IMPELLA® or Extracorporeal Membrane Oxygenation for Left Ventricular Dominant Refractory Cardiogenic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Design
2.2. CS Definition
2.3. Devices Implantation and CS Management
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Outcomes
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aissaoui, N.; Puymirat, E.; Delmas, C.; Ortuno, S.; Durand, E.; Bataille, V.; Drouet, E.; Bonello, L.; Bonnefoy-Cudraz, E.; Lesmeles, G.; et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 2020, 22, 664–672. [Google Scholar] [CrossRef]
- Thiele, H.; Ohman, E.M.; De Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Hear. J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef]
- Rao, P.; Khalpey, Z.; Smith, R.; Burkhoff, D.; Kociol, R.D. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef]
- Stretch, R.; Sauer, C.M.; Yuh, D.D.; Bonde, P. National Trends in the Utilization of Short-Term Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2014, 64, 1407–1415. [Google Scholar] [CrossRef]
- Schurtz, G.; Laine, M.; Delmas, C.; Kerbaul, F.; Puymirat, E.; Lemesle, G.; Bonello, L. Mechanical Support in Cardiogenic Shock Complicating Acute Coronary Syndrome: Ready for Prime Time? Curr. Vasc. Pharmacol. 2018, 16, 418–426. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump with In-Hospital Mortality and Major Bleeding Among Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734. [Google Scholar] [CrossRef]
- Kormos, R.L.; Antonides, C.F.J.; Goldstein, D.J.; Cowger, J.A.; Starling, R.C.; Kirklin, J.K.; Rame, J.E.; Rosenthal, D.; Mooney, M.L.; Caliskan, K.; et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: A consensus statement of the mechanical circulatory support academic research consortium. J. Heart Lung Transpl. 2020, 39, 735–750. [Google Scholar] [CrossRef]
- Hicks, K.A.; Mahaffey, K.W.; Mehran, R.; Nissen, S.E.; Wiviott, S.D.; Dunn, B.; Solomon, S.D.; Marler, J.R.; Teerlink, J.R.; Farb, A.; et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. Circulation 2018, 137, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Karatolios, K.; Chatzis, G.; Markus, B.; Luesebrink, U.; Ahrens, H.; Divchev, D.; Syntila, S.; Jerrentrup, A.; Schieffer, B. Comparison of mechanical circulatory support with venoarterial extracorporeal membrane oxygenation or Impella for patients with cardiogenic shock: A propensity-matched analysis. Clin. Res. Cardiol. