A Novel Language Paradigm for Intraoperative Language Mapping: Feasibility and Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Language Paradigm
2.2. Patient and Subject Sample
2.2.1. Healthy Subjects—Evaluation Study
2.2.2. Patient Group—Feasibility Study
2.3. Procedures and Data Analysis
2.3.1. Evaluation Study
2.3.2. Feasibility Study
3. Results
3.1. Evaluation Study
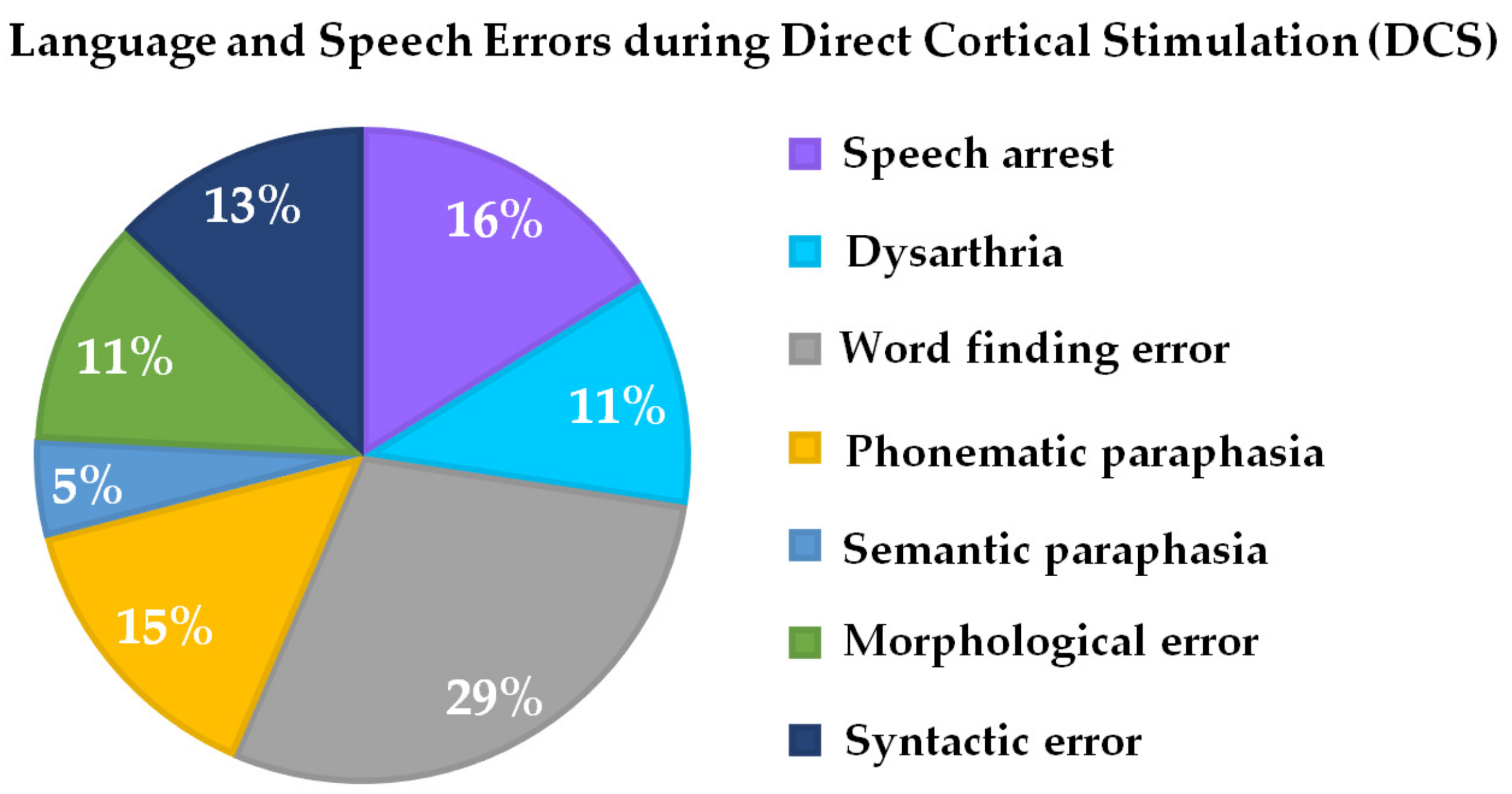
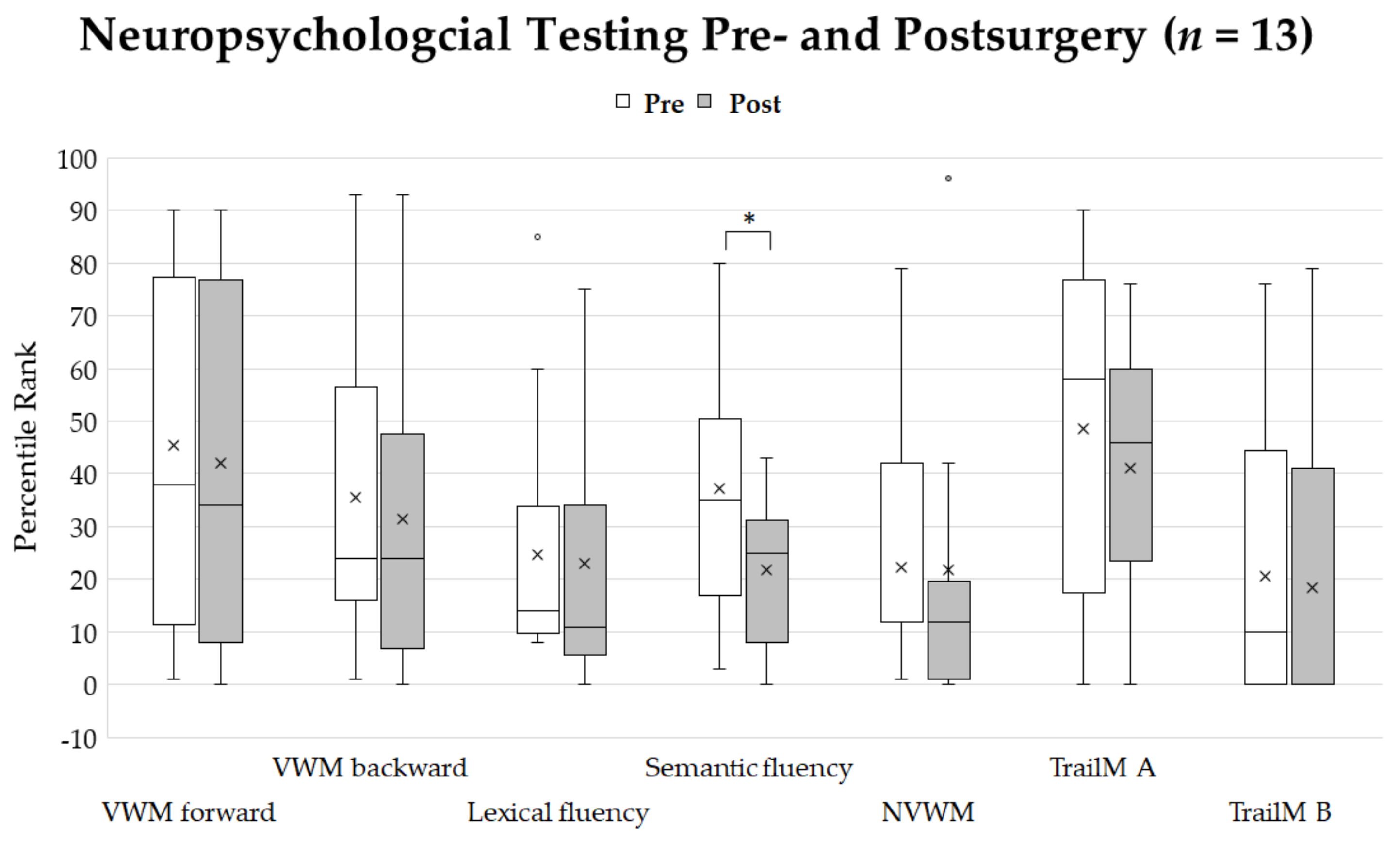
3.2. Feasibility Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bu, L.-H.; Zhang, J.; Lu, J.-F.; Wu, J.-S. Glioma surgery with awake language mapping versus generalized anesthesia: A systematic review. Neurosurg. Rev. 2020, 1–15. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Lingzhong, M.; Lau, D.; Molinaro, A.M.; Perry, D.W.; Meng, L.; Berger, M.S. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J. Neurosurg. 2015, 123, 325–339. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quiñones-Hinojosa, A.R. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef] [PubMed]
- De Witte, E.; Mariën, P. The neurolinguistic approach to awake surgery reviewed. Clin. Neurol. Neurosurg. 2013, 115, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Tang, V.; Rathbone, M.; Dorsay, J.P.; Jiang, S.; Harvey, D. Rehabilitation in primary and metastatic brain tumours. J. Neurol. 2008, 255, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Hamberger, M.J.; Tamny, T.R. Auditory naming and temporal lobe epilepsy. Epilepsy Res. 1999, 35, 229–243. [Google Scholar] [CrossRef]
- Ojemann, J.G.; Ojemann, G.A.; Lettich, E. Cortical stimulation mapping of language cortex by using a verb generation task: Effects of learning and comparison to mapping based on object naming. J. Neurosurg. 2002, 97, 33–38. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics 2009, 6, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Ojemann, G.; Mateer, C. Human language cortex: Localization of memory, syntax, and sequential motor-phoneme identification systems. Science 1979, 205, 1401–1403. [Google Scholar] [CrossRef]
- Bello, L.; Gambini, A.; Castellano, A.; Carrabba, G.; Acerbi, F.; Fava, E.; Giussani, C.; Cadioli, M.; Blasi, V.; Casarotti, A.; et al. Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. NeuroImage 2008, 39, 369–382. [Google Scholar] [CrossRef]
- Bello, L.; Acerbi, F.; Giussani, C.; Baratta, P.; Taccone, P.; Songa, V. Technique application. Neurosurgery 2006, 58, 115–125. [Google Scholar] [CrossRef]
