Transanal Irrigation for Neurogenic Bowel Disease, Low Anterior Resection Syndrome, Faecal Incontinence and Chronic Constipation: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Outcomes
2.3. Search Strategy and Data Extraction
2.4. Risk of Bias and Quality Assessment
2.5. Data Synthesis
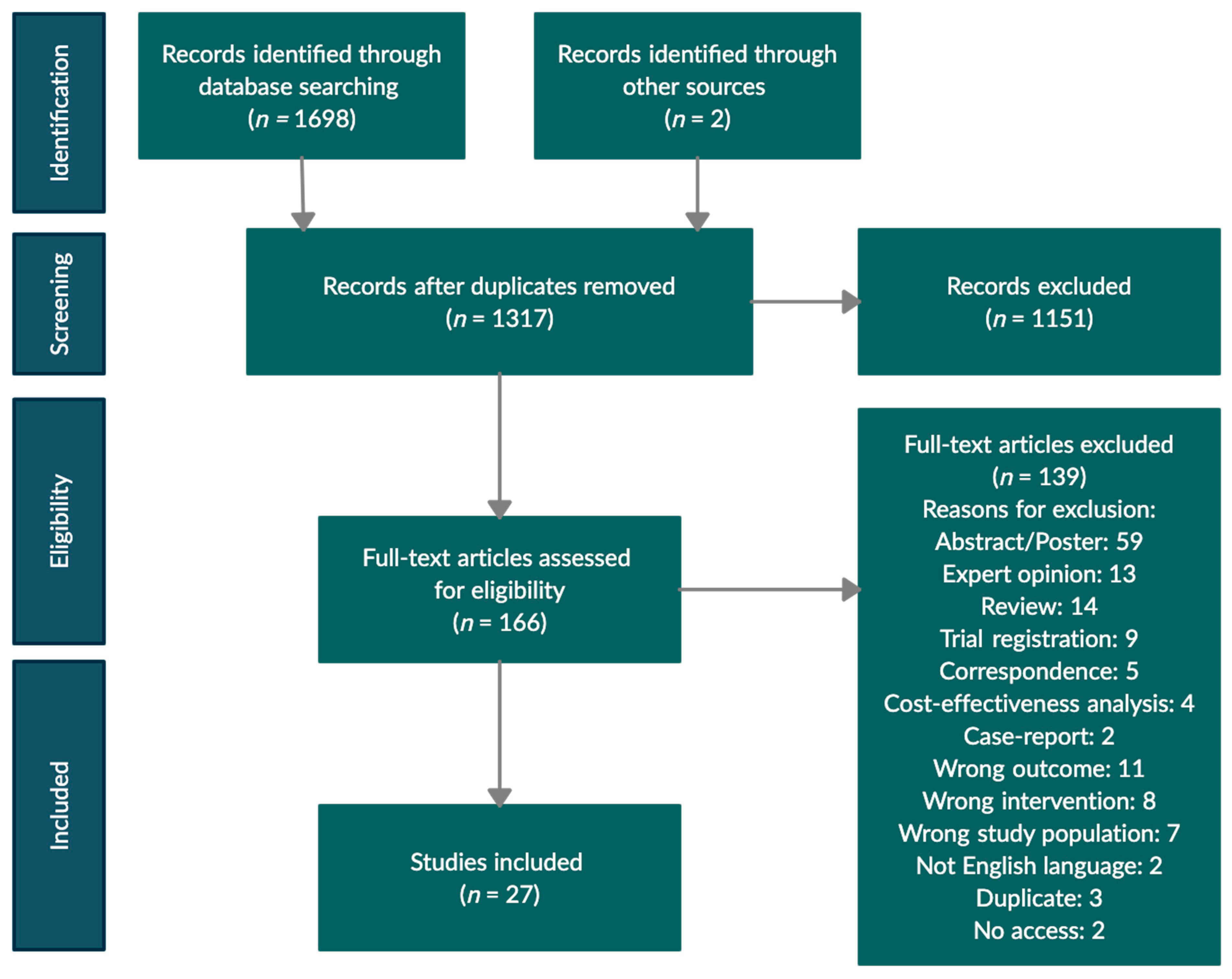
3. Results
3.1. Neurogenic Bowel Dysfunction
3.2. Low Anterior Resection Syndrome
3.2.1. Transanal Irrigation as Treatment for LARS
3.2.2. Transanal Irrigation as a Prophylactic Treatment for LARS
3.3. Faecal Incontinence and Constipation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coggrave, M.; Norton, C.; Cody, J.D. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst. Rev. 2014, 13, CD002115. [Google Scholar] [CrossRef]
- Dale, M.; Morgan, H.; Carter, K.; White, J.; Carolan-Rees, G. Peristeen Transanal Irrigation System to Manage Bowel Dysfunction: A NICE Medical Technology Guidance. Appl. Health Econ. Health Policy 2019, 17, 25–34. [Google Scholar] [CrossRef]
- Christensen, P.; Olsen, N.; Krogh, K.; Bacher, T.; Laurberg, S. Scintigraphic assessment of retrograde colonic washout in fecal incontinence and constipation. Dis. Colon Rectum 2003, 46, 68–76. [Google Scholar] [CrossRef]
- Emmanuel, A. Neurogenic bowel dysfunction. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Emmanuel, A.V.; Krogh, K.; Bazzocchi, G.; Leroi, A.M.; Bremers, A.; Leder, D.; van Kuppevelt, D.; Mosiello, G.; Vogel, M.; Perrouin-Verbe, B.; et al. Consensus review of best practice of transanal irrigation in adults. Spinal Cord 2013, 51, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Glickman, S.; Kamm, M.A. Bowel dysfunction in spinal-cord-injury patients. Lancet 1996, 347, 1651–1653. [Google Scholar] [CrossRef]
- Christensen, P.; Bazzocchi, G.; Coggrave, M.; Abel, R.; Hultling, C.; Krogh, K.; Media, S.; Laurberg, S. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology 2006, 131, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Fearnhead, N.S.; Martellucci, J. Transanal irrigation: Another hope for patients with LARS. Tech. Coloproctol. 2020, 24, 1231–1232. [Google Scholar] [CrossRef]
- Dulskas, A.; Smolskas, E.; Kildusiene, I.; Samalavicius, N.E. Treatment possibilities for low anterior resection syndrome: A review of the literature. Int. J. Colorectal Dis. 2018, 33, 251–260. [Google Scholar] [CrossRef]
- Pieniowski, E.H.A.; Palmer, G.J.; Juul, T.; Lagergren, P.; Johar, A.; Emmertsen, K.J.; Nordenvall, C.; Abraham-Nordling, M. Low Anterior Resection Syndrome and Quality of Life After Sphincter-Sparing Rectal Cancer Surgery: A Long-term Longitudinal Follow-up. Dis. Colon Rectum 2019, 62, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Wiltink, L.M.; Nout, R.A.; Meershoek-Klein Kranenbarg, E.; Laurberg, S.; Marijnen, C.A.; van de Velde, C.J. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: Report of a multicenter randomized trial. Clin. Colorectal Cancer 2015, 14, 106–114. [Google Scholar] [CrossRef]
- Christensen, P.; Krogh, K. Transanal irrigation for disordered defecation: A systematic review. Scand. J. Gastroenterol. 2010, 45, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Krogh, K.; Buntzen, S.; Payandeh, F.; Laurberg, S. Long-term outcome and safety of transanal irrigation for constipation and fecal incontinence. Dis. Colon Rectum 2009, 52, 286–292. [Google Scholar] [CrossRef]
- Emmanuel, A. Managing neurogenic bowel dysfunction. Clin. Rehabil. 2010, 24, 483–488. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 1 December 2020).
