phys. Ther. 2004, 84, 1144–1156. [Google Scholar] [CrossRef]
- Heineman, K.R.; Bos, A.F.; Hadders-Algra, M. The Infant Motor Profile: A standardized and qualitative method to assess motor behaviour in infancy. Dev. Med. Child Neurol. 2008, 50, 275–282. [Google Scholar] [CrossRef]
- Heineman, K.R.; Bos, A.F.; Hadders-Algra, M. Infant Motor Profile and cerebral palsy: Promising associations. Dev. Med. Child Neurol. 2011, 53 (Suppl. 4), 40–45. [Google Scholar] [CrossRef]
- Rizzi, R.; Menici, V.; Cioni, M.L.; Cecchi, A.; Barzacchi, V.; Beani, E.; Giampietri, M.; Cioni, G.; Sgandurra, G.; Clinical CareToy-R Consortium. Concurrent and predictive validity of the infant motor profile in infants at risk of neurodevelopmental disorders. BMC Pediatr. 2021, 21, 68. [Google Scholar] [CrossRef]
- Heineman, K.R.; Schendelaar, P.; Van den Heuvel, E.R.; Hadders-Algra, M. Motor development in infancy is related to cognitive function at 4 years of age. Dev. Med. Child Neurol. 2018, 60, 1149–1155. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Heineman, K.R.; la Bastide-van Gemert, S.; Kuiper, D.; Drenth Olivares, M.; Hadders-Algra, M. Motor behaviour in infancy is associated with cognitive, neurological and behavioural function in 9-year-old children born to parents with reduced fertility. Dev. Med. Child Neurol. 2020, 62, 1089–1095. [Google Scholar] [CrossRef]
- Green, E.; Stroud, L.; O’Connell, R.; Bloomfield, S.; Cronje, J.; Foxcroft, C.; Hurter, K.; Lane, H.; Marais, R.; Marx, C.; et al. Griffiths Scales of Child. Development, 3rd ed.; Hogrefe: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Mullen, E.M. Mullen Scales of Early Learning; American Guidance Service: Circle Pines, MN, USA, 1995. [Google Scholar]
- Luttikhuizen dos Santos, E.S.; de Kieviet, J.F.; Königs, M.; van Elburg, R.M.; Oosterlaan, J. Predictive value of the Bayley scales of infant development on development of very preterm/very low birth weight children: A meta-analysis. Early Hum. Dev. 2013, 89, 487–496. [Google Scholar] [CrossRef]
- Griffiths, A.; Toovey, R.; Morgan, P.E.; Spittle, A.J. Psychometric properties of gross motor assessment tools for children: Systematic review. BMJ Open 2018, 8, e021734. [Google Scholar] [CrossRef]
- Maitre, N.L.; Slaughter, J.C.; Aschner, J.L. Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum. Dev. 2013, 89, 781–786. [Google Scholar] [CrossRef]
- Jones, E.J.; Gliga, T.; Bedford, R.; Charman, T.; Johnson, M.H. Developmental pathways to autism: A review of prospective studies of infants at risk. Neurosci. Biobehav. Rev. 2014, 39, 1–33. [Google Scholar] [CrossRef]
- Ozonoff, S.; Iosif, A.M. Changing conceptualizations of regression: What prospective studies reveal about the onset of autism spectrum disorder. Neurosci. Biobehav. Rev. 2019, 100, 296–304. [Google Scholar] [CrossRef]
- Wan, M.W.; Green, J.; Scott, J. A systematic review of parent-infant interaction in infants at risk of autism. Autism 2019, 23, 811–820. [Google Scholar] [CrossRef]
- Tanner, A.; Dounavi, K. The emergence of autism symptoms prior to 18 months of age: A systematic literature review. J. Autism Dev. Disord. 2020. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Penner, M. Autism spectrum disorder: Advances in diagnosis and evaluation. BMJ 2018, 361, k1674. [Google Scholar] [CrossRef]
- Robins, D.L.; Fein, D.; Barton, M.L.; Green, J.A. The Modified Checklist for Autism in Toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. J. Autism. Dev. Disord. 2001, 31, 131–144. [Google Scholar] [CrossRef]