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.; Hellgren, L.; Vikholm, P. Survival after refractory cardiogenic shock is comparable in patients with Impella and veno-arterial extracorporeal membrane oxygenation when adjusted for SAVE score. Eur. Heart J. Acute Cardiovasc. Care 2017, 8, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, Y.; Cheung, A.; Ignaszewski, A.; Higgins, J.; Kaan, A.; Griesdale, D.E.; Moss, R. Comparative outcomes in cardiogenic shock patients managed with Impella microaxial pump or extracorporeal life support. J. Thorac. Cardiovasc. Surg. 2011, 142, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Garan, A.R.; Takeda, K.; Salna, M.; Vandenberge, J.; Doshi, D.; Karmpaliotis, D.; Kirtane, A.J.; Takayama, H.; Kurlansky, P. Prospective Comparison of a Percutaneous Ventricular Assist Device and Venoarterial Extracorporeal Membrane Oxygenation for Patients with Cardiogenic Shock Following Acute Myocardial Infarction. J. Am. Heart Assoc. 2019, 8, e012171. [Google Scholar] [CrossRef] [PubMed]
- Karami1, M.; den Uil, C.A.; Ouweneel, D.M.; Scholte, N.T.B.; Engström, A.E.; Akin, S.; Lagrand, W.K.; Vlaar, A.P.J.; Jewbali, L.S.; Henriques, J.P.S. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: Impella CP/5.0 versus ECMO. Eur. Heart J. Acute Cardiovasc. Care 2019, 33. [Google Scholar] [CrossRef]
- Mourad, M.; Gaudard, P.; De La Arena, P.; Eliet, J.; Zeroual, N.; Rouvière, P.; Roubille, F.; Albat, B.; Colson, P.H. Circulatory Support with Extracorporeal Membrane Oxygenation and/or Impella for Cardiogenic Shock during Myocardial Infarction. ASAIO J. 2018, 64, 708–714. [Google Scholar] [CrossRef]
- Akanni, O.J.; Takeda, K.; Truby, L.K.; Kurlansky, P.A.; Chiuzan, C.; Han, J.; Topkara, V.K.; Yuzefpolskaya, M.; Colombo, P.C.; Karmpaliotis, D.; et al. EC-VAD: Combined Use of Extracorporeal Membrane Oxygenation and Percutaneous Microaxial Pump Left Ventricular Assist Device. ASAIO J. 2019, 65, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Møller-Helgestad, O.K.; Hyldebrandt, J.A.; Banke, A.; Rud, C.S.; Udesen, N.L.; Linde, L.; Okkels-Jensen, L.; Schmidt, H.; Ravn, H.B.; Møller, J.E. Impella CP or VA-ECMO in profound cardiogenic shock: Left ventricular unloading and organ perfusion in a large animal model. EuroIntervention 2019, 14, e1585–e1592. [Google Scholar] [CrossRef]