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Hooper, P.; Jutai, J.W.; Strong, G.; Russell-Minda, E. Age-related macular degeneration and low-vision rehabilitation: A systematic review. Can. J. Ophthalmol. 2008, 43, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, A.; Marshall, J.; Duthie, G. Rectal irrigation for relief of functional bowel disorders. Nurs. Stand. 2004, 19, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Bazzocchi, G.; Coggrave, M.; Abel, R.; Hulting, C.; Krogh, K.; Media, S.; Laurberg, S. Outcome of transanal irrigation for bowel dysfunction in patients with spinal cord injury. J. Spinal Cord Med. 2008, 31, 560–567. [Google Scholar] [CrossRef]
- Del Popolo, G.; Mosiello, G.; Pilati, C.; Lamartina, M.; Battaglino, F.; Buffa, P.; Redaelli, T.; Lamberti, G.; Menarini, M.; Di Benedetto, P.; et al. Treatment of neurogenic bowel dysfunction using transanal irrigation: A multicenter Italian study. Spinal Cord 2008, 46, 517–522. [Google Scholar] [CrossRef]
- Loftus, C.; Wallace, E.; McCaughey, M.; Smith, E. Transanal irrigation in the management of neurogenic bowel dysfunction. Ir. Med. J. 2012, 105, 241–243. [Google Scholar]
- Kim, H.R.; Lee, B.S.; Lee, J.E.; Shin, H.I. Application of transanal irrigation for patients with spinal cord injury in South Korea: A 6-month follow-up study. Spinal Cord 2013, 51, 389–394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamonet-Torny, J.; Bordes, J.; Daviet, J.C.; Dalmay, F.; Joslin, F.; Salle, J.Y. Long-term transanal irrigation’s continuation at home. Preliminary study. Ann. Phys. Rehabil. Med. 2013, 56, 134–142. [Google Scholar] [CrossRef]
- Adriaansen, J.J.; van Asbeck, F.W.; van Kuppevelt, D.; Snoek, G.J.; Post, M.W. Outcomes of neurogenic bowel management in individuals living with a spinal cord injury for at least 10 years. Arch. Phys. Med. Rehabil. 2015, 96, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, G.; Gosling, J.; Raeburn, A.; Storrie, J.; Panicker, J.; Emmanuel, A. Transanal irrigation for bowel symptoms in patients with multiple sclerosis. Dis. Colon Rectum 2012, 55, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Passananti, V.; Wilton, A.; Preziosi, G.; Storrie, J.B.; Emmanuel, A. Long-term efficacy and safety of transanal irrigation in multiple sclerosis. Neurogastroenterol. Motil. 2016, 28, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Brochard, C.; Peyronnet, B.; Hascoet, J.; Olivier, R.; Manunta, A.; Jezequel, M.; Alimi, Q.; Ropert, A.; Neunlist, M.; Bouguen, G.; et al. Defecation disorders in Spina Bifida: Realistic goals and best therapeutic approaches. Neurourol. Urodyn. 2019, 38, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Agachan, F.; Chen, T.; Pfeifer, J.; Reissman, P.; Wexner, S.D. A constipation scoring system to simplify evaluation and management of constipated patients. Dis. Colon Rectum 1996, 39, 681–685. [Google Scholar] [CrossRef]
- Vaizey, C.J.; Carapeti, E.; Cahill, J.A.; Kamm, M.A. Prospective comparison of faecal incontinence grading systems. Gut 1999, 44, 77–80. [Google Scholar] [CrossRef]
- Krogh, K.; Christensen, P.; Sabroe, S.; Laurberg, S. Neurogenic bowel dysfunction score. Spinal Cord 2006, 44, 625–631. [Google Scholar] [CrossRef]
- Rockwood, T.H.; Church, J.M.; Fleshman, J.W.; Kane, R.L.; Mavrantonis, C.; Thorson, A.G.; Wexner, S.D.; Bliss, D.; Lowry, A.C. Fecal Incontinence Quality of Life Scale: Quality of life instrument for patients with fecal incontinence. Dis. Colon Rectum 2000, 43, 9–16, discussion 16–17. [Google Scholar] [CrossRef]
- International Classification of Functioning, Disability, and Health; ICF World Health Organization: Geneva, Switzerland, 2001.
- Jorge, J.M.; Wexner, S.D. Etiology and management of fecal incontinence. Dis. Colon Rectum 1993, 36, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Fallon, A.; Westaway, J.; Moloney, C. A systematic review of psychometric evidence and expert opinion regarding the assessment of faecal incontinence in older community-dwelling adults. Int. J. Evid. Based Healthc. 2008, 6, 225–259. [Google Scholar] [CrossRef][Green Version]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- EQ-5D-5L User Guide. EuroQol Research Foundation. 2019. Available online: https://euroqol.org/publications/user-guides (accessed on 1 December 2020).
