Predicting the Risk of Postoperative Complications in Patients Undergoing Minimally Invasive Resection of Primary Liver Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Evaluation and Surgical Approaches
2.2. Data Collection and Study Endpoints
2.3. Statistical Analysis
3. Results
3.1. Cohort Characteristics
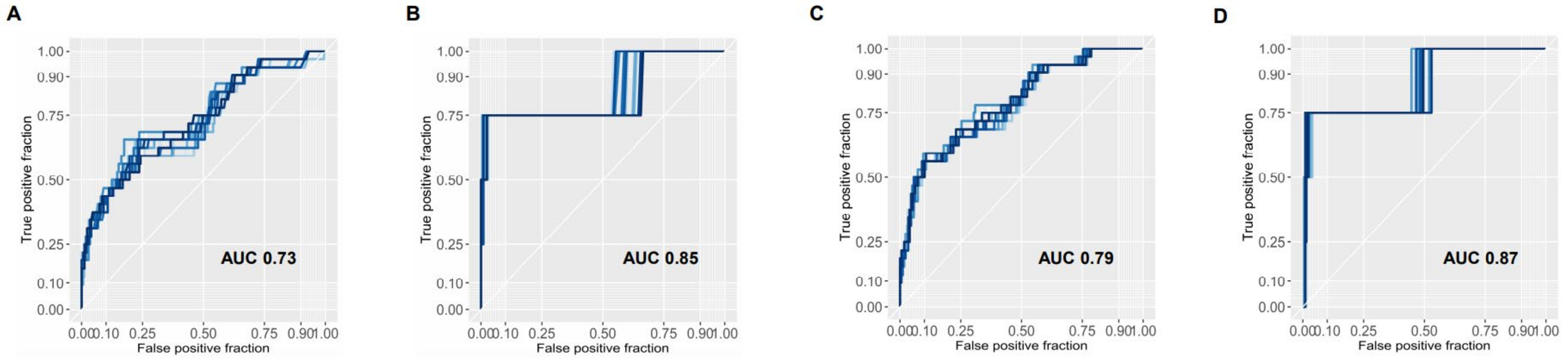
3.2. Model Generation for the Prediction of Major Complications
3.3. Prediction of Pre- and Postoperative Model for Primary and Secondary Endpoints
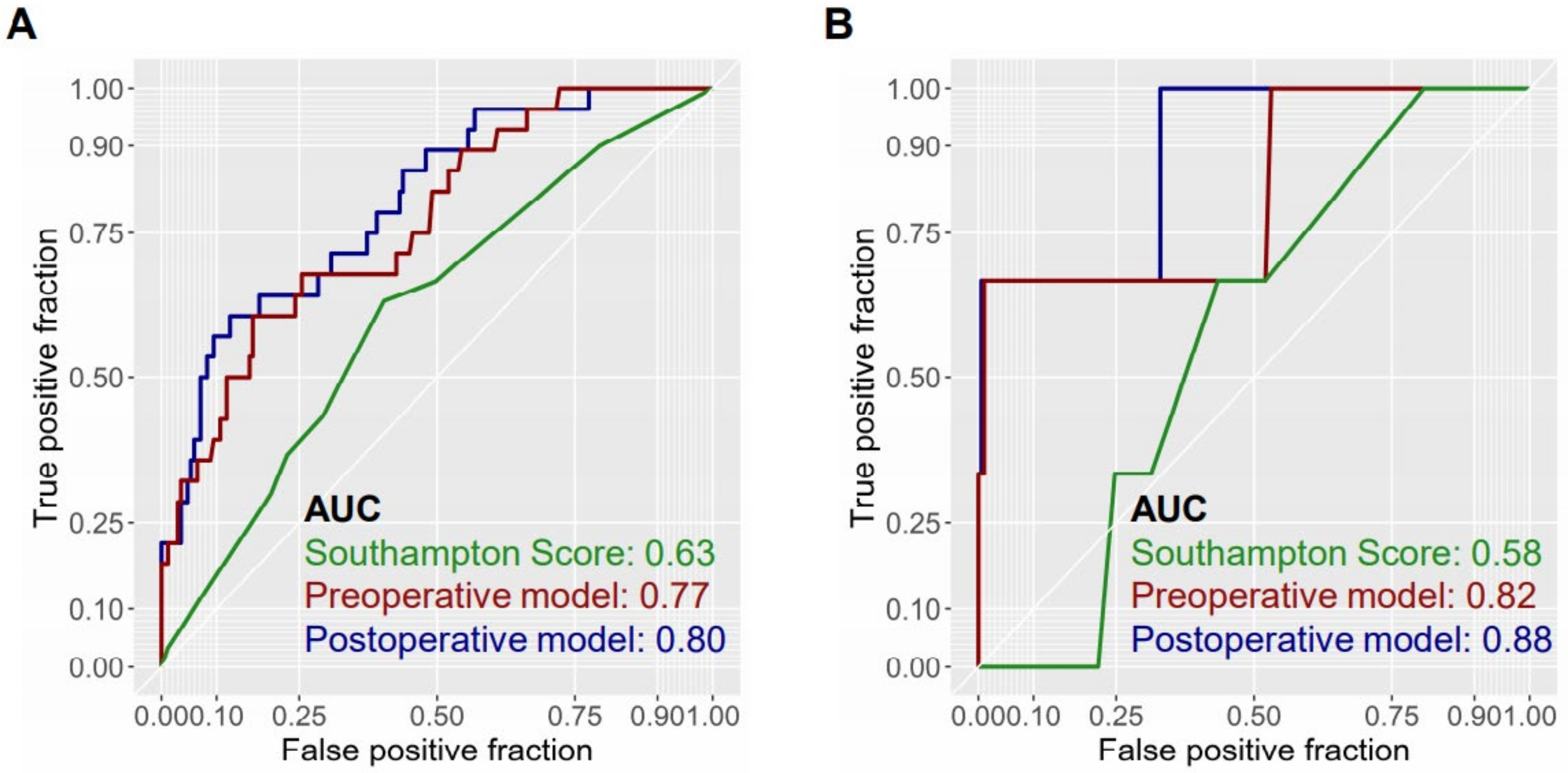
3.4. Predictive Ability of the Two Models in Patients Undergoing Major Hepatectomy
3.5. Comparison of Predictive Models with Previously Reported Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilal, M.A.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J.; et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.-W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.M.; Lee, J.Y.; Kim, S.H.; Yoon, J.H.; Kim, Y.J.; Han, J.K.; Choi, B.I. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: Long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014, 270, 900–909. [Google Scholar] [CrossRef]
- Sala, M.; Llovet, J.M.; Vilana, R.; Bianchi, L.; Sole, M.; Ayuso, C.; Bru, C.; Bruix, J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004, 40, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Li, H.; Wen, N.; Chen, J.; Wei, Y.; Li, B. Laparoscopic liver resection: A review of current indications and surgical techniques. Hepatobiliary Surg. Nutr. 2018, 7, 277–288. [Google Scholar] [CrossRef]
- Haber, P.K.; Wabitsch, S.; Krenzien, F.; Benzing, C.; Andreou, A.; Schöning, W.; Öllinger, R.; Pratschke, J.; Schmelzle, M. Laparoscopic liver surgery in cirrhosis—Addressing lesions in posterosuperior segments. Surg. Oncol. 2019, 28, 140–144. [Google Scholar] [CrossRef]