- Schrage, B.; Burkhoff, D.; Rübsamen, N.; Becher, P.M.; Schwarzl, M.; Bernhardt, A.; Grahn, H.; Lubos, E.; Söffker, G.; Clemmensen, P.; et al. Unloading of the Left Ventricle during Venoarterial Extracorporeal Membrane Oxygenation Therapy in Cardiogenic Shock. JACC Heart Fail. 2018, 6, 1035–1043. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Deseda, G.C.; Dabboura, S.; Eckner, D.; et al. Left Ventricular Unloading Is Associated with Lower Mortality in Patients with Cardiogenic Shock Treated with Venoarterial Extracorporeal Membrane Oxygenation. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Tongers, J.; Sieweke, J.-T.; Kühn, C.; Napp, L.C.; Flierl, U.; Röntgen, P.; Schmitto, J.D.; Sedding, D.G.; Haverich, A.; Bauersachs, J.; et al. Early Escalation of Mechanical Circulatory Support Stabilizes and Potentially Rescues Patients in Refractory Cardiogenic Shock. Circ. Heart Fail. 2020, 13, e005853. [Google Scholar] [CrossRef]
- Freund, A.; Jobs, A.; Lurz, P.; Feistritzer, H.-J.; De Waha-Thiele, S.; Meyer-Saraei, R.; Montalescot, G.; Huber, K.; Noc, M.; Windecker, S.; et al. Frequency and Impact of Bleeding on Outcome in Patients with Cardiogenic Shock. JACC Cardiovasc. Interv. 2020, 13, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Basir, M.B.; Schreiber, T.L.; Grines, C.L.; Dixon, S.R.; Moses, J.W.; Maini, B.S.; Khandelwal, A.K.; Ohman, E.M.; O’Neill, W.W. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am. J. Cardiol. 2017, 119, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Bonello, L.; Delmas, C.; Schurtz, G.; Leurent, G.; Bonnefoy, E.; Aissaoui, N.; Henry, P. Mechanical circulatory support in patients with cardiogenic shock in intensive care units: A position paper of the ‘‘Unité de Soins Intensifs de Cardiologie’’ group of the French Society of Cardiology, endorsed by the “‘Groupe Athérome et Cardiologie Interventionnelle’” of the French Society of Cardiology. Arch. Cardiovasc. Dis. 2018, 111, 601–612. [Google Scholar] [PubMed]


| Total Population n = 128 | VA-ECMO First n = 97 | IMPELLA® First n = 31 | p | |
|---|---|---|---|---|
| Demographics and Medical History | ||||
| Age (years) | 53.8 ± 13.1 | 52 ± 12.4 | 59.4 ± 13.8 | 0.006 |
| Sex (male), n (%) | 93 (72.7%) | 70 (72.2%) | 23 (74.2%) | 0.825 |
| Body mass index (Kg/m2) | 27 ± 4.9 | 27 ± 5.1 | 26.7 ± 4.3 | 0.735 |
| Diabetes, n (%) | 25 (19.5%) | 18 (18.6%) | 7 (22.6%) | 0.660 |
| History of coronary artery disease, n (%) | 34 (26.6%) | 26 (26.8%) | 8 (25.8%) | 0.913 |
| History of stroke, n (%) | 4 (3.1%) | 2 (2.1%) | 2 (6.5%) | 0.260 |
| Peripheral artery disease, n (%) | 3 (2.3%) | 1 (1%) | 2 (6.5%) | 0.154 |