- Bello, L.; Gallucci, M.; Fava, M.; Carrabba, G.; Giussani, C.; Acerbi, F.; Baratta, P.; Songa, V.; Conte, V.; Branca, V.; et al. Intraoperative Subcortical Language Tract Mapping Guides Surgical Removal of Gliomas Involving Speech Areas. Neurosurgery 2007, 60, 67–82. [Google Scholar] [CrossRef]
- Lubrano, V.; Filleron, T.; Démonet, J.-F.; Roux, F.-E. Anatomical correlates for category-specific naming of objects and actions: A brain stimulation mapping study. Hum. Brain Mapp. 2012, 35, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, A.; Blasi, V.; Falini, A.; Ferroli, P.; Cadioli, M.; Danesi, U.; Aquino, D.; Marras, C.; Caldiroli, D.; Broggi, G. Presurgical Functional MR Imaging of Language and Motor Functions: Validation with Intraoperative Electrocortical Mapping. Radiology 2008, 248, 579–589. [Google Scholar] [CrossRef]
- Herholz, K.; Reulen, H.-J.; Von Stockhausen, H.-M.; Thiel, A.; Llmberger, J.; Kessler, J.; Eisner, W.; Yousry, T.A.; Heiss, W.-D. Preoperative Activation and Intraoperative Stimulation of Language-related Areas in Patients with Glioma. Neurosurgery 1997, 41, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Papagno, C.; Gallucci, M.; Casarotti, A.; Castellano, A.; Falini, A.; Fava, E.; Giussani, C.; Carrabba, G.; Bello, L.; Caramazza, A. Connectivity constraints on cortical reorganization of neural circuits involved in object naming. NeuroImage 2011, 55, 1306–1313. [Google Scholar] [CrossRef]
- Rofes, A.; Miceli, G. Language Mapping with Verbs and Sentences in Awake Surgery: A Review. Neuropsychol. Rev. 2014, 24, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Rau, S.; Fesl, G.; Bruhns, P.; Havel, P.; Braun, B.; Tonn, J.-C.; Ilmberger, J. Reproducibility of Activations in Broca Area with Two Language Tasks: A Functional MR Imaging Study. Am. J. Neuroradiol. 2007, 28, 1346–1353. [Google Scholar] [CrossRef]
- Coello, A.F.; Moritz-Gasser, S.; Martino, J.; Martinoni, M.; Matsuda, R.; Duffau, H. Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J. Neurosurg. 2013, 119, 1380–1394. [Google Scholar] [CrossRef] [PubMed]
- Polczynska, M. New tests for language mapping with intraoperative electrical New tests for language mapping with intraoperative electrical stimulation of the brain to preserve language in individuals with tumors and epilepsy: A preliminary follow-up study. Pozn. Stud. Contemp. 2009, 45, 261–279. [Google Scholar]
- Rofes, A.; Spena, G.; Miozzo, A.; Fontanella, M.M.; Miceli, G. Advantages and disadvantages of intraoperative language tasks in awake surgery: A three-task approach for prefrontal tumors. J. Neurosurg. Sci. 2015, 59, 337–349. [Google Scholar] [PubMed]