- Iwama, T.; Imajo, M.; Yaegashi, K.; Mishima, Y. Self washout method for defecational complaints following low anterior rectal resection. Jpn. J. Surg. 1989, 19, 251–253. [Google Scholar] [CrossRef]
- Koch, S.M.; Rietveld, M.P.; Govaert, B.; van Gemert, W.G.; Baeten, C.G. Retrograde colonic irrigation for faecal incontinence after low anterior resection. Int. J. Colorectal Dis. 2009, 24, 1019–1022. [Google Scholar] [CrossRef]
- Rosen, H.; Robert-Yap, J.; Tentschert, G.; Lechner, M.; Roche, B. Transanal irrigation improves quality of life in patients with low anterior resection syndrome. Colorectal Dis. 2011, 13, e335–e338. [Google Scholar] [CrossRef]
- Martellucci, J.; Sturiale, A.; Bergamini, C.; Boni, L.; Cianchi, F.; Coratti, A.; Valeri, A. Role of transanal irrigation in the treatment of anterior resection syndrome. Tech. Coloproctol. 2018, 22, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Navascues, J.M.; Labaka-Arteaga, I.; Aguirre-Allende, I.; Artola-Etxeberria, M.; Saralegui-Ansorena, Y.; Elorza-Echaniz, G.; Borda-Arrizabalaga, N.; Placer-Galan, C. A randomized trial comparing transanal irrigation and percutaneous tibial nerve stimulation in the management of low anterior resection syndrome. Colorectal Dis. 2020, 22, 303–309. [Google Scholar] [CrossRef]
- Rosen, H.R.; Kneist, W.; Fürst, A.; Krämer, G.; Hebenstreit, J.; Schiemer, J.F. Randomized clinical trial of prophylactic transanal irrigation versus supportive therapy to prevent symptoms of low anterior resection syndrome after rectal resection. BJS Open 2019, 3, 461–465. [Google Scholar] [CrossRef]
- Rosen, H.R.; Boedecker, C.; Fürst, A.; Krämer, G.; Hebenstreit, J.; Kneist, W. “Prophylactic” transanal irrigation (TAI) to prevent symptoms of low anterior resection syndrome (LARS) after rectal resection: Results at 12-month follow-up of a controlled randomized multicenter trial. Tech. Coloproctol. 2020, 24, 1247–1253. [Google Scholar] [CrossRef]
- Williams, N.S.; Patel, J.; George, B.D.; Hallan, R.I.; Watkins, E.S. Development of an electrically stimulated neoanal sphincter. Lancet 1991, 338, 1166–1169. [Google Scholar] [CrossRef]
- Emmertsen, K.J.; Laurberg, S. Low anterior resection syndrome score: Development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann. Surg. 2012, 255, 922–928. [Google Scholar] [CrossRef]
- Juul, T.; Battersby, N.J.; Christensen, P.; Janjua, A.Z.; Branagan, G.; Laurberg, S.; Emmertsen, K.J.; Moran, B. Validation of the English translation of the low anterior resection syndrome score. Colorectal Dis. 2015, 17, 908–916. [Google Scholar] [CrossRef]
- Juul, T.; Ahlberg, M.; Biondo, S.; Emmertsen, K.J.; Espin, E.; Jimenez, L.M.; Matzel, K.E.; Palmer, G.; Sauermann, A.; Trenti, L.; et al. International validation of the low anterior resection syndrome score. Ann. Surg. 2014, 259, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Temple, L.K.; Bacik, J.; Savatta, S.G.; Gottesman, L.; Paty, P.B.; Weiser, M.R.; Guillem, J.G.; Minsky, B.D.; Kalman, M.; Thaler, H.T.; et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis. Colon Rectum 2005, 48, 1353–1365. [Google Scholar] [CrossRef]
- Altomare, D.F.; Spazzafumo, L.; Rinaldi, M.; Dodi, G.; Ghiselli, R.; Piloni, V. Set-up and statistical validation of a new scoring system for obstructed defaecation syndrome. Colorectal Dis. 2008, 10, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Briel, J.W.; Schouten, W.R.; Vlot, E.A.; Smits, S.; van Kessel, I. Clinical value of colonic irrigation in patients with continence disturbances. Dis. Colon Rectum 1997, 40, 802–805. [Google Scholar] [CrossRef]
- Crawshaw, A.P.; Pigott, L.; Potter, M.A.; Bartolo, D.C. A retrospective evaluation of rectal irrigation in the treatment of disorders of faecal continence. Colorectal Dis. 2004, 6, 185–190. [Google Scholar] [CrossRef]
- Cazemier, M.; Felt-Bersma, R.J.; Mulder, C.J. Anal plugs and retrograde colonic irrigation are helpful in fecal incontinence or constipation. World J. Gastroenterol. 2007, 13, 3101–3105. [Google Scholar] [CrossRef]
- Koch, S.M.; Melenhorst, J.; van Gemert, W.G.; Baeten, C.G. Prospective study of colonic irrigation for the treatment of defaecation disorders. Br. J. Surg. 2008, 95, 1273–1279. [Google Scholar] [CrossRef]
- Vollebregt, P.F.; Elfrink, A.K.; Meijerink, W.J.; Felt-Bersma, R.J. Results of long-term retrograde rectal cleansing in patients with constipation or fecal incontinence. Tech. Coloproctol. 2016, 20, 633–639. [Google Scholar] [CrossRef]
- Juul, T.; Christensen, P. Prospective evaluation of transanal irrigation for fecal incontinence and constipation. Tech. Coloproctol. 2017, 21, 363–371. [Google Scholar] [CrossRef]
- Bildstein, C.; Melchior, C.; Gourcerol, G.; Boueyre, E.; Bridoux, V.; Vérin, E.; Leroi, A.M. Predictive factors for compliance with transanal irrigation for the treatment of defecation disorders. World J. Gastroenterol. 2017, 23, 2029–2036. [Google Scholar] [CrossRef]
- van der Hagen, S.J.; van der Meer, W.; Soeters, P.B.; Baeten, C.G.; van Gemert, W.G. A prospective non-randomized two-centre study of patients with passive faecal incontinence after birth trauma and patients with soiling after anal surgery, treated by elastomer implants versus rectal irrigation. Int. J. Colorectal Dis. 2012, 27, 1191–1198. [Google Scholar] [CrossRef][Green Version]
- Etherson, K.J.; Minty, I.; Bain, I.M.; Cundall, J.; Yiannakou, Y. Transanal Irrigation for Refractory Chronic Idiopathic Constipation: Patients Perceive a Safe and Effective Therapy. Gastroenterol. Res. Pract. 2017, 2017, 3826087. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.G. Royal Society of Medicine, Section of Proctology; Meeting 27 November 1974. President’s Address. Anorectal incontinence. Proc. R. Soc. Med. 1975, 68, 681–690. [Google Scholar] [PubMed]