- Schmelzle, M.; Krenzien, F.; Schöning, W.; Pratschke, J. Laparoscopic liver resection: Indications, limitations, and economic aspects. Langenbeck’s Arch. Surg. 2020, 405, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Shehta, A.; Ahn, S.; Yoon, Y.S.; Cho, J.Y.; Choi, Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: Case-matched study with propensity score matching. J. Hepatol. 2015, 63, 643–650. [Google Scholar] [CrossRef]
- Haber, P.K.; Wabitsch, S.; Kästner, A.; Andreou, A.; Krenzien, F.; Schöning, W.; Pratschke, J.; Schmelzle, M. Laparoscopic Liver Resection for Intrahepatic Cholangiocarcinoma: A Single-Center Experience. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 1354–1359. [Google Scholar] [CrossRef]
- Ratti, F.; Rawashdeh, A.; Cipriani, F.; Primrose, J.; Fiorentini, G.; Abu Hilal, M.; Aldrighetti, L. Intrahepatic cholangiocarcinoma as the new field of implementation of laparoscopic liver resection programs. A comparative propensity score-based analysis of open and laparoscopic liver resections. Surg. Endosc. 2020, 1–12. [Google Scholar] [CrossRef]
- Krenzien, F.; Schöning, W.; Brunnbauer, P.; Benzing, C.; Öllinger, R.; Biebl, M.; Bahra, M.; Raschzok, N.; Cherqui, D.; Geller, D.; et al. The ILLS Laparoscopic Liver Surgery Fellow Skills Curriculum. Ann. Surg. 2020, 272, 786–792. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [PubMed]
- Buell, J.F.; Gayet, B.; Han, H.S.; Wakabayashi, G.; Kim, K.H.; Belli, G.; Cannon, R.; Saggi, B.; Keneko, H.; Koffron, A.; et al. Evaluation of stapler hepatectomy during a laparoscopic liver resection. HPB 2013, 15, 845–850. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guerrini, G.P.; Esposito, G.; Tarantino, G.; Serra, V.; Olivieri, T.; Catellani, B.; Assirati, G.; Guidetti, C.; Ballarin, R.; Magistri, P.; et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: The first meta-analysis. Langenbeck’s Arch. Surg. 2020, 405, 265–275. [Google Scholar] [CrossRef]
- Daskalaki, D.; Gonzalez-Heredia, R.; Brown, M.; Bianco, F.M.; Tzvetanov, I.; Davis, M.; Kim, J.; Benedetti, E.; Giulianotti, P.C. Financial Impact of the Robotic Approach in Liver Surgery: A Comparative Study of Clinical Outcomes and Costs Between the Robotic and Open Technique in a Single Institution. J. Laparoendosc. Adv. Surg. Tech. 2017, 27, 375–382. [Google Scholar] [CrossRef]