- Yuen, T.; Penner, M.; Carter, M.T.; Szatmari, P.; Ungar, W.J. Assessing the accuracy of the Modified Checklist for Autism in Toddlers: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 1093–1100. [Google Scholar] [CrossRef]
- Robins, D.L. Screening for autism spectrum disorders in primary care settings. Autism 2008, 12, 537–556. [Google Scholar] [CrossRef]
- Bradbury, K.; Robins, D.L.; Barton, M.; Ibañez, L.V.; Stone, W.L.; Warren, Z.E.; Fein, D. Screening or autism spectrum disorder in high-risk younger siblings. J. Dev. Behav. Pediatr. 2020, 41, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, W.; Wallis, K.; Bennett, A.; Brooks, E.; Dudley, J.; Gerdes, M.; Pandey, J.; Levy, S.E.; Schultz, R.T.; Miller, J.S. Accuracy of autism screening in a large pediatric network. Pediatrics 2019, 144, e20183963. [Google Scholar] [CrossRef]
- Carbone, P.S.; Campbell, K.; Wilkes, J.; Stoddard, G.J.; Huynh, K.; Young, P.C.; Gabrielsen, T.P. Primary care autism screening and later autism diagnosis. Pediatrics 2020, 146, e20192314. [Google Scholar] [CrossRef]
- Wetherby, A.M.; Woods, J.; Allen, L.; Cleary, J.; Dickinson, H.; Lord, C. Early indicators of autism spectrum disorders in the second year of life. J. Autism Dev. Disord. 2004, 34, 473–493. [Google Scholar] [CrossRef]
- Wetherby, A.M.; Brosnan-Maddox, S.; Peace, V.; Newton, L. Validation of the Infant-Toddler Checklist as a broadband screener for autism spectrum disorders from 9 to 24 months of age. Autism 2008, 12, 487–511. [Google Scholar] [CrossRef]
- Parikh, C.; Iosif, A.M.; Ozonoff, S. Brief report: Use of the Infant-Toddler Checklist in infant siblins of children with autism spectrum disorder. J. Autism Dev. Disord. 2020. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Adolphs, R. The social brain: Neural basis of social knowledge. Annu. Rev. Psychol. 2009, 60, 693–716. [Google Scholar] [CrossRef]
- Schurz, M.; Radua, J.; Tholen, M.G.; Maliske, L.; Margulies, D.S.; Mars, R.B.; Sallet, J.; Kanske, P. Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol. Bull. 2020. [Google Scholar] [CrossRef]
- Van Overwalle, F.; D’aes, T.; Mariën, P. Social cognition and the cerebellum: A meta-analytic connectivity analysis. Hum. Brain Mapp. 2015, 36, 5137–5154. [Google Scholar] [CrossRef]
- Müller, R.A.; Fishman, I. Brain connectivity and neuroimaging of social networks in autism. Trends Cogn. Sci. 2018, 22, 1103–1116. [Google Scholar] [CrossRef]
- Happé, F.; Frith, U. Annual research review: Looking back to look forward—Changes in the concept of autism and implications for future research. J. Child. Psychol. Psychiatry 2020, 61, 218–232. [Google Scholar] [CrossRef]
- Rosenbaum, P.; King, S.; Law, M.; King, G.; Evans, J. Family-centred services: A conceptual framework and research review. Phys. Occup. Ther. Pediatr. 1998, 18, 1–20. [Google Scholar] [CrossRef]
- Law, M.; Teplicky, R.; King, S.; King, G.; Kertoy, M.; Moning, T.; Rosenbaum, P.; Burke-Gaffney, J. Family-Centred Service: Moving Ideas into Practice. Child Care Health Dev. 2005, 31, 633–642. [Google Scholar] [CrossRef]
- Akhbari Ziegler, S.; Dirks, T.; Hadders-Algra, M. Coaching in early physical therapy intervention: The COPCA program as an example of translation of theory into practice. Disabil. Rehabil. 2019, 41, 1846–1854. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Bauman, M.L.; Choueiri, R.; Kasari, C.; Carter, A.; Granpeesheh, D.; Mailloux, Z.; Smith Roley, S.; Wagner, S.; Fein, D.; et al. Early intervention for children with autism spectrum disorder under 3 years of age: Recommendations for practice and research. Pediatrics 2015, 136 (Suppl. 1), S60–S81. [Google Scholar] [CrossRef] [PubMed]