- Habashy, E.; Mahdy, A.E. Patient-Reported Outcome Measures (PROMs) in Pelvic Floor Disorders. Curr. Urol. Rep. 2019, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, G.; Emmanuel, A. Neurogenic bowel dysfunction: Pathophysiology, clinical manifestations and treatment. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Faaborg, P.M.; Christensen, P.; Krassioukov, A.; Laurberg, S.; Frandsen, E.; Krogh, K. Autonomic dysreflexia during bowel evacuation procedures and bladder filling in subjects with spinal cord injury. Spinal Cord 2014, 52, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Faaborg, P.M.; Christensen, P.; Kvitsau, B.; Buntzen, S.; Laurberg, S.; Krogh, K. Long-term outcome and safety of transanal colonic irrigation for neurogenic bowel dysfunction. Spinal Cord 2009, 47, 545–549. [Google Scholar] [CrossRef] [PubMed]

| Reference | Study Design | TAI Cohort (Total Cohort) | Follow-Up Time | Inclusion Criteria | Patient Characteristics | Details on TAI | Bowel Function Outcome | Quality of Life Outcome | Discontinuation | Adverse Events | Quality Assessment θ [17] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gardiner 2004 [19] | Prospective cohort | 4 | 6 weeks | N/A | 2 with MS, 1 with epilepsy, 1 with transverse myelitis | N/A | Successful outcome in all patients | N/A | No one discontinued | N/A | Reporting: 2 External: 1 Internal: 4 Power: 0 Total score: 7 |
| Christensen 2006 [7] | Multicentre randomised controlled trial TAI or conservative treatment (CT) | 42 (87) | 10 weeks | At least 3 months after SCI Presence of one of four predefined bowel symptoms | SCI and SB Age (years), mean (SD): 47.5 (12.8) Male/female: 29/13 Predominant symptoms: CC: 76% FI: 21% Other: 3% Duration of bowel symptoms (months), median (range): 54 (4–780) American Spine Injury Association score (complete/incomplete): T9 and above: 21/10 T10-L2: 3/5 L3-S1: 1/1 S2 and below: 0/1 | Peristeen® (Coloplast A/S, Denmark) Volume (mL), median (range): 700 (200–1500) Frequency: 16% every day, 49% every second day, 35% 1–3 times/week 62% self-administered Trained by a specialist nurse | Termination scores: CCCS * [29], mean (SD): TAI: 10.3 (4.4) CT: 13.2 (3.4) (p = 0.0016) FIGS score * [30], mean (SD): TAI: 5.0 (4.6) CT: 7.3 (4.0) (p = 0.015) NBD score * [31], mean (SD): TAI: 10.4 (6.8) CT: 13.3 (6.4) (p = 0.48) Total time spent on bowel management daily (min), mean (SD): TAI: 47.0 (25.0) CT: 74.4 (59.8) (p = 0.040) | Termination scores, modified FIQLS * [32], mean (SD): Lifestyle: TAI: 3.0 (0.7) CT: 2.8 (0.8) (p = 0.13) Coping/ behaviour: TAI: 2.8 (0.8) CT: 2.4 (0.7) (p = 0.013) Depression/ self-perception: TAI: 3.0 (0.8) CT: 2.7 (0.8) (p = 0.055) Embarrassment: TAI: 3.2 (0.8) CT: 2.8 (0.9) (p = 0.024) | 12 (29%) patients discontinued: 25% repeated expulsions of catheter, 17% prior to training, 17% lost to follow-up, 8% lack of compliance, 8% dislike of TAI, 8% burst of rectal balloons, 8% inefficacy, 8% adverse events | 14 (36%) patients experienced side effects: 15.7% abdominal pain, 10.5% sweating, 7.0% chills, 5.9% pronounced general discomfort, 5.4% dizziness, 3.0% pounding headache, 2.7% flushing, 1.4% anorectal pain No significant difference in the proportion of patients experiencing side effects between the groups (p = 0.052) 4 adverse events in TAI group. 3 serious adverse events | Reporting: 11 External: 3 Internal: 11 Power: 1 Total score: 26 |
| Christensen 2008 [20] | Multicentre prospective cohort | 62 42 overlap- ping with Christensen 2006 [7] | 10 weeks | At least 3 months after SCI Presence of one of four predefined bowel symptoms | SCI and SB Age (years), mean (range): 47.5 (25–76) Male/female: 45/17 Predominant symptoms: CC: 76% FI: 18% Other: 6% Duration of bowel symptoms (months), median (range): 60 (4–776) Complete/incomplete: 37/25 Level of injury: Supraconal: 61 Conal/cauda equina (S2–S4): 1 | Peristeen® (Coloplast A/S, Denmark) Volume (mL), median (range): 650 (0–1500) Frequency: 20% every day, 48% every second day, 30% 1–3 times/week, 2% never In patients irrigating daily, 40% need assistance; 60% of those who irrigated every second day needed assistance Trained by a specialist nurse | Post-treatment–pre-treatment score, mean (95% CI): CCCS: −3.4 (−4.6; −2.2) (p < 0.0001) FIGS score: −4.1 (−5.2; −2.9) (p < 0.0001) NBD score: −4.5 (−6.6; −2.4) (p < 0.0001) | N/A | 17 (27%) patients discontinued: 29% repeated expulsions, 24% lost to follow-up, 12% prior to training, 12% inefficacy, 6% leakage of water around catheter, 6% dislike of treatment, 6% bursts of rectal balloons, 6% adverse events, | N/A | Reporting: 11 External: 3 Internal: 8 Power: 0 Total score: 22 |
| Del Popolo 2008 [21] | Multicentre prospective cohort | 33 | 3 weeks | Congenital SCI or acquired SCI at least 6 months previously Severe NBD with unsatisfactory bowel management | SCI, MS and SB Age (years), median (SD): 31.6 (13.3) Male/female: 18/15 Predominant symptoms: FI: 13% CC: 84% Not recorded: 3% Complete/incomplete: 13/14 | Peristeen® (Coloplast A/S, Denmark) Volume (mL), mean (SD): 789 (222) Frequency: 15% ≥1 time a day, 55% every second day, 30% 1–3 times a week 100% self- administered Trained by a specialist nurse | Pre/post-treatment: Likert like scale: Abdominal discomfort (p < 0.001) Incomplete evacuation (p < 0.001) Leakage of faeces (p = 0.002) Gas incontinence (p = 0.002) 11-point Likert scale: Increase in opinion of bowel function p = 0.001 Defaecation time: Decrease in time spent on evacuation p = 0.004 | 11-point Likert scale: Increase in QoL score p = 0.001 | 1 (3%) patient discontinued: 3% lost to follow-up | No adverse events recorded | Reporting: 10 External: 3 Internal: 7 Power: 0 Total score: 20 |
| Loftus 2012 [22] | Prospective cohort | 11 | 3–28 months | NBD Unsatisfactorily treated with conservative management | SCI and SB Age (years), mean (range): 44 (27–72) Male/female: 7/4 Complete/incomplete: 4/5 Level of injury: 1 C4, 2 C7, 1 T4, 1 T5, 2 T6, 2 L1 | Peristeen® (Coloplast A/S, Denmark) Trained by a specialist nurse | Post-treatment–pre-treatment score, mean: CCCS: −7.55 (p < 0.001) FIGS score: −5.36 (p < 0.001) NBD score: −10.32 (p < 0.005) | N/A | N/A | No major adverse events | Reporting: 7 External: 3 Internal: 4 Power: 0 Total score: 14 |