- Cheung, T.T.; Dai, W.C.; Tsang, S.H.; Chan, A.C.; Chok, K.S.; Chan, S.C.; Lo, C.M. Pure Laparoscopic Hepatectomy Versus Open Hepatectomy for Hepatocellular Carcinoma in 110 Patients With Liver Cirrhosis: A Propensity Analysis at a Single Center. Ann. Surg. 2016, 264, 612–620. [Google Scholar] [CrossRef]
- Fretland, Å.A.; Dagenborg, V.J.; Bjørnelv, G.M.W.; Kazaryan, A.M.; Kristiansen, R.; Fagerland, M.W.; Hausken, J.; Tønnessen, T.I.; Abildgaard, A.; Barkhatov, L.; et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann. Surg. 2018, 267, 199–207. [Google Scholar] [CrossRef]
- Cauchy, F.; Fuks, D.; Nomi, T.; Schwarz, L.; Barbier, L.; Dokmak, S.; Scatton, O.; Belghiti, J.; Soubrane, O.; Gayet, B. Risk factors and consequences of conversion in laparoscopic major liver resection. Br. J. Surg. 2015, 102, 785–795. [Google Scholar] [CrossRef]
- Costi, R.; Scatton, O.; Haddad, L.; Randone, B.; Andraus, W.; Massault, P.P.; Soubrane, O. Lessons learned from the first 100 laparoscopic liver resections: Not delaying conversion may allow reduced blood loss and operative time. J. Laparoendosc. Adv. Surg. Tech. 2012, 22, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ban, D.; Tanabe, M.; Ito, H.; Otsuka, Y.; Nitta, H.; Abe, Y.; Hasegawa, Y.; Katagiri, T.; Takagi, C.; Itano, O.; et al. A novel difficulty scoring system for laparoscopic liver resection. J. Hepato Biliary Pancreat. Sci. 2014, 21, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Krenzien, F.; Wabitsch, S.; Haber, P.; Kamali, C.; Brunnbauer, P.; Benzing, C.; Atanasov, G.; Wakabayashi, G.; Öllinger, R.; Pratschke, J.; et al. Validity of the Iwate criteria for patients with hepatocellular carcinoma undergoing minimally invasive liver resection. J. Hepato Biliary Pancreat. Sci. 2018, 25, 403–411. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Fuks, D.; Kokudo, N.; Gayet, B. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann. Surg. 2018, 267, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Halls, M.C.; Berardi, G.; Cipriani, F.; Barkhatov, L.; Lainas, P.; Harris, S.; D’Hondt, M.; Rotellar, F.; Dagher, I.; Aldrighetti, L.; et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. BJS Br. J. Surg. 2018, 105, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Struecker, B.; Haber, P.; Öllinger, R.; Bahra, M.; Pascher, A.; Pratschke, J.; Schmelzle, M. Comparison of Single-Port Versus Standard Multiport Left Lateral Liver Sectionectomy. Surg. Innov. 2018, 25, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, S.; Schöning, W.; Kästner, A.; Haber, P.K.; Benzing, C.; Krenzien, F.; Lenz, K.; Schmelzle, M.; Pratschke, J. A propensity-matched study of full laparoscopic versus hand-assisted minimal-invasive liver surgery. Surg. Endosc. 2020, 1–8. [Google Scholar] [CrossRef]
- Benzing, C.; Krenzien, F.; Atanasov, G.; Seehofer, D.; Sucher, R.; Zorron, R.; Pratschke, J.; Schmelzle, M. Single incision laparoscopic liver resection (SILL)—A systematic review. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2015, 4. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Prodeau, M.; Drumez, E.; Duhamel, A.; Vibert, E.; Farges, O.; Lassailly, G.; Mabrut, J.-Y.; Hardwigsen, J.; Régimbeau, J.-M.; Soubrane, O.; et al. An ordinal model to predict the risk of symptomatic liver failure in patients with cirrhosis undergoing hepatectomy. J. Hepatol. 2019, 71, 920–929. [Google Scholar] [CrossRef]
- Krenzien, F.; Schmelzle, M.; Struecker, B.; Raschzok, N.; Benzing, C.; Jara, M.; Bahra, M.; Öllinger, R.; Sauer, I.M.; Pascher, A.; et al. Liver Transplantation and Liver Resection for Cirrhotic Patients with Hepatocellular Carcinoma: Comparison of Long-Term Survivals. J. Gastrointest. Surg. 2018, 22, 840–848. [Google Scholar] [CrossRef]
- Complication Prediction Tool Shinyapps by RStudio. Available online: https://laprs.shinyapps.io/RiskScore/ (accessed on 9 February 2021).