- Hadders-Algra, M. Early intervention in the first two years post-term. In Early Detection and Early Intervention in Developmental Disorders—From Neuroscience to Participation; Hadders-Algra, M., Ed.; Mac Keith Press: London, UK, 2021; pp. 198–227. [Google Scholar]
- Hutchon, B.; Gibbs, D.; Harniess, P.; Jary, S.; Crossley, S.L.; Moffat, J.V.; Basu, N.; Basu, A.P. Early intervention programmes for infants at high risk of atypical neurodevelopmental outcome. Dev. Med. Child Neurol. 2019, 61, 1362–1367. [Google Scholar] [CrossRef]
- Akhbari Ziegler, S.; Hadders-Algra, M. Coaching approaches in early intervention and paediatric rehabilitation. Dev. Med. Child Neurol. 2020, 62, 569–574. [Google Scholar] [CrossRef]
- Gooding, J.S.; Cooper, L.G.; Blaine, A.I.; Franck, L.S.; Howse, J.L.; Berns, S.D. Family support and family-centered care in the neonatal intensive care unit: Origins, advances, impact. Semin. Perinatol. 2011, 35, 20–28. [Google Scholar] [CrossRef]
- O’Brien, K.; Bracht, M.; Robson, K.; Ye, X.Y.; Mirea, L.; Cruz, M.; Ng, E.; Monterrosa, L.; Soraisham, A.; Alvaro, R.; et al. Evaluation of the Family Integrated Care model of neonatal intensive care: A cluster randomized controlled trial in Canada and Australia. BMC Pediatr. 2015, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Hadders-Algra, M. Early intervention in the neonatal period. In Early Detection and Early Intervention in Developmental Disorders—From Neuroscience to Participation; Hadders-Algra, M., Ed.; Mac Keith Press: London, UK, 2021; pp. 185–197. [Google Scholar]
- Brett, J.; Staniszewska, S.; Newburn, M.; Jones, N.; Taylor, L. A systematic mapping review of effective interventions for communicating with, supporting and providing information to parents of preterm infants. BMJ Open 2011, 1, e000023. [Google Scholar] [CrossRef] [PubMed]
- Benzies, K.M.; Magill-Evans, J.E.; Hayden, K.A.; Ballantyne, M. Key components of early intervention programs for preterm infants and their parents: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2013, 13 (Suppl. 1), S10. [Google Scholar] [CrossRef]
- Mackay, L.J.; Benzies, K.M.; Barnard, C.; Hayden, K.A. A scoping review of parental experiences caring for their hospitalized medically fragile infants. Acta Paediatr. 2020, 109, 266–275. [Google Scholar] [CrossRef]
- Als, H.; Lawhon, G.; Brown, E.; Gibes, R.; Duffy, F.H.; McAnulty, G.; Blickman, J.G. Individualized behavioral and environmental care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: Neonatal intensive care unit and developmental outcome. Pediatrics 1986, 113, 1123–1132. [Google Scholar]
- Als, H.; Lawhon, G.; Duffy, F.H.; McAnulty, G.B.; Gibes-Grossman, R.; Blickman, J.G. Individualized developmental care for the very low-birth-weight preterm infant. Medical and neurofunctional effects. JAMA 1994, 272, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Jacobs, S.E. NIDCAP: A systematic review and meta-analyses of randomized controlled trials. Pediatrics 2013, 131, e881–e893. [Google Scholar] [CrossRef]
- Puthussery, S.; Chutiyami, M.; Tseng, P.C.; Kilby, L.; Kapadia, J. Effectiveness of early intervention programs for parents of preterm infants: A meta-review of systematic reviews. BMC Pediatr. 2018, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.; Orton, J.; Anderson, P.J.; Boyd, R.; Doyle, L.W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 2015, CD005495. [Google Scholar] [CrossRef]