| Renal failure before admission, n (%) | 7 (5.5%) | 5 (5.2%) | 2 (6.5%) | 0.999 |
| Admission | ||||
| Acute myocardial infarction, n (%) | 72 (56.3%) | 51 (52.6%) | 21 (67.7%) | 0.138 |
| Left ventricular ejection fraction (LVEF,%) | 21.8 ± 15.5 | 19.1 ± 13.8 | 27.2 ± 17.6 | 0.017 |
| Creatinine (mg/L) | 18 ± 10.1 | 18.5 ± 10.3 | 16.5 ± 9.6 | 0.353 |
| Hemoglobin (g/dL) | 11.9 ± 2.6 | 11.3 ± 2.4 | 13.5 ± 2.6 | <0.001 |
| Lactate (mmol/L) | 5.82 ± 4.93 | 6.84 ± 5.33 | 3.03 ± 1.57 | <0.001 |
| ASAT (IU/L) | 460 ± 749 | 498.3 ± 835 | 348.8 ± 397.9 | 0.340 |
| ALAT (IU/L) | 269.5 ± 552.1 | 325.6 ± 627.5 | 104.8 ± 101.9 | 0.054 |
| PT (%) | 63.2 ± 31.2 | 61.7 ± 33.9 | 69 ± 17 | 0.322 |
| Bilirubin (mg/L) | 13.2 ± 14.5 | 14 ± 15.2 | 10.3 ± 11.7 | 0.289 |
| CRP-us (mg/L) | 68.7 ± 75.9 | 68.2 ± 71.7 | 70.3 ± 88.7 | 0.903 |
| Mechanical support | ||||
| VA-ECMO, n (%) | 107 (83.6%) | 97 (100%) | 10 (32.3%) | - |
| IMPELLA®, n (%) | 41 (32%) | 10 (10.3%) | 31 (100%) | - |
| IMPELLA-CP®, n (%) | 34 (26.6%) | 7 (7.2%) | 27 (87.1%) | - |
| VA-ECMO as first device, n (%) | 97 (75.8%) | 97 (100%) | - | - |
| IMPELLA® as first device, n (%) | 31 (24.2%) | - | 31 (100%) | - |
| Duration of MCS (days) | 8.6 ± 9.2 | 9.4 ± 10.1 | 6 ± 5 | 0.077 |
| Total Population n = 128 | VA-ECMO First n = 97 | IMPELLA First n = 31 | p | |
|---|---|---|---|---|
| 30-Day Outcomes | ||||
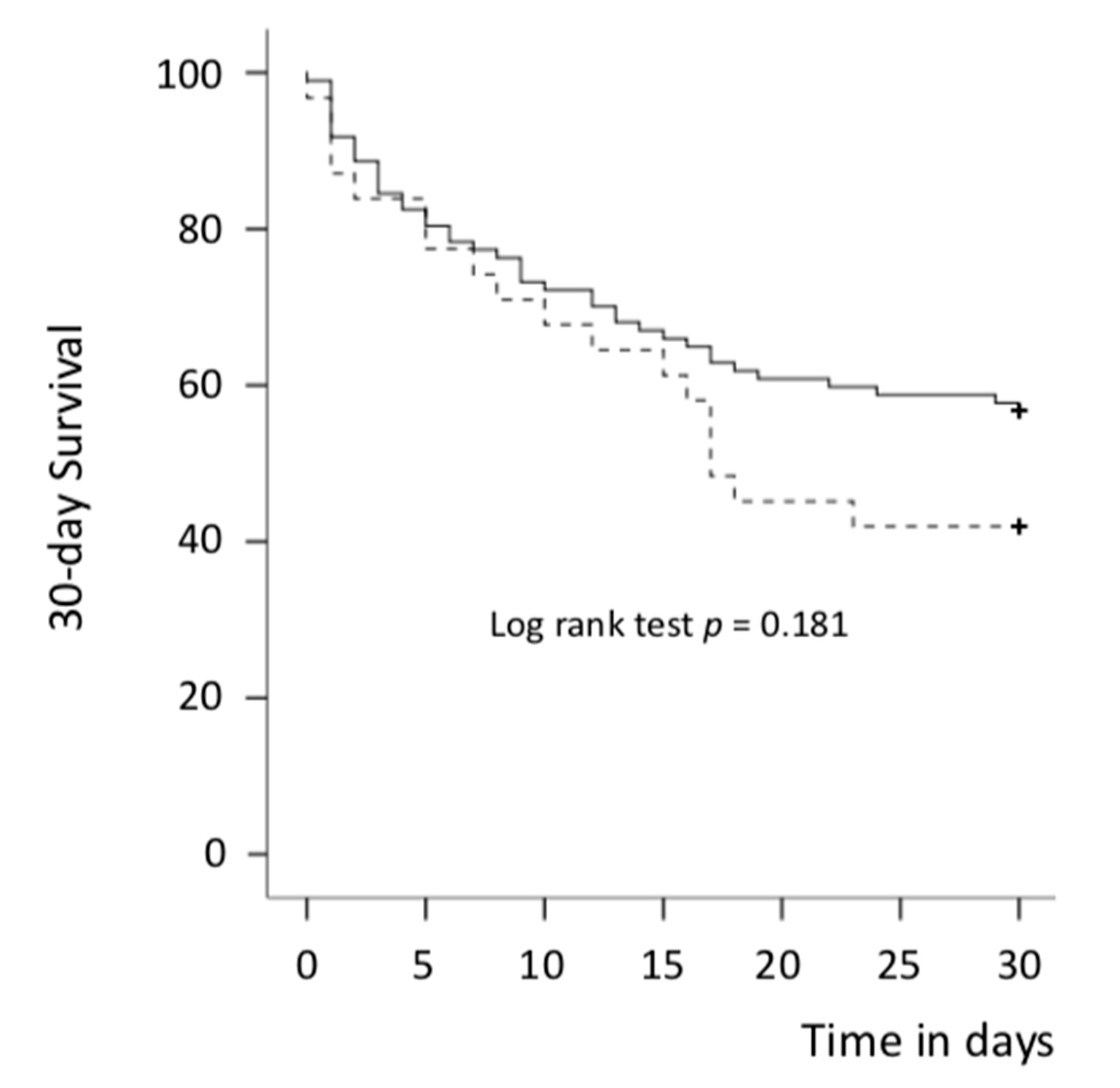
| 30-day mortality, n (%) | 60 (46.9%) | 42 (43.3%) | 18 (58.1%) | 0.152 |
| Bridge to left ventricular assist device (LVAD, n %) | 18 (14.1%) | 14 (14.4%) | 4 (12.9%) | 0.831 |
| Bridge to transplantation, n (%) | 14 (10.9%) | 14 (14.4%) | 0 | 0.025 |
| Composite of all-cause death, LVAD, transplantation, n (%) | 88 (68.8%) | 67 (69.1%) | 21 (67.7%) | 0.889 |
| Need for MCS escalation, n (%) | 20 (15.6%) | 10 (10.3%) | 10 (32.3%) | 0.003 |
| Time between first MCS and escalation (Days) | 3.1 ± 4 | 5 ± 5 | 1.1 ± 0.9 | 0.026 |
| Stroke, n (%) | 15 (11.7%) | 13 (13.4%) | 2 (6.5%) | 0.295 |
| Sepsis, n (%) | 46 (35.9%) | 39 (40.2%) | 7 (22.6%) | 0.075 |
| Renal replacement therapy, n (%) | 60 (46.9%) | 43 (44.3%) | 17 (54.8%) | 0.307 |
| Major bleeding, n (%) | 48 (37.5%) | 39 (40.2%) | 9 (29%) | 0.263 |
| Vascular complication related to MCS insertion, n (%) | 21 (16.5%) | 15 (15.5%) | 6 (19.4%) | 0.627 |
| Myocardial recovery, n (%) | 37 (28.9%) | 29 (29.9%) | 8 (25.8%) | 0.662 |
| Six-Month Outcomes | ||||