- Mandonnet, E.; Winkler, P.A.; Duffau, H. Direct electrical stimulation as an input gate into brain functional networks: Principles, advantages and limitations. Acta Neurochir. 2010, 152, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Mandonnet, E.; Nouet, A.; Gatignol, P.; Capelle, L.; Duffau, H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain 2007, 130, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Kayama, T.; Guidelines committee of the Japan Awake Surgery Conference. The Guidelines for Awake Craniotomy Guidelines committee of the Japan awake surgery conference. Neurol. Med. Chir. 2012, 52, 119–141. [Google Scholar] [CrossRef]
- Deloche, G.; Hannequin, D. Test de Dénomination Orale d‘Images DO 80; Les Editions du Centre de Psychologie Appliquee: Paris, France, 1997; Volume 7. [Google Scholar]
- Presentation. Available online: https://www.neurobs.com (accessed on 7 February 2021).
- Hansen, E.; Seemann, M.; Zech, N.; Doenitz, C.; Luerding, R.; Brawanski, A. Awake craniotomies without any sedation: The awake-awake-awake technique. Acta Neurochir. 2013, 155, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Adult Intelligence Scale—Fourth Edition; Pearson: New York, NY, USA, 2012. [Google Scholar]
- Corsi, P.M. Human Memory and the Medial Temporal Region of the Brain; McGill University: Montreal, QC, Canada, 1972. [Google Scholar]
- Aschenbrenner, S.; Tucha, O.; Lange, K.W. Regensburger Wortflüssigkeits—Test: RWT Handanweisung; Hogrefe, Verlag für Psychologie: Göttingen, Germany, 2000. [Google Scholar]
- Spreen, O.; Strauss, E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary; Oxford University Press: New York, NY, USA; Oxford, UK, 1991. [Google Scholar]
- Reitan, R.; Wolfson, D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation; Reitan Neuropsychology; Springer: Berlin/Heidelberg, Germany, 1985; Volume 4. [Google Scholar]
- Microsoft PowerPoint. Available online: https://www.microsoft.com (accessed on 7 February 2021).
- Sanai, N.; Mirzadeh, Z.; Berger, M.S. Functional Outcome after Language Mapping for Glioma Resection. N. Engl. J. Med. 2008, 358, 18–27. [Google Scholar] [CrossRef]
- Brainlab Transforms Healthcare with Software-Based Technology. Available online: https://www.brainlab.com (accessed on 7 February 2021).
- Hickok, G.; Poeppel, D. Neural basis of speech perception. Handb. Clin. Neurol. 2015, 129, 149–160. [Google Scholar] [CrossRef]