| Kim 2013 [23] | Multicentre prospective cohort | 52 | 6 months | SCI at least 6 months previously Unsatisfactorily treated with conservative management | SCI Age (years), median (range): 45.5 (18–65) Male/female: 41/10 Predominant symptoms, multiple choice: FI: 29% CC: 54% Pain/discomfort during defaecation:38% Haemorrhoid or anal bleeding: 35% Autonomic dysreflexia: 17% Injury type: Tetraplegia: 28 Paraplegia: 24 | Peristeen® (Coloplast A/S, Denmark) Volume (mL), mean (SD): 789 (153) Frequency: 11% every day, 17% every second day, 72% twice every week 33% self-administered Trained by an investigator | Pre/post-treatment: Self-reported impact of bowel function on QoL increased measured with a ICF qualifier scale * [33] (p = 0.003) Decreased defaecation time (p = 0.003) At 6 months FU: Satisfaction of TAI (10-point Likert scale (10 = perfect satisfaction), mean (SD): 8.33 (1.37) | At 6 months FU: Impact of TAI on QoL (10-point Likert scale (10 = perfect satisfaction), mean (SD): 8.44 (1.34) | 34 (66%) patients discontinued (reasons, multiple choice): 26% time-consuming, 25% personal reasons, 24% inefficacy, 15% adverse events, 12% expulsion of catheter, 6% difficulties cleaning up after TAI, 6% dislike of treatment, 3% leakage of irrigation fluid | 15 (29%) patients experienced side effects: 17% abdominal pain or discomfort, 6% minor anal bleeding, 2% hot flash, 2% headache, 2% perianal discomfort, 2% perspiration, 2% general discomfort, 2% fatigue | Reporting: 11 External: 3 Internal: 9 Power: 0 Total score: 22 |
| Hamonet-Torny 2013 [24] | Retrospective | 16 | Mean (range): 31 (7.5–66) months | Patients benefitting from TAI | SCI, MS, SB, multiple system atrophy Age (years), mean: 49 Predominant symptoms: CC: 75% CC + FI: 19% CC + perianal pain: 6% Injury type: Tetraplegia: 3 Paraplegia: 2 | Peristeen® (Coloplast A/S, Denmark) Volume (mL), mean: 922 Mean irrigation frequency: twice a week 38% self-administered Irrigation time (min), mean: 20.3 Time to obtain defaecation after irrigation (min), mean: 18.33 Formal education, except one | NBD score, mean: 6.25 CCIS * [34,35]: 0.50 62.5% irrigated after a mean of 31 months Time spent on bowel management < 30 min for 60% of patients Difference in consumption of laxatives, mean: Before: 1.66 After: 1.4 (p = 0.6783) | N/A | 6 (38%) patients discontinued: 50% inefficacy, 13% heavy administration, 13% vomiting following administration | 1 (6%) patients experienced anal bleeding 1 adverse event | Reporting: 9 External: 1 Internal: 6 Power: 0 Total score: 16 |
| Adriaansen 2015 [25] | Multicentre cross-sectional | 29 (258) | N/A | SCI with time since injury of ≥10 years Age at injury 18–35 years Current age 28–65 years Using a wheelchair ≥ 500 m | SCI Age (years), mean (range): 45 (29–64) Male: 77% Time since injury (years), mean (range): 22 (10–46) Injury type: Tetraplegia: 12 Paraplegia: 17 | N/A | Severe NBD: 41.4% Dissatisfied/very dissatisfied with TAI, 5-point Likert scale: 17.2% Perianal problems: 41.4% CC: 27.6% FI at least once a month: 34.5% Average > 60 min required for defaecation: 24.1% | N/A | N/A | N/A | Reporting: 7 External: 2 Internal: 6 Power: 0 Total score: 15 |
| Preziosi 2012 [26] | Prospective cohort | 37 | 6 weeks | Failure of biofeedback Not eligible for biofeedback No response to conservative treatment MS and NBD | MS Age (years), median (range): 49 (42–56) Male/female: 3/27 | Peristeen® (Coloplast A/S, Denmark) Recommended volume between 500–1500 mL Recommended irrigation frequency every third day adjusted according to response 93% self-administered Trained by a specialist nurse | Pre/post-treatment: CCCS, median (IQR): Pre: 12 (8.75–16) Post: 8 (4–12.5) (p = 0.001) CCIS, median (IQR): Pre: 12 (4.75–16) Post: 4 (2–8) (p < 0.001) | Pre/post-treatment: SF-36 * [36], mean (SD): Pre: 51.3 (7.8) Post: 50.4 (7.8) (p = 0.051) | 7 (19%) patients discontinued prior to irrigation training 14 (47%) patients discontinued during trial At 6 months of follow-up, all responders continued using the irrigation, with the exception of 2 patients | N/A | Reporting: 10 External: 3 Internal: 9 Power: 0 Total score: 22 |
| Passananti 2016 [27] | Multicentre prospective cohort | 49 | Minimum 1 year with a mean of 40 months | MS and NBD for ≥6 months Bowel symptoms for ≥6 months not responding to conservative management | MS Age (years), mean (range): 51 (26–80) Male/female: 12/37 Predominant symptoms: FI: 33% CC: 67% | Peristeen® (Coloplast A/S, Denmark) Frequency: 48% irrigating daily, 48% every second day, 4% every third day 98% self-administered Trained by a specialist nurse | Pre/post-treatment: FI (weekly episodes), mean (range): Pre: 4.8 (1–21) Post: 0.9 (0–7) (p < 0.005) Severe NBD: Pre: 47% Post: 18% | Pre/post-treatment: EQ-5D * [37] utility score, mean (95% CI): Pre: 0.57 (0.5;0.65) Post: 0.52 (0.4;0.63) EQ-VAS score, mean (95% CI): Pre: 44.5 (41.26;47.73) Post: 63.4 (58.41;68.49) | 22 (45%) patients discontinued: 55% dislike of treatment, 14% inefficacy, 9% adverse events, 9% other pathology, 9% lost to follow-up, 5% burst of rectal balloons | N/A | Reporting: 10 External: 3 Internal: 8 Power: 0 Total score: 21 |
| Brochard 2019 [28] | Prospective cohort | 15 (57) | Not specified for TAI group. FU for entire cohort: 46 (±36) months | Spinal dysraphism Evaluation by gastroenterologist | SB Not specified for the TAI cohort | N/A | Pre/post-treatment: Improvement of CCIS * ≥ 50%: 46.7% Variation of CCIS <50%: 19.5% (p = 0.016) | N/A | N/A | N/A | Reporting: 10 External: 3 Internal: 8 Power: 0 Total score: 21 |
| Reference | Study Sesign | TAI Cohort (Total Cohort) | Follow-Up Time | Inclusion Criteria | Patient Characteristics | Details on TAI | Bowel Cunction Outcome | Quality of Life Outcome | Discontinuation | Adverse Events | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iwama 1989 [38] | Prospective cohort | 10 | N/A | N/A | LARS 2 Turnbull-Cutait, 2 extra anal staple sutures, 1 pull-through operation, 5 anterior resections Age (years), mean (range): 61.4 (38–75) Male/female: 7/3 Predominant symptom: Frequent urge to defecate | Colostomy wash-out set (Hollister Incorporated, USA or Eisai Company, Japan) Irrigation volume (mL), range: 200–1000 Irrigation time (min), range: 20–50 Frequency of irrigation: 10% twice a day, 60% every day, 10% every second day, 20% once a week | In all cases, the frequent urge to defecate disappeared | N/A | Two patients continued using irrigation for more than 5 years, approximately once a week without any complications. | N/A | Reporting: 6 External: 1 Internal: 3 Power: 0 Total score: 10 |