| 6-month mortality | 67 (52.3%) | 47 (48.5%) | 20 (64.5%) | 0.119 |
| Bridge to LVAD, n (%) | 20 (15.6%) | 15 (15.5%) | 5 (16.1%) | 0.999 |
| Bridge to transplantation, n (%) | 16 (12.5%) | 16 (16.5%) | 0 | 0.016 |
| Composite of all-cause death, LVAD, transplantation | 94 (73.4%) | 71 (73.2%) | 23 (74.2%) | 0.913 |
| Need for MCS escalation, n (%) | 20 (15.6%) | 10 (10.3%) | 10 (32.3%) | 0.003 |
| Time between first MCS and escalation (Days) | 3.1 ± 4 | 5 ± 5 | 1.1 ± 0.9 | 0.026 |
| Stroke, n (%) | 16 (12.5%) | 14 (14.4%) | 2 (6.5%) | 0.353 |
| Sepsis, n (%) | 53 (41.4%) | 43 (44.3%) | 10 (32.3%) | 0.089 |
| Renal replacement therapy, n (%) | 63 (49.2%) | 44 (45.4%) | 19 (61.3%) | 0.279 |
| Major bleeding, n (%) | 52 (40.6%) | 40 (41.2%) | 12 (38.7%) | 0.771 |
| Vascular complication related to MCS insertion, n (%) | 21 (16.5%) | 15 (15.5%) | 6 (19.4%) | 0.627 |
| Myocardial recovery, n (%) | 38 (29.7%) | 30 (30.9%) | 8 (25.8%) | 0.587 |
| Total Population n = 128 | Alive at 30 Days n = 68 | Dead at 30 Days n = 60 | p | |
|---|---|---|---|---|
| Demographics and Medical History | ||||
| Age (years) | 53.8 ± 13.1 | 51 ± 13.6 | 56.9 ± 11.8 | 0.011 |
| Sex (male), n (%) | 93 (72.7%) | 43 (63.2%) | 50 (83.3%) | 0.011 |
| Body mass index (Kg/m2) | 27 ± 4.9 | 26.6 ± 4.8 | 27.4 ± 5 | 0.404 |
| Diabetes, n (%) | 25 (19.5%) | 10 (14.7%) | 15 (25%) | 0.118 |
| History of coronary artery disease, n (%) | 34 (26.6%) | 18 (26.5%) | 16 (26.7%) | 0.980 |
| History of stroke, n (%) | 4 (3.1%) | 0 | 4 (6.7%) | 0.042 |
| Peripheral artery disease, n (%) | 3 (2.3%) | 0 | 3 (5%) | 0.089 |
| Renal failure before admission, n (%) | 7 (5.5%) | 5 (7.4%) | 2 (3.3%) | 0.456 |
| Admission | ||||
| Acute myocardial infarction, n (%) | 72 (56.3%) | 32 (47.1%) | 40 (66.7%) | 0.026 |
| LVEF (%) | 21.8 ± 15.5 | 20.8 ± 14 | 23.1± 17.4 | 0.472 |
| Creatinine (mg/L) | 18 ± 10.1 | 16.1± 8.3 | 20.2 ± 11.6 | 0.021 |
| Hemoglobin (g/dL) | 11.9 ± 2.6 | 11.7 ± 2.6 | 12.1 ± 2.6 | 0.503 |
| Lactate (mmol/L) | 5.82 ± 4.93 | 4.83 ± 3.48 | 7.09 ± 6.12 | 0.013 |
| ASAT (IU/L) | 460 ± 749 | 442.3 ± 878.9 | 482.7 ± 546.3 | 0.770 |
| ALAT (IU/L) | 269.5 ± 552.1 | 209.6 ± 484.6 | 340.1 ± 619.3 | 0.194 |
| PT (%) | 63.2 ± 31.2 | 67.9 ± 36.5 | 57.1 ± 21.4 | 0.070 |
| Bilirubin (mg/L) | 13.2 ± 14.5 | 11.2 ± 11.4 | 15.9 ± 17.7 | 0.098 |
| CRP-us (mg/L) | 68.7 ± 75.9 | 57.7 ± 83.9 | 83.9 ± 86.1 | 0.086 |
| Mechanical Support | ||||
| VA-ECMO, n (%) | 107 (83.6%) | 57 (83.8%) | 50 (83.3%) | 0.940 |