| Complication Grading According to Clavien–Dindo | ||||
|---|---|---|---|---|
| CD 0-2 (n = 178) | CD 3-5 (n = 32) | Odds Ratio (95% CI) | p | |
| General Variables | ||||
| Age in years | 67.1 (65.6–68.7) | 65.9 (61.6–70.1) | 0.981 (0.949–1.016) | 0.26 |
| Male gender | 121 (68.0) | 22 (68.8) | 1.036 (0.471–2.421) | 0.93 |
| BMI in kg/m2 | 26.8 (26.1–27.5) | 26.5 (24.7–28.4) | 0.999 (0.92–1.082) | 0.98 |
| Diabetes | 49 (27.7) | 14 (43.8) | 2.047 (0.934–4.425) | 0.07 |
| HCC | 136 (76.4) | 19 (59.4) | 0.451 (0.207–1.0) | 0.047 |
| HCV | 37 (21.3) | 11 (36.7) | 2.144 (0.914–4.847) | 0.07 |
| HBV | 23 (13.2) | 3 (10.0) | 0.729 (0.165–2.288) | 0.63 |
| Previous hepatectomy | 8 (4.6) | 4 (13.3) | 3.935 (1.119–12.712) | 0.02 |
| Previous abdominal surgery | 84 (47.2) | 17 (53.1) | 1.268 (0.596–2.723) | 0.54 |
| ASA 3/4 | 97 (55.4) | 22 (68.8) | 1.769 (0.791–3.956) | 0.16 |
| Surgical variables | ||||
| Number of previously performed LLRs | 279 (255–302) | 268 (210–326) | 1.0 (0.997–1.002) | 0.72 |
| Major hepatectomy | 40 (22.7) | 12 (37.5) | 2.04 (0.899–4.489) | 0.08 |
| Simultaneous ablation | 5 (2.8) | 3 (9.4) | 3.579 (0.704–15.402) | 0.09 |
| MILL | 143 (80.3) | 17 (54.8) | 0.315 (0.143–0.701) | 0.004 |
| HALS | 31 (17.4) | 12 (38.7) | 1.362 (0.535–3.183) | 0.49 |
| SILS | 4 (2.2) | 2 (6.5) | 2.9 (0.390–15.552) | 0.23 |
| Length of surgery (LOS) in min. | 226.9 (213.6–240.1) | 296.7 (260.4–333.0) | 1.008 (1.004–1.012) | <0.001 |
| Conversion | 1 (0.6) | 2 (6.3) | 11.8 (1.097–258.613) | 0.046 |
| R1 Status | 15 (8.6) | 10 (31.3) | 4.818 (1.891–12.011) | <0.001 |
| Perioperative RBCs transfused | 0.11 (0.0–0.21) | 0.31 (0.0–0.65) | 3.821 (0.736–16.915) | 0.18 |
| Maximum tumor diameter in cm | 4.5 (4.0–4.9) | 4.2 (3.4–5.1) | 0.974 (0.848–1.10) | 0.69 |
| Liver function variables | ||||
| ALT, U/L | 41.3 (33.8–48.9) | 52.1 (26.8–77.3) | 1.008 (1.0–1.016) | 0.037 |
| AST, U/L | 48.4 (38.9–58.0) | 56.7 (22.7–90.7) | 1.004 (0.997–1.01) | 0.25 |
| Thrombocytes | 192.1 (173.8–210.5) | 200.5 (139.9–261.22) | 1 (0.995–1.004) | 0.98 |
| Thrombocytes <100/uL | 18 (10.3) | 7 (23.3) | 2.638 (0.941–6.809) | 0.052 |
| Albumin mg/dl | 41.2 (40.0–42.4) | 40.0 (38.4–42.7) | 0.979 (0.901–1.070) | 0.62 |
| Bilirubin mg/dl | 0.67 (0.59–0.76) | 0.63 (0.51–0.75) | 0.772 (0.249–1.59) | 0.59 |
| INR | 1.1 (1.08–1.13) | 1.1 (1.07–1.16) | 3.772 (0.098–107.931) | 0.45 |
| ALBI score | −2.85 (−2.97−(−2.74)) | −2.76 (−3.06–(−2.48)) | 1.278 (0.516–2.935) | 0.575 |
| FIB−4 | 3.24 (2.83–3.66) | 3.28 (2.25–4.3) | 1.005 (0.853–1.145) | 0.95 |
| LiMAX µg/kg/h | 322 (289–355) | 379 (306–451) | 1.002 (0.999–1.005) | 0.15 |
| Cirrhosis in imaging | 119 (70.8) | 18 (62.1) | 0.674 (0.30–1.570) | 0.35 |
| Cirrhosis in pathology | 98 (58.0) | 14 (46.7) | 0.634 (0.287–1.385) | 0.25 |