- Blauw-Hospers, C.H.; Hadders-Algra, M. A systematic review of the effects of early intervention on motor development. Dev. Med. Child Neurol. 2005, 47, 421–432. [Google Scholar] [CrossRef]
- Blauw-Hospers, C.H.; Dirks, T.; Hulshof, L.J.; Bos, A.F.; Hadders-Algra, M. Pediatric physical therapy in infancy: From nightmare to dream? A two-arm randomized trial. Phys. Ther. 2011, 91, 1323–1338. [Google Scholar] [CrossRef]
- Morgan, C.; Novak, I.; Dale, R.C.; Badawi, N. Optimising motor learning in infants at high risk of cerebral palsy: A pilot study. BMC Pediatr. 2015, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Novak, I.; Dale, R.C.; Guzzetta, A.; Badawi, N. Single blind randomised controlled trial of GAME (Goals–Activity – Motor Enrichment) in infants at high risk of cerebral palsy. Res. Dev. Disabil. 2016, 55, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Holmström, L.; Eliasson, A.C.; Almeida, R.; Furmark, C.; Weiland, A.L.; Tedroff, K.; Löwing, K. Efficacy of the small step program in a randomized controlled trial for infants under 12 months old at risk of cerebral palsy (CP) and other neurological disorders. J. Clin. Med. 2019, 8, 1016. [Google Scholar] [CrossRef]
- Hielkema, T.; Boxum, A.G.; Hamer, E.G.; La Bastide-Van Gemert, S.; Dirks, T.; Reinders-Messelink, H.A.; Maathuis, C.G.B.; Verheijden, J.; Geertzen, J.H.B.; Hadders-Algra, M. LEARN2MOVE 0–2 years, a randomized early intervention trial for infants at very high risk of cerebral palsy: Family outcome and infant’s functional outcome. Disabil. Rehabil. 2020, 42, 3762–3770. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.C.; Nordstrand, L.; Ek, L.; Lennartsson, F.; Sjöstrand, L.; Tedroff, K.; Krumlinde-Sundholm, L. The effectiveness of Baby-CIMT in infants younger than 12 months with clinical signs of unilateral cerebral palsy; an explorative study with randomized design. Res. Dev. Disabil. 2018, 72, 191–201. [Google Scholar] [CrossRef]
- Chamudot, R.; Parush, S.; Rigbi, A.; Horovitz, R.; Gross-Tsur, V. Effectiveness of modified constraint-induced movement therapy compared with bimanual therapy home programs for infants with hemiplegia: A randomized controlled trial. Am. J. Occup. Ther. 2018, 72, 7206205010p1–7206205010p9. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Namara, M.M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic review of interventions for preventing and treating children with cerebral palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Hielkema, T.; Hamer, E.G.; Boxum, A.G.; La Bastide-Van Gemert, S.; Dirks, T.; Reinders-Messelink, H.A.; Maathuis, C.G.B.; Verheijden, J.; Geertzen, J.H.B.; Hadders-Algra, M.; et al. LEARN2MOVE 0–2 years, a randomized early intervention trial for infants at very high risk of cerebral palsy: Neuromotor, cognitive, and behavioural outcome. Disabil. Rehabil. 2020, 42, 3752–3761. [Google Scholar] [CrossRef]
- Mayston, M. Bobath and NeuroDevelopmental Therapy: What is the future? Dev. Med. Child Neurol. 2016, 58, 994. [Google Scholar] [CrossRef]
- Green, J.; Garg, S. Annual research review: The state of autism intervention science: Progress, target psychological and biological mechanisms and future prospects. J. Child Psychol. Psychiatry 2018, 59, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Trembath, D.; Gurm, M.; Scheerer, N.E.; Trevisan, D.A.; Paynter, J.; Bohadana, G.; Roberts, J.; Iarocci, G. Systematic review of factors that may influence the outcomes and generalizability of parent-mediated interventions of young children with autism spectrum disorder. Autism Res. 2019, 12, 1304–1321. [Google Scholar] [CrossRef]