- Friederici, A.D.; Gierhan, S.M.E. The language network. Curr. Opin. Neurobiol. 2013, 23, 250–254. [Google Scholar] [CrossRef]
- Vigneau, M.; Beaucousin, V.; Hervé, P.-Y.; Duffau, H.; Crivello, F.; Houdé, O.; Mazoyer, B.; Tzourio-Mazoyer, N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage 2006, 30, 1414–1432. [Google Scholar] [CrossRef]
- Berg, T.; Levelt, W.J.M. Speaking: From Intention to Articulation. Am. J. Psychol. 1990, 103, 409. [Google Scholar] [CrossRef]
- Petro, A.; Pinker, S. The Language Instinct. TESOL Q. 1996, 30, 365. [Google Scholar] [CrossRef]
- Friederici, A.D. Towards a neural basis of auditory sentence processing. Trends Cogn. Sci. 2002, 6, 78–84. [Google Scholar] [CrossRef]
- Binder, J.R. The Wernicke area. Neurology 2015, 85, 2170–2175. [Google Scholar] [CrossRef]
- Duffau, H.; Lopes, M.; Arthuis, F.; Bitar, A.; Sichez, J.-P.; Van Effenterre, R.; Capelle, L. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985-96) and with (1996-2003) functional mapping in the same institution. J. Neurol. Neurosurg. Psychiatry 2005, 76, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Capelle, L.; Denvil, D.; Sichez, N.; Gatignol, P.; Taillandier, L.; Lopes, M.; Mitchell, M.-C.; Roche, S.; Muller, J.-C.; et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: Functional results in a consecutive series of 103 patients. J. Neurosurg. 2003, 98, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Sefcikova, V.; Sporrer, J.K.; Ekert, J.O.; Kirkman, M.A.; Samandouras, G. High Interrater Variability in Intraoperative Language Testing and Interpretation in Awake Brain Mapping Among Neurosurgeons or Neuropsychologists: An Emerging Need for Standardization. World Neurosurg. 2020, 141, e651–e660. [Google Scholar] [CrossRef]
- Shao, Z.; Ejanse, E.; Evisser, K.; Meyer, A.S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol. 2014, 5, 772. [Google Scholar] [CrossRef]
- Santini, B.; Talacchi, A.; Squintani, G.; Casagrande, F.; Capasso, R.; Miceli, G. Cognitive outcome after awake surgery for tumors in language areas. J. Neuro Oncol. 2012, 108, 319–326. [Google Scholar] [CrossRef]
- Satoer, D.; Visch-Brink, E.; Smits, M.; Kloet, A.; Looman, C.; Dirven, C.; Vincent, A. Long-term evaluation of cognition after glioma surgery in eloquent areas. J. Neuro Oncol. 2014, 116, 153–160. [Google Scholar] [CrossRef]
- Satoer, D.; Vork, J.; Visch-Brink, E.; Smits, M.; Dirven, C.; Vincent, A. Cognitive functioning early after surgery of gliomas in eloquent areas. J. Neurosurg. 2012, 117, 831–838. [Google Scholar] [CrossRef]