| Advanced fibrosis (grade III-IV) | 115 (68.0) | 20 (66.7) | 0.939 (0.412–2.143) | 0.88 |
| Portal Hypertension in imaging | 50 (29.8) | 9 (31.0) | 1.062 (0.434–2.435) | 0.89 |
| MELD | 8.2 (7.7–8.6) | 9.1 (6.9–11.3) | 1.077 (0.922–1.234) | 0.30 |
| Preoperative ascites | 2 (1.3) | 1 (3.8) | 3.14 (0.142–33.970) | 0.36 |
| Clavien–Dindo Grade | Frequency |
|---|---|
| 3a | 17 (8.1%) |
| Biliary leakage | 6 |
| Intraabdominal abscess | 5 |
| Pleural effusion | 3 |
| Pneumothorax | 2 |
| Wound infection | 1 |
| 3b | 11 (5.2%) |
| Biliary leakage | 2 |
| Burst abdomen | 3 |
| Ileus | 1 |
| Intraabdominal abscess | 2 |
| Postoperative hemorrhage | 1 |
| Wound infection | 2 |
| 4 | 0 (0%) |
| 5 | 4 (1.9%) |
| Congestive heart failure | 1 |
| Pneumonia | 1 |
| Pulmonary embolism | 1 |
| ISGLS C post hepatectomy liver failure | 1 |
| Odds Ratio | p Value | |
|---|---|---|
| Preoperative model | ||
| Diabetes | 2.74 (1.13–6.63) | 0.026 |
| Rehepatectomy | 5.79 (1.46–22.92) | 0.013 |
| ALT | 1.01 (1.0–1.02) | 0.033 |
| Non-HCC | 3.27 (1.28–8.36) | 0.014 |
| Standard multiport approach | 0.26 (0.11–0.63) | 0.003 |
| Postoperative variables | ||
| Length of surgery (LOS) | 1.01 (1.0–1.01) | 0.002 |
| Conversion | 23.4 (1.57–350.1) | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haber, P.K.; Maier, C.; Kästner, A.; Feldbrügge, L.; Ortiz Galindo, S.A.; Geisel, D.; Fehrenbach, U.; Biebl, M.; Krenzien, F.; Benzing, C.; et al. Predicting the Risk of Postoperative Complications in Patients Undergoing Minimally Invasive Resection of Primary Liver Tumors. J. Clin. Med. 2021, 10, 685. https://doi.org/10.3390/jcm10040685
Haber PK, Maier C, Kästner A, Feldbrügge L, Ortiz Galindo SA, Geisel D, Fehrenbach U, Biebl M, Krenzien F, Benzing C, et al. Predicting the Risk of Postoperative Complications in Patients Undergoing Minimally Invasive Resection of Primary Liver Tumors. Journal of Clinical Medicine. 2021; 10(4):685. https://doi.org/10.3390/jcm10040685
Chicago/Turabian StyleHaber, Philipp K., Christoph Maier, Anika Kästner, Linda Feldbrügge, Santiago Andres Ortiz Galindo, Dominik Geisel, Uli Fehrenbach, Matthias Biebl, Felix Krenzien, Christian Benzing, and et al. 2021. "Predicting the Risk of Postoperative Complications in Patients Undergoing Minimally Invasive Resection of Primary Liver Tumors" Journal of Clinical Medicine 10, no. 4: 685. https://doi.org/10.3390/jcm10040685
APA StyleHaber, P. K., Maier, C., Kästner, A., Feldbrügge, L., Ortiz Galindo, S. A., Geisel, D., Fehrenbach, U., Biebl, M., Krenzien, F., Benzing, C., Schöning, W., Pratschke, J., & Schmelzle, M. (2021). Predicting the Risk of Postoperative Complications in Patients Undergoing Minimally Invasive Resection of Primary Liver Tumors. Journal of Clinical Medicine, 10(4), 685. https://doi.org/10.3390/jcm10040685