| IMPELLA®, n (%) | 41 (32%) | 16 (23.5%) | 25 (41.7%) | 0.028 |
| IMPELLA-CP®, n (%) | 34 (26.6%) | 15 (22.1%) | 19 (31.7%) | 0.141 |
| VA-ECMO as first device, n (%) | 97 (75.8%) | 55 (80.9%) | 42 (70%) | 0.152 |
| IMPELLA® as first device, n (%) | 31 (24.2%) | 13 (19.1%) | 18 (30%) | 0.152 |
| Duration of MCS (Days) | 8.6 ± 9.2 | 9.5 ± 11.1 | 7.5 ± 6.4 | 0.230 |
| Hazard Ratio | 95% Confidence Interval | p | |
|---|---|---|---|
| Age (per year) | 1.03 | 0.99–1.07 | 0.073 |
| Sex male | 5.70 | 1.85–17.51 | 0.002 |
| Diabetes | 1.51 | 0.68–3.56 | 0.309 |
| Acute myocardial infarction at admission | 2.79 | 1.29–6.03 | 0.009 |
| LVEF (per %) | 0.99 | 0.97–.01 | 0.349 |
| Lactate level (per one unit) | 1.17 | 1.10–1.25 | <0.001 |
| Creatinine level (per one unit) | 1.04 | 1.01–1.08 | 0.026 |
| Hemoglobin level (per one unit) | 0.92 | 0.81–1.05 | 0.236 |
| VA-ECMO first | 0.25 | 0.10–.65 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schurtz, G.; Rousse, N.; Saura, O.; Balmette, V.; Vincent, F.; Lamblin, N.; Porouchani, S.; Verdier, B.; Puymirat, E.; Robin, E.; et al. IMPELLA® or Extracorporeal Membrane Oxygenation for Left Ventricular Dominant Refractory Cardiogenic Shock. J. Clin. Med. 2021, 10, 759. https://doi.org/10.3390/jcm10040759
Schurtz G, Rousse N, Saura O, Balmette V, Vincent F, Lamblin N, Porouchani S, Verdier B, Puymirat E, Robin E, et al. IMPELLA® or Extracorporeal Membrane Oxygenation for Left Ventricular Dominant Refractory Cardiogenic Shock. Journal of Clinical Medicine. 2021; 10(4):759. https://doi.org/10.3390/jcm10040759
Chicago/Turabian StyleSchurtz, Guillaume, Natacha Rousse, Ouriel Saura, Vincent Balmette, Flavien Vincent, Nicolas Lamblin, Sina Porouchani, Basile Verdier, Etienne Puymirat, Emmanuel Robin, and et al. 2021. "IMPELLA® or Extracorporeal Membrane Oxygenation for Left Ventricular Dominant Refractory Cardiogenic Shock" Journal of Clinical Medicine 10, no. 4: 759. https://doi.org/10.3390/jcm10040759
APA StyleSchurtz, G., Rousse, N., Saura, O., Balmette, V., Vincent, F., Lamblin, N., Porouchani, S., Verdier, B., Puymirat, E., Robin, E., Van Belle, E., Vincentelli, A., Aissaoui, N., Delhaye, C., Delmas, C., Cosenza, A., Bonello, L., Juthier, F., Moussa, M. D., & Lemesle, G. (2021). IMPELLA® or Extracorporeal Membrane Oxygenation for Left Ventricular Dominant Refractory Cardiogenic Shock. Journal of Clinical Medicine, 10(4), 759. https://doi.org/10.3390/jcm10040759