- French, L.; Kennedy, E.M.M. Annual research review: Early intervention for infants and young children with, or at-risk of, autism spectrum disorder: A systematic review. J. Child Psychol. Psychiatr. 2018, 59, 444–456. [Google Scholar] [CrossRef]
- Sandbank, M.; Bottema-Beutel, K.; Crowley, S.; Cassidy, M.; Dunham, K.; Feldman, J.I.; Crank, J.; Albarran, S.A.; Raj, S.; Mahbub, P.; et al. Project AIM: Autism intervention meta-analysis for studies of young children. Psychol. Bull. 2020, 146, 1–29. [Google Scholar] [CrossRef]
- Reichow, B.; Hume, K.; Barton, E.E.; Boyd, B.A. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2018, 5, CD009260. [Google Scholar] [CrossRef] [PubMed]
- Fuller, E.A.; Kaiser, A.P. The effects of early intervention on social communication outcomes for children with autism spectrum disorder: A meta-analysis. J. Autism. Dev. Disord. 2020, 50, 1683–1700. [Google Scholar] [CrossRef]
- Rodgers, M.; Marshall, D.; Simmonds, M.; Le Couteur, A.; Biswas, M.; Wright, K.; Rai, D.; Palmer, S.; Stewart, L.; Hodgson, R. Interventions based on early intensive applied behaviour analysis for autistic children: A systematic review and cost-effectivenesss analysis. Health Technol. Assess. 2020, 24, 1–306. [Google Scholar] [CrossRef]
- Granpeesheh, D.; Dixon, D.R.; Tarbox, J.; Kaplan, A.M.; Wilke, A.E. The effects of age and treatment intensity on behavioral intervention outcomes for children with autism spectrum disorders. Res. Autism Spect. Dis. 2009, 3, 1014–1022. [Google Scholar] [CrossRef]
- Holmes, J.M.; Levi, D.M. Treatment of amblyopia as a function of age. Vis. Neurosci. 2018, 35, E015. [Google Scholar] [CrossRef]
- Tomasello, M. How children come to understand false beliefs: A shared intentionality account. Proc. Natl. Acad. Sci. USA 2018, 115, 8491–8498. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Charman, T.; Pickles, A.; Wan, M.W.; Elsabbagh, M.; Slonims, V.; Taylor, C.; McNally, J.; Booth, R.; Gliga, T.; et al. Parent-mediated intervention versus no intervention for infants at high risk of autism: A parallel, single-blind, randomised trial. Lancet Psychiatry. 2015, 2, 133–140. [Google Scholar] [CrossRef]
- Green, J.; Pickles, A.; Pasco, G.; Bedford, R.; Wan, M.W.; Elsabbagh, M.; Slonims, V.; Gliga, T.; Jones, E.; Cheung, C.; et al. Randomised trial of a parent-mediated intervention for infants at high risk for autism: Longitudinal outcomes to age 3 years. J. Child Psychol. Psychiatry 2017, 58, 1330–1340. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadders-Algra, M. Early Diagnostics and Early Intervention in Neurodevelopmental Disorders—Age-Dependent Challenges and Opportunities. J. Clin. Med. 2021, 10, 861. https://doi.org/10.3390/jcm10040861
Hadders-Algra M. Early Diagnostics and Early Intervention in Neurodevelopmental Disorders—Age-Dependent Challenges and Opportunities. Journal of Clinical Medicine. 2021; 10(4):861. https://doi.org/10.3390/jcm10040861
Chicago/Turabian StyleHadders-Algra, Mijna. 2021. "Early Diagnostics and Early Intervention in Neurodevelopmental Disorders—Age-Dependent Challenges and Opportunities" Journal of Clinical Medicine 10, no. 4: 861. https://doi.org/10.3390/jcm10040861
APA StyleHadders-Algra, M. (2021). Early Diagnostics and Early Intervention in Neurodevelopmental Disorders—Age-Dependent Challenges and Opportunities. Journal of Clinical Medicine, 10(4), 861. https://doi.org/10.3390/jcm10040861