- Bonifazi, S.; Passamonti, C.; Vecchioni, S.; Trignani, R.; Martorano, P.; Durazzi, V.; Lattanzi, S.; Mancini, F.; Ricciuti, R. Cognitive and linguistic outcomes after awake craniotomy in patients with high-grade gliomas. Clin. Neurol. Neurosurg. 2020, 198, 106089. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, E.; Snijders, T.J.; Baumfalk, A.E.; Ruis, C.; Van Baarsen, K.M.; Broekman, M.L.; Van Zandvoort, M.J.E.; Robe, P.A. Neurocognitive changes after awake surgery in glioma patients: A retrospective cohort study. J. Neuro Oncol. 2020, 146, 97–109. [Google Scholar] [CrossRef] [PubMed]

| Patient Number | Age | Handedness | Tumor Location | Diagnosis | Presurgical Karnofsky | Presurgical Seizures | Presurgical Deficit | Postsurgical Deficit | Intraoperative Deficit |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | Right | IF | OD II, recurrent | 100 | Yes | None | None | WF, SA, PP, SP, SE, ME |
| 2 | 21 | Right | SG, AG | AA III | 90 | Yes | Mild AP | Mild AP | WF, SA |
| 3 | 61 | Right | SG, AG | GBM | 100 | Yes | None | None | WF, SA |
| 4 | 28 | Right | IF | A II | 100 | Yes | None | None | WF, SA |
| 5 | 47 | Right | IF | AOD III | 100 | Yes | None | Mild DA | SA, DA |
| 6 | 26 | Right | STG | AA III | 100 | Yes | None | None | WF, SE, ME |
| 7 | 36 | Right | STG, MTG | AA III | 90 | No | WF | None | DA, PP |
| 8 | 38 | Right | Premotor area | AA III | 100 | Yes | None | None | WF, SA, DA, ME |
| 9 | 32 | Right | STG, MTG | AA III, recurrent | 90 | Yes | Mild DA | None | WF, PP, SE, ME |
| 10 | 27 | Right | IF | AA III, recurrent | 100 | No | None | None | WF, PP, SE |
| 11 | 27 | Left | IF | AA III | 100 | Yes | None | None | WF |
| 12 | 37 | Right | Premotor area | AA III | 100 | No | None | None | SA |
| 13 | 52 | Right | IF | AA III | 100 | No | None | None | WF, DA, PP, SE, ME |
| 14 | 55 | Right | SG | AA III | 90 | Yes | Mild DA | Mild DA | DA |
| 15 | 68 | Right | IC, premotor area | AA III | 90 | Yes | Mild AP | None | WF, SA |
| 16 | 76 | Right | IF | AOD III | 100 | No | None | None | WF, PP |
| 17 | 12 | Right | MTG | GG I | 90 | Yes | WF | None | WF, SA, PP, SP |
| 18 | 20 | Right | STG | PA | 90 | Yes | WF | None | WF |
| 19 | 53 | Right | Premotor area | GBM | 90 | Yes | Mild DA | Mild DA | WF, ME, SE |
| 20 | 59 | Right | SG, postG | GBM | 90 | Yes | WF | Mild DA | DA |
| 21 | 41 | Right | IF | AA III | 100 | Yes | None | None | WF, SA |