| Koch 2009 [39] | Prospective cohort | 26 | Mean (SD): 1.6 (1.1) years | FI after LAR for rectal cancer | LARS 30 rectal cancer Age (years), mean (SD): 67.6 (7.4) Male/female: 21/5 FU (years) after LAR, mean (SD): 4.7 (3.5) | Biotrol® Irrimatic pump (B. Braun Medical A/S, Germany) Irrigation volume (mL), mean (SD): 1500 (600) Irrigation time + defaecation time (min), mean (SD): 43.9 (27.3) Frequency (day), mean (SD): 1.8 (0.7) | Pre-/post-treatment: William’s Incontinence Score * [45], mean (SD): Pre: 4.5 (0.6) Post: 1.7 (0.9) (p < 0.0001) 57% pseudo continent, 14% incontinent for flatus, 29% incontinent for liquid stools | N/A | 5 (19%) discontinued: 10% improved and stopped TAI, 80% were not satisfied | 16 (62%) patients experienced side effects: 27% abdominal cramps, 23% leakage after irrigation, 7% time-consuming, 30% other (nausea, pain inserting cone etc.) | Reporting: 10 External: 1 Internal: 8 Power: 0 Total score: 19 |
| Rosen 2011 [40] | Multicentre Prospective cohort | 14 | Median (range): 29 (15–46) months | LARS Minimum 9 months after stoma reversal Insufficient conservative treatment | LARS 12 rectal cancer, 2 large villous adenomas Age (years), median (range): 68 (45–80) Male/Female: 11/3 Time (months) from LAR or stoma reversal to assessment, median (range): 19 (9–48) Neoadjuvant radiotherapy (n): 10 | Peristeen® (Coloplast A/S, Denmark) (2 used a Foley catheter) Volume (mL), median (range): 900 (500–1500) Irrigation frequency: 64% every day, 28% every second day, 7% every third day 100% self-administered Trained by a specialist nurse | Pre-/post-treatment: Defaecation episodes (n)/day, median (range): 8 (4–12) to 1 (1–2) (p < 0.001) Defaecation episodes (n)/night, median (range): 3 (2–5) to 0 (0–0) (p < 0.0001) CCIS, median (range): 17 (15–20) to 5 (4–9) (p < 0.01) | Pre-/post-treatment: MCS SF-36 *: 46 (35–55) to 55 (45–60) (p < 0.01) PCS SF-36 *: 55 (41–60) to 56 (49–62) (p = 0.3061) All domains of FIQLS were improved (p < 0.001) | No patients discontinued | 3 (21%) patients experienced transient abdominal pain, 4 (29%) patients experienced minor rectal bleeding | Reporting: 11 External: 2 Internal: 7 Power: 0 Total score: 20 |
| Martellucci 2018 [41] | Prospective cohort | 33 | 6 months TAI following 3 months enema treatment | Short-term or long-term LARS with a LARS score ≥ 30 Failed conservative treatment | LARS 25 rectal cancer, 1 ulcerative colitis, 1 diverticular disease Age (years), median (range): 61 (29–83) Male/Female: 17/10 Neoadjuvant RT (n): 18 21 total mesorectal excision, 3 partial mesorectal excision, sigmoid resection 2, 1 total colectomy | Peristeen® (Coloplast A/S, Denmark) Volume (mL), median (range): 450 (300–1000) Frequency: 3–4 times per week Trained by a specialist nurse | Pre-/post-treatment: Daily number of bowel movements, median (range): Pre: 7 (0–14) Post: 1 (0–4) Post enema: 4 (0–13) LARS score * [46,47,48], median (range): Pre: 35.1 (30–42) Post: 12.2 (0–21) (p < 0.0001) Post enema: 27 (5–39) (p < 0.0001) MSKCC BFI * [49]: Significant improvement in frequency items, urgency items, incomplete emptying, and clustering of the No difference in effect between short-term and long-term LARS | Four scales of SF-36 significantly improved (mental health, social functioning, role emotional and bodily pain). | 6 (18%) patients discontinued: 17% refused participation, 50% cancer recurrence, 17% proctitis, 17% dissatisfaction with protocol 85% continued TAI after the study | N/A | Reporting: 10 External: 3 Internal: 9 Power: 0 Total score: 22 |
| Enriquez-Navascues 2019 [42] | Randomised controlled trial TAI or percutaneous tibial nerve stimulation | 13 (27) | 6 months | LARS score > 29 Total mesorectal excision for rectal cancer 1 year since LARS or stoma reversal | LARS 13 rectal cancer Age (years), mean (range): 68 (48–71) Male/female: 9/4 Duration (months) of LARS, median (range): 30 (13–84) Neoadjuvant chemoradiotherapy: 6 | Peristeen® (Coloplast A/S, Denmark) Volume: Adjusted for each patient Frequency of irrigation: Initially once a day then adjusted to 3–4 times a week 100% self-administered Trained by a specialist nurse | Intention-to-treat: Reduction in LARS grade in at least 50% of patients: 8 out of 13 patients fell from major to minor LARS Per-protocol: LARS score, median (IQR): 35 (32–39) to 12 (12–26) (p = 0.021) 80% of patients treated with TAI reported a reduction of at least 50% in the FIGS score No significant improvement in the ODS * [50] score | For EORTC-QLQ-C30 * [51] VAS scores of Global health status improved (p = 0.020) | 3 (23%) discontinued: 23% no acceptability of TAI | No significant adverse events | Reporting: 11 External: 3 Internal: 9 Power: 0 Total score: 23 |
| Rosen 2019 [43] | Multicentre randomised controlled trial TAI or best supportive care (BS) as prophylaxisfor LARS immediately after ileostomy closure | 18 (37) Rectal resection for rectal cancer | One week, 1 month, 3 months | Rectal resection for rectal cancer Anastomotic height < 5 cm above dentate line Complete healing of anastomosis Informed consent and physical and mental capability to perform TAI | LARS 18 rectal cancer Age (years), median (range): 58.5 (52–70) Male/female: 12/6 Neoadjuvant radiotherapy: 15 | Peristeen® (Coloplast A/S, Denmark) or Foley catheter (28 French) Irrigation volume: 1000 mL Irrigation frequency: Every 24 h Irrigation time (min), median (range): 45 (30–60) 100% self-administered Trained by a specialist | Maximum number of defaecation episodes during daytime at 1 month, median (range): TAI: 3 (1–10) vs BS: 7 (3–30) (p = 0.003) Maximum number of defaecation episodes during night at 3 months, median (range): TAI: 0 (0–2) vs. BS: 1 (1–5) (p = 0.002) LARS score at 3 months, median (range): TAI: 9 (0–34) vs. BS: 31 (3–42) (p = 0.001) CCIS at 3 months, median (range): TAI: 2 (0–11) vs. BS: 6 (0–17) (p = 0.046) | MCS SF-36 at 3 months, median (range): TAI: 55 (31–60) vs. BS: 57 (26–63) (p = 0.436) PCS SF-36 at 3 months, median (range): TAI: 50 (39–64) vs. BS: 51 (37–61) (p = 0.741) | 1 (6%) patients discontinued | No complications related to TAI | Reporting: 11 External: 2 Internal: 11 Power: 1 Total score: 25 |