| 22 | 61 | Right | IF | AA III | 90 | Yes | Mild DA | None | WF, DA, PP, ME, SE |
| 23 | 27 | Right | IF | AA III | 100 | Yes | None | None | WF |
| 24 | 42 | Right | MTG | OD II | 100 | Yes | None | None | PP, SP, SE |
| MNI Coordinates | Cluster Size | Region | Brodmann Area | Hemisphere | T-Value | pFWE | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| 34 | −86 | 18 | 2598 | Fusiform gyrus, MOG, IOG, SOG, lingual gyrus, SPG, MTG | 7, 18, 19, 37 | R | 21.85 | 0.000 |
| −18 | 2 | 8 | 342 | Putamen, pallidum | L | 17.87 | 0.000 | |
| −38 | 2 | 34 | 835 | Precentral gyrus, postcentral gyrus, IFG | 4, 6 | L | 17.46 | 0.000 |
| −8 | −24 | −10 | 881 | Thalamus, hippocampus | L | 16.31 | 0.000 | |
| −40 | −84 | −4 | 2497 | MOG, fusiform gyrus, IOG, SPG, ITG, IPG, SOG | 7, 19, 37 | L | 16.16 | 0.000 |
| 24 | −30 | −2 | 268 | Thalamus | R | 13.35 | 0.000 | |
| −6 | 14 | 44 | 424 | SMA | 6 | L and R | 13.19 | 0.000 |
| −24 | 26 | 12 | 541 | IFG (PT), IFG (PO), Insula | L | 10.80 | 0.000 | |
| 32 | 28 | −2 | 96 | Insula | R | 9.59 | 0.000 | |
| −2 | −94 | 2 | 50 | Fissura calcarina | L | 8.97 | 0.000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosengarth, K.; Pai, D.; Dodoo-Schittko, F.; Hense, K.; Tamm, T.; Ott, C.; Lürding, R.; Bumes, E.; Greenlee, M.W.; Schebesch, K.M.; et al. A Novel Language Paradigm for Intraoperative Language Mapping: Feasibility and Evaluation. J. Clin. Med. 2021, 10, 655. https://doi.org/10.3390/jcm10040655
Rosengarth K, Pai D, Dodoo-Schittko F, Hense K, Tamm T, Ott C, Lürding R, Bumes E, Greenlee MW, Schebesch KM, et al. A Novel Language Paradigm for Intraoperative Language Mapping: Feasibility and Evaluation. Journal of Clinical Medicine. 2021; 10(4):655. https://doi.org/10.3390/jcm10040655
Chicago/Turabian StyleRosengarth, Katharina, Delin Pai, Frank Dodoo-Schittko, Katharina Hense, Teele Tamm, Christian Ott, Ralf Lürding, Elisabeth Bumes, Mark W Greenlee, Karl Michael Schebesch, and et al. 2021. "A Novel Language Paradigm for Intraoperative Language Mapping: Feasibility and Evaluation" Journal of Clinical Medicine 10, no. 4: 655. https://doi.org/10.3390/jcm10040655
APA StyleRosengarth, K., Pai, D., Dodoo-Schittko, F., Hense, K., Tamm, T., Ott, C., Lürding, R., Bumes, E., Greenlee, M. W., Schebesch, K. M., Schmidt, N. O., & Doenitz, C. (2021). A Novel Language Paradigm for Intraoperative Language Mapping: Feasibility and Evaluation. Journal of Clinical Medicine, 10(4), 655. https://doi.org/10.3390/jcm10040655