| Rosen 2020 [44] | Multicentre prospective cohort | 19 (37) | 12 months FU from Rosen 2019 [43] | See Rosen 2019 [43] | See Rosen 2019 [43] | Peristeen® (Coloplast A/S, Denmark) or Foley catheter Volume (mL), median (range): 600 (range 200–1000) Irrigation frequency: 50% every day, 30% every second day, 20% not on a regular schedule but at least 2/week. 100% self-administered | Maximum number of defaecation episodes during, median (range): Day: TAI: 3 (1–6) vs. BS: 5 (2–10) (p = 0.018) Night: TAI: 0 (0–1) vs. BS 1 (0–5) (p = 0.004) LARS score, median (range): TAI: 18 (9–32) vs. 30 (3–39) (p = 0.063) CCIS: TAI: 4 (0–12) vs. BS: 7 (0–16) (p = 0.151) | MCS SF-36, median (range): TAI: 52 (34–59) vs. BS: 56 (28–62) (p = 0.325) PCS SF-36, median (range): TAI: 55 (50–67) vs. 5 (31–59) (p = 0.460) | 9 (47%) patients discontinued: 89% time-consuming, 11% pain during TAI | N/A | Reporting: 10 External: 3 Internal: 9 Power: 0 Total score: 23 |
| Reference | Study Design | TAI Cohort (Total Cohort) | Follow-Up Time | Inclusion Criteria | Patient Characteristics | Details on TAI | Bowel Function Outcome | Quality of Life Outcome | Discontinuation | Adverse Events | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Briel 1996 [52] | Prospective cohort | 16 | Median of 18 months | Impaired continence | Heterogeneous aetiology Age (years), median (range): 52 (25–72) Male/female: 5/11 FI: 16 | System unspecified Irrigation time (min), median (range): 30 (10–90) Irrigation frequency: 87% ≥ 1 time a day Trained by enterostomal therapist | 38% reported a successful outcome | N/A | 6 (38%) patients discontinued | N/A | Reporting: 4 External: 1 Internal: 4 Power: 0 Total score: 9 |
| Crawshaw 2003 [53] | Prospective cohort | 48 | Median (range): 11 (4–27) months | Absence of correctable pathology or the failure of medical and surgical treatment | Heterogeneous aetiology Age (years), median (IQR): 54 (41–61) Male/female: 13/35 Symptoms: FI: 33 CC: 15 | Equipment adapted from a Coloplast Stoma Irrigation set (Coloplast A/S, Denmark) Irrigation volume: 1500 mL Irrigation frequency: 5% twice a day, 38% daily, 17% on alternate days, 15% every 3–7 days, 19% as required Trained by specialist nurse | Bowel control, visual analogue scale: Successful response to TAI in 24 (50%) patients. Bowel rating among these 24 patients, VAS 100 maximum (100 = full control), median (IQR): Pre: 15 (3–24) Post: 50 (34–65) | QoL among 24 patients with successful outcome, median (IQR): 59.16 (46.55–67.43) No difference compared to the 24 patients without successful response | 4 (8%) patients discontinued: 50% unacceptable, 50% relief of symptoms with rectopexy | N/A | Reporting: 8 External: 2 Internal: 8 Power: 0 Total score: 18 |
| Gardiner 2004 [19] | Prospective cohort | 57 | 6 weeks | Symptoms: FI: 16 CC: 41 | N/A | Proportion of patients with successful outcome: FI: 75% CC: 51% Slow transit CC (n = 15): 57% Obstructed defaecation (n = 26): 42% | N/A | FI: 2 (12.5%) patients discontinued: 6.25% not severe enough symptoms to continue TAI, 6.25% still under review | N/A | Reporting: 2 External: 1 Internal: 4 Power: 0 Total score: 7 | |
| Cazemier 2007 [54] | Cross-sectional | 40 | Time (y) using irrigation, mean (range): 8.5 (2.5–18) | FI or CC TAI No response to medical treatment or biofeedback | Heterogeneous aetiology Includes NBD FI: 28 Age (years): 42 Male/Female: 5/23 CC: 12 Age (years): 45 Male/Female: 3/9 | Iryflex® (B. Braun Medical A/S, Germany) Irrigation volume: 500–1000 mL Frequency: 32% daily, 36% 3 times/week, 32% twice or less/week | 25 (63%) patients still used TAI Overall satisfaction (n = 40): 29 (73%) Actual users (n = 25), satisfaction: 22 (88%) | N/A | Overall, 15 (38%) discontinued: FI: 5 (29%) CC: 7 (58%) | Side effects: 37.5% abdominal cramps | Reporting: 9 External: 3 Internal: 10 Power: 0 Total score: 22 |
| Koch 2008 [55] | Prospective cohort | 39 | 3, 6 and 12 months | FI or CC or both after failed conservative treatment or after (partially) unsuccessful surgical treatment for defaecation disorder | Heterogeneous aetiology Age (years), mean (SD): 58 (13.5) Male/Female: 13/26 Symptoms: FI: 18 CC: 11 FI + CC: 10 | Biotrol® Irrimatic pump (B. Braun Medical A/S, Germany) or irrigation bag Braun (B. Braun Medical A/S, Germany) 1-year FU: Irrigation volume (L), mean (SD): 1.75 (0.79) Irrigation time (min), mean (SD): 36.39 (16.02) Frequency (time/day), mean (SD): 1.1 (0.49) Trained by physician | 3 months FU, number (%) pseudo continent: FI: 11 (61%) (p < 0.001) FI + CC: 6 (60%) (p = 0.009) Baseline compared with 1-year FU: FI: Park’s score [61]: 3.61 (0.5) to 1.6 (0.92) (p < 0.005) CCCS: Feeling of incomplete evacuation: 1.60 (2.47) to 2.75 (1.36) (p = 0.036) | Improvement in overall QoL measured with SF-36 and the FIQLS (p = 0.012) | 9 (23%) patients discontinued: 78% unsatisfactory results, 22% appendicostomy | 23 (59%) experienced side effects: 7% leakage after irrigation, 16% abdominal cramps, 22% abdominal bloating, 13% combination of the above side effects, 2% other | Reporting: 11 External: 2 Internal: 8 Power: 0 Total score: 21 |
| Vollebregt 2016 [56] | Prospective cohort | 60 | Median FU: 12 months | Chronic defaecatory disorders not responding to conservative treatment | Heterogeneous aetiology Includes NBD and colorectal surgery Age (years), median (range): 49 (21–74) Male/female: 15/45 Symptoms: FI: 8 CC: 44 FI + CC: 8 | Peristeen® (Coloplast A/S, Denmark) or Biotrol® Irrimatic pump (B. Braun Medical A/S, Germany) Irrigation volume (mL), median (range): 875 (250–2200) Frequency: 6% twice/day, 52% daily, 33% every second day, 6% when needed Trained by enterostomal therapist | First FU: FIQLS score did not differ between patients continuing or discontinuing TAI | First FU: Using SF-36 patients continuing TAI had more energy and were less fatigued compared with patients discontinuing TAI (p = 0.01) Patients continuing TAI had a tendency to have a higher SF-36 social functioning and a higher total SF-36 score, but this was non-significant | 33 (55%) of patients had discontinued at the first FU, 37 (62%) at second FU and 38 (63%) at last FU | N/A | Reporting: 10 External: 3 Internal: 8 Power: 0 Total score: 21 |
| Juul 2017 [57] | Prospective cohort | 507 | Mean (range): 1.06 (0.52–1.46) years | Intractable FI and/or CC with unsatisfactory results after conservative treatment | Heterogeneous aetiology Includes NBD and anorectal surgery Age (years), median (range): 56 (19–86) Male/female: 84/423 Symptoms: FI: 238 CC: 171 FI + CC: 98 | Coloplast irrigation bag ®/Colotip® (Coloplast A/S, Denmark) (majority), Coloplast irrigation bag® (Coloplast A/S, Denmark)/Qufora cone® (MBH International A/S), Aqua colon enema tip with silicone balloon ch 24® (Runfold Plastics Ltd., UK) or Peristeen® (Coloplast A/S, Denmark) Irrigation volume (mL), median (IQR): 1000 (750–1000) Irrigation time (min), median (IQR): 20 (15–30) Frequency: 35% daily, 16% every second day, 20% 2–3 times/week, 21% < once a week Self-administered 99%, assistance 1% Trained by specialist nurse | Patients with FI, pre-/post-treatment, mean change (95% CI): 11-point Likert, FI: 2.7 (2.2–3.2) (p < 0.001) CCIS: 2.2 (1.6–2.8) (p < 0.001) FIGS score: 2.2 (1.5–2.9) (p < 0.001) 65% improvement of FI, 29% stability, and 6% deterioration. Patients with CC, pre/post-treatment, mean change (95% CI): 11-point Likert, CC: 1.6 (0.9–2.4) (p < 0.001) CCCS: 1.9 (1.1–2.7) (p < 0.001) ODS score: 3.3 (2.0–4.5) (p < 0.001). 48% improvement of CC, 40% stability and 12% deterioration. | Patients with FI and CC, pre-/post-treatment, mean change (95% CI): 11-point Likert, QoL: 1.8 (1.4–2.2) (p < 0.001) | 174 (34%) discontinued: 49% inefficacy, 18% dislike treatment, 16% symptoms resolved, 13% time consumption, 12% side effects, 8% practical problems, 21% other, 8% undetermined | 120 (58%) patients experienced side effects: 23% abdominal pain, 15% anorectal pain, 6% chills/shivering, 11% nausea, 8% dizziness, 13% sweating | Reporting: 11 External: 2 Internal: 8 Power: 0 Total score: 21 |
| Bildstein 2017 [58] | Retrospective | 108 | 1-year FU | FI or CC Refractory to conservative treatment | Heterogeneous aetiology Includes NBD Age (years), mean (range): 55 (18–83) Male/female: 21/87 Symptoms CC: 51 FI + CC: 47 FI: 10 | Peristeen® (Coloplast A/S, Denmark) Trained by specialist nurse | 1-year FU: 46 (42.6%) patients still irrigated 62 (57%) discontinued: 44 had discontinued, 5 failed during first training, 12 lost to follow-up and 1 died | N/A | Reasons for discontinuation: 36.4% technical problems, 40.9% inefficacy, and 22.7% constraints (primary time-consuming) Median (range) time before discontinuation: 3 (0.2–11) months | 25 (54.3%) reported minor 47 minor and self-limiting adverse events: 34% leakage of fluid around catheter, 29.9% pain when inserting catheter or water, 19.1% catheter expulsion, 10.6% rectal balloon burst, 6.4% water retention | Reporting: 11 External: 3 Internal: 9 Power: 0 Total score: 23 |
| van der Hagen 2012 [59] | Multicentre non-randomised trial | 35 (70) | 6 months | History of birth trauma Passive faecal incontinence CCIS ≤ 8 after anal sphincter exercise and biofeedback Defect of the internal anal sphincter | Sphincter damage after birth trauma Age (years), mean (range): 53 (38–74) | REPROP® Clyster Trained by specialist nurse | In 3 (9%) patients faecal incontinence resolved completely Baseline 6-month FU: CCIS, average number of days per week with incontinence for solid or liquid stools, and average number of pads used did not change significantly | N/A | 3 (9%) patients discontinued | No severe adverse effects | Reporting: 11 External: 2 Internal: 7 Power: 0 Total score: 20 |
| Etherson 2017 [60] | Prospective cohort | 102 | Length of therapy use, median (range): 30.15 (1–460) weeks | Fulfilled Rome II criteria Past or present TAI treatment Received TAI for chronic idiopathic constipation (CIC) Failed all medical and behavioural therapies | Chronic idiopathic constipation (CIC) Age (years), median (range): 45 (25–84) Male/female: 7/95 Duration (years) of CIC, mean (SD): 21.8 (16.9) | Peristeen® (Coloplast A/S, Denmark) (majority), Qufora ® (MBH International A/S) Biotrol® Irrimatic pump (B. Braun Medical A/S, Germany) Frequency: on average every second day | Overall symptom improvement: Bowel frequency: 42% Clearance of rectum: 63% Abdominal pain: 48% Bloating: 49% General well-being: 65% Awareness of urge: 25% Overall satisfaction with TAI was reported by 67% as either moderately better or very much better | N/A | 48 (47%) patients discontinued | 22 (22%) patients experienced side effects: 6% rectal bleeding, 3% painful irrigations, 2% painful haemorrhoids, 2% new anal fissure, 10% bursting balloons, 3% splitting of catheter | Reporting: 10 External: 2 Internal: 8 Power: 0 Total score: 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekhael, M.; Kristensen, H.Ø.; Larsen, H.M.; Juul, T.; Emmanuel, A.; Krogh, K.; Christensen, P. Transanal Irrigation for Neurogenic Bowel Disease, Low Anterior Resection Syndrome, Faecal Incontinence and Chronic Constipation: A Systematic Review. J. Clin. Med. 2021, 10, 753. https://doi.org/10.3390/jcm10040753
Mekhael M, Kristensen HØ, Larsen HM, Juul T, Emmanuel A, Krogh K, Christensen P. Transanal Irrigation for Neurogenic Bowel Disease, Low Anterior Resection Syndrome, Faecal Incontinence and Chronic Constipation: A Systematic Review. Journal of Clinical Medicine. 2021; 10(4):753. https://doi.org/10.3390/jcm10040753
Chicago/Turabian StyleMekhael, Mira, Helle Ø Kristensen, Helene Mathilde Larsen, Therese Juul, Anton Emmanuel, Klaus Krogh, and Peter Christensen. 2021. "Transanal Irrigation for Neurogenic Bowel Disease, Low Anterior Resection Syndrome, Faecal Incontinence and Chronic Constipation: A Systematic Review" Journal of Clinical Medicine 10, no. 4: 753. https://doi.org/10.3390/jcm10040753
APA StyleMekhael, M., Kristensen, H. Ø., Larsen, H. M., Juul, T., Emmanuel, A., Krogh, K., & Christensen, P. (2021). Transanal Irrigation for Neurogenic Bowel Disease, Low Anterior Resection Syndrome, Faecal Incontinence and Chronic Constipation: A Systematic Review. Journal of Clinical Medicine, 10(4), 753. https://doi.org/10.3390/jcm10040753






